Abstract
Background
Tuberculosis (TB) remains a significant global public health problem. China has the second highest TB burden in the world. With a growing TB population with diabetes mellitus (DM), the TB control system faces mounting challenges. To date, evidence remains inconclusive regarding the association between TB-DM co-morbidity and delayed diagnosis of TB patients. This study aims to assess the diagnostic delay of TB patients with known DM and identify the factors associated with this delay.
Methods
Data was collected from China’s Tuberculosis information management system in two counties of Zhejiang province, China. Patient delay, health system delay and total diagnostic delay are defined as follows: 1) the interval between the onset of TB symptoms and first visit to any health facility; 2) from the first visit to the health facility to the confirmed TB diagnosis in the designated hospital; 3) the sum of patient and health system’s respective delays. Comparison of these delays was made between TB patients with and without DM using Mann-Whitney U test and Chi-square test. Univariate and multivariate regression analysis was used to identify factors influencing delays among TB patients with DM.
Results
Of 969 TB patients, 67 (7%) TB patients had DM co-morbidity. Compared with TB patients without DM, TB patients with DM experienced significantly shorter health system delays (p < 0.05), and there was a significantly lower proportion of patients whose health system delayed> 14 days (7.0% vs. 18%, p < 0.05). However, no significant difference was observed between both patient categories regarding patient delay and total diagnostic delay. The multivariate regression analysis suggested that TB patients with DM who were aged < 60 years (AOR = 3.424, 95%CI: 1.008–11.627, p < 0.05) and non-severe cases (AOR = 9.725, 95%CI: 2.582–36.626, p < 0.05) were more likely to have a total diagnostic delay of> 14 days.
Conclusions
Our study suggests that DM does not contribute to further diagnostic delay as expected. Instead, we observed significantly improved health system delay among TB patients with DM. The findings indicate the importance of early screening and diagnosis for TB among diabetic patients and of strengthening the integrated control and management of TB and diabetic programs.
Similar content being viewed by others
Background
Tuberculosis (TB) remains a significant global public health problem. In 2018, 10 million people were newly diagnosed with TB with 1.24 million deaths [1]. Early diagnosis and timely treatment of TB plays an important role in TB control, especially in controlling the spread of TB within the community [2] and avoiding poor disease prognosis [3]. However, health-care seeking delays in TB patients are common. Studies have reported the socioeconomic factors associated with delayed health-seeking among TB patients, such as older age, being female, low education level, and income [4,5,6] as well as clinic characteristics such as smear positive results, pulmonary cavity, cough, and night sweats [3, 7, 8].
Diabetes mellitus (DM) is a severe chronic disease characterized by hyperglycemia, which leads to disabling and life-threatening health complications [9]. Global figures for 2019 show that an estimated 463 million adults in the 20–79 age range live with DM, while the total number is predicted to rise to 578 million by 2030 [9]. Furthermore, the deaths attributed to DM and related complications in 2019 is estimated to surpass 4 million [9]. DM is an important risk factor for TB; people with DM were reported to have a threefold higher probability of getting TB compared to people without DM [10]. Worldwide research studies indicate that 1.9–50% of TB patients have DM [11, 12]. It is generally believed that TB is a disease of poverty, as 97% of TB cases were reported from 119 low-and middle-income countries in 2018 [1]. With DM co-morbidity, TB patients face even greater financial burden and the co-morbidity could seriously impoverish both patients and fragile health systems. TB patients with DM have to deal with more complicated problems than general TB patients such as contradictory dietary recommendations and low adherence to medication. The screening, treatment and care of patients with both TB and DM also require coordinated planning and service delivery across communicable and non-communicable disease programs [13]. Thus, the International Union against Tuberculosis and Lung Disease (IUATLD) issued guidelines for management of patients with TB-DM co-morbidity following the launch of a collaborative framework to advocate and implement joint care and control of TB and DM with the World Health Organization (WHO) in 2011 [14, 15].
China has the second highest TB burden in the world [1]. In 2018, an estimated 860,000 people fell ill with TB, accounting for 9% of the world’s TB case burden [1]. TB caused nearly 40,000 deaths in the same year. China also has one of the largest burdens in DM in the world, with 9.2% age-adjusted comparative prevalence and a population living with diabetes of 100 million in 2019 [9]. In a pilot project conducted in TB clinics /hospitals in China, the overall prevalence of DM in patients with TB was 12.4% [16]. Another community-based cohort study showed that TB patients had a higher odds ratio (OR: 3.17) of having DM than non-TB controls [17]. In most of China, and since the year 2000, TB patients receive standardized diagnosis and treatment in the TB clinic that is integrated in the county’s designated general hospital [18]. Other health facilities, including township hospitals and village clinics refer TB patients or presumptive TB patients to the designated TB hospitals for standardized diagnosis and treatment [18].
To date, most studies on TB and DM are epidemiological studies [11, 16, 17]. Very few studies have focused on the management of patients with TB-DM co-morbidity [13]. In addition, few studies examine delays in health-care seeking among TB-DM co- morbidity patients or explore factors that might explain these delays within this patient group. In an observational study conducted at community level, Wang et al. found that hyperglycemia was significantly associated with a higher risk of total diagnostic delay in TB patients over 30 years. This study also found that older age and lack of TB awareness are associated with a significantly higher risk of such delay in TB patients [3]. Chen et al. reported that DM was associated with a longer patient delay of TB patients in two TB dispensaries of Beijing, and smear positivity was positively associated with patient delay among TB patients with DM [7]. While both studies have reported that DM is associated with more serious diagnostic delay in TB patients [3, 7], more evidence is needed to verify this relationship. This study examines delayed diagnosis of TB patients with DM as compared to those without DM and identifies factors causing these delays to provide the evidence base for improved case detection and management of TB patients with DM co-morbidity.
Methods
Study design
This is a retrospective study of delayed diagnosis of TB patients with DM as compared to those without DM. Data were collected from patient records exported from China’s Tuberculosis information management system (TBIMS, TB special reporting system version 2.0).
Study setting
This study was conducted in Cangnan and Yongjia, two counties of Wenzhou City, Zhejiang province. Table 1 shows the socioeconomic, demographic and health service information for the two counties under study and Wenzhou city. The permanent population of both studied counties are 1.35 million and 0.98 million respectively (8.25 million in Wenzhou City). Both counties have an average per capita GDP of US $5677 and $5672 in 2017 respectively, lower than that of Wenzhou city ($9761), and much lower than that of Zhejiang province($13,638). Similarly, the per capita disposable income for both counties is also lower than that of Wenzhou City and Zhejiang Province [19, 20]. The number of beds, practicing (assistant) physicians, and registered nurses per 1000 people in the two counties are also lower than that of Wenzhou city [19] (Table 1). In both counties, there is a designated general hospital where all TB cases are either referred to or self-presented for standardized diagnosis and treatment.
Data collection
This study was coordinated by Center for Disease Prevention and Control (CDCs) of Zhejiang Province, Cangnan CDC and Yongjia CDC. The CDC staff in these two counties exported patient data in 2017 from TBIMS to the Microsoft Excel (Microsoft, Redmond, WA, USA). Data covers general characteristics of TB patients such as age, sex, household registration status, level of hospital for initial TB diagnosis; clinical information such as TB severity (e.g. with large cavities or lesions in more than two lung lobes), cavity, treatment duration, smear sputum results, treatment classification, DM status; and health service-related data such as time of onset of TB symptoms, time of first health-care visit, time of confirmed TB diagnosis. Most of these data (including DM status) were collected and recorded during TB consultation by the TB health workers at the time of TB registration. DM is routinely screened through self-report of TB patients during TB consultation, and laboratory examination of blood sugar and glycated hemoglobin (HbA1C) is not compulsory.
Definitions
In this paper, we study patient delay, health system delay and total diagnostic delay. Patient delay is defined as the interval between the onset of TB symptoms and first visit to a health facility. Health system delay is defined as the interval between the first visit to a health facility and confirmed TB diagnosis in the TB designated hospital. Total diagnostic delay (hereinafter referred to as total delay) is defined as the interval between the onset of TB symptoms and TB confirmation diagnosis, which is the sum of the patient delay and health system delay. In the meantime, we use 14 days as a cut-off point for analysis of patient delay, health system delay and total delay based on previous studies [21, 22]. Diagnosis is mainly based on sputum smear examination, supplemented by sputum culture and X-Ray. Patients with TB, patients with presumptive TB, and those with presumptive TB symptoms would have sputum checked three times, that is, to examine a sample of “instant sputum” in the outpatient clinic on the same day, and “night sputum” and “morning sputum” for examination the next day [23].
Data analysis
Data was analyzed using SPSS 21.0 (SPSS, Inc., Chicago, USA). Descriptive statistics were adopted to report general and delay characteristics of TB patients with and without DM (including patient delay, health system delay and total delay). Median and Interquartile Range (IQR) was used for continuous variables, while counts and proportions were used for categorical variables. The univariate analysis including Mann-Whitney U Test and Chi-square test was employed to compare general characteristics and delay between TB patients with and without DM, and identify factors that are associated with delay of TB patients with DM. The multivariate regression analysis including linear regression and binary logistic regression was used to confirm factors associated with delay of TB patients with DM. The dependent variables were number of days of patient delay, health system delay and total delay in the linear regression, and patient delay, health system delay and total delay > 14 days (Yes = 1, No = 0) in the binary logistic regression, respectively. The independent variables were general and clinical characteristics of patients. The independent variables of delay with a p value < 0.2 in the Mann-Whitney U Test and Chi-square test were included in the subsequent multivariate regression analysis, using backward method, to adjust for potential confounding and identify those which were statistically associated with delay. The results were presented as adjusted ORs with 95% CI. The significant level was set at 5%.
Results
General and clinical characteristics of TB patients with DM as compared to those without DM
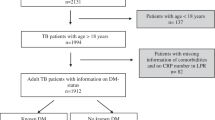
Of all 969 TB patients, 67 (7%) had TB-DM co-morbidity. The median (IQR) age was 47 (30–62), 27% were above 60 years, 70% were male, 66% were local residents, 36% were smear positive patients, 89% were newly registered patients, 23% were severe cases, 33% have cavity. The median (IQR) treatment duration was 330 (187–366) days, 81% received initial TB diagnosis at county-level hospital, 31% were cured and 61% completed treatment. The DM prevalence among male and female patients was 7.8% (53/681) and 4.9% (14/288), and no significant difference was found between these two groups.
Compared with TB patients without DM, TB patients with DM had significantly higher median age (58 vs. 46, p < 0.05), higher proportion of patients who were above 60 years (43% vs. 26%, p < 0.05), local residents (88% vs. 65%, p < 0.05), smear positive (51% vs. 35%, p < 0.05), new patients (100% vs. 88%, p < 0.05), severe cases(36% vs. 22%, p < 0.05), with cavity (54% vs. 32%, p < 0.05), and who had initially been diagnosed at county-level general hospitals (94% vs. 80%, p < 0.05) (Table 2).
Patient delay, health system delay and total delay of TB patients with DM as compared to those without DM
Of all 969 patients, the median (IQR) patient delay was 15 (5–35) days, while 52% had patient delay> 14 days. The median (IQR) health system delay was 0 (0–4) days, while 17% had system delay> 14 days. The median (IQR) total delay was 20 (7–48) days, while 61% had total delay> 14 days.
Compared with TB patients without DM, TB patients with DM experienced significantly shorter health system delays (0 vs. 0, p < 0.05), had significantly lower proportion of patients whose health system delay> 14 days (7.0% vs. 18%, p < 0.05). No significant difference was found between these two patient categories regarding patient delay (17 vs. 15, p > 0.05), total delay (19 vs. 21, p > 0.05), proportion of patients with patient delay (54% vs. 52%, p > 0.05) and total delay> 14 days (55% vs. 61%, p > 0.05) (Table 3).
In addition, TB patients with DM co-morbidity had higher proportion of health system delays of 0 day, as compared to those without DM co-morbidity (82% vs. 58%, p < 0.05, See Table 3).
Factors influencing patient delay, health system delay and total delay of TB patients with DM
In TB patients with DM, univariate analysis showed that the variable of severe case or not was significantly associated with the number of days of patient delay (p < 0.05). The variables of household registration status and level of hospital for initial TB diagnosis (county-level or prefectural-level) were significantly associated with the number of days of health system delays (p < 0.05). The variables of age (> 60 years or not), household registration status, severe case or not and level of hospital for initial TB diagnosis (county-level or prefectural-level) were significantly associated with the number of days of total delays (p < 0.05). Further linear regression showed that, TB-DM patients who were initially diagnosed at prefectural-level hospital (AOR = 25.179, 95%CI: 14.698–35.659) tended to have longer health system delays (Table 4).
Similarly, univariate analysis showed that the variable of severe case or not was significantly associated with patient delay> 14 days (p < 0.05). The variables of household registration status, and level of hospital for initial TB diagnosis (county-level or prefectural-level) were significantly associated with health system delay> 14 days (p < 0.05). The variable of severe cases or not were significantly associated with the total delay> 14 days (p < 0.05). Further multivariate regression analysis showed that, TB patients with DM who were non-severe cases (AOR = 5.031, 95%CI: 1.696–14.918) were more likely to have patient delay> 14 days. TB patients with DM who were < 60 years (AOR = 3.424, 95%CI: 1.008–11.627), non-severe cases (AOR = 9.725, 95%CI: 2.582–36.626) were more likely to have total delay> 14 days (Table 5).
Discussion
Summary of findings
In the present study, we found that of 969 TB patients, 7% patients had TB-DM co- morbidity, and TB patients with DM tended to be of older age, local residents, smear positive patients, new patients, severe cases and have cavity. The median total delay of TB patients with DM was 19 days, as compared to 21 days for TB patients without DM. Compared with TB patients without DM, TB patients with DM experienced shorter health system delays and lower probability of health system delay> 14 days. TB patients with DM who were aged< 60 years, non-severe cases were more likely to have total diagnostic delay > 14 days.
Comparison with literature
In recent years China has undergone a rapid increase in DM burden in the context of high TB burden, thus, the TB control system faces a double challenge posed by TB- DM co-morbidity. Our study found that prevalence of DM in TB patients was 7%, which is similar to prevalence reported in Wang et al.’s community-based cohort study (6.3%) [17], but much lower than that in Li et al.’s study (12.4%) [16]. One possible explanation for the inconsistency in different DM prevalence studies is that our analysis is based on the data recorded from routine TB clinical consultations, where DM information is collected based on TB patients’ self-report and blood sugar screening is not routinely conducted among TB patients. In Wang et al.’s study, most TB patients were screened for DM in 2–3 weeks after the initiation of TB treatment, to avoid a potential over-diagnosis of hyperglycemia induced by TB temporarily [17]. It is noteworthy that DM prevalence among male TB patients was higher than that among female patients (7.8% vs. 4.9%), although without significant difference. It is worth exploring the underlying causes of this pattern of prevalence variation, taking into consideration, for example, the sex factor or access variables between male and female patients.
It was previously reported that DM patients are susceptible to lower respiratory tract infections [3]. The frequent symptoms of cough, fever, and chills would overlap typical TB symptoms, resulting in longer health-seeking delays in TB patients with DM. However, contrary to previous studies [3, 7], we found that TB patients with DM are associated with shorter patient delay and total delay as compared to TB patients without DM (although without statistical significance). One possible explanation for this is that TB patients with DM, especially when there was a higher proportion of severe cases among them as compared to those without DM in our study, may tend to seek health services earlier as they suffer from more serious clinical symptoms. In addition, we found significantly shorter health system delay among TB patients with DM as compared to TB patients without DM. This pattern is also true for the proportions of patients whose patient, health system and total delays were > 14 days. The significantly improved health system delay for TB patients with DM may be partly due to the improved knowledge and awareness of risk factors (including DM) for TB and improved referrals of patients with high risk to TB among health providers. In some places, TB screening is conducted among high-risk populations, including the elderly and diabetic patients. However, health providers should also recognize other common risk factors to TB such as HIV, smoking, alcoholism, malnutrition [1], and provide timely screening, diagnosis of TB or referral to the TB designated hospital. It is noteworthy that we identified a higher proportion of TB patients with DM with a health system delay of 0 days. As they are likely to seek diabetic care in the same general hospital where the designated TB clinic is located, it becomes easier for them to receive an internal referral to the TB clinic, where they are diagnosed and treated without further delays. Our study suggests the importance of health education and promotion to improve risk awareness for TB among diabetic patients, since we did not find significant difference between these two categories of patients with regards to patient delay and total diagnostic delay (which is mainly contributed by the patient delay). However, although we did not observe more serious delay in TB patients with DM, one should not neglect the challenges of managing patients with TB-DM co-morbidity [13].
Very few studies focused on factors affecting delay of patients with TB-DM co- morbidity. Chen et al. reported that smear positivity was positively associated with patient delay> 30 days for TB patients with DM [7]. Other studies have also reported that a higher risk of patient delay> 14 days was associated with smear positivity for TB patients [8, 24]. However, we did not observe that smear positivity had significant influence on patient delay among TB patients with DM in the present study.
Previous studies have reported that TB patients with mild TB symptoms, particularly those without hemoptysis, were more likely to have patient delay [3, 25]. Similarly, both of our univariate and multivariate regression analysis suggests that non-severe cases tended to have longer patient delay, total delay as well as a higher risk of patient delay and total delay > 14 days. It may be because severe cases may seek health services earlier as they suffered more serious clinical symptoms, while the non-severe cases tend to delay their care seeking due to milder symptoms. These findings suggest that intensive TB case finding among diabetic patients is important for early detection of TB cases, especially because some TB patients may be asymptomatic or have mild symptoms of TB, making it difficult to detect them by other means.
Similar to previous studies on migrant TB delay [26,27,28,29], our study showed that migrant TB patients with DM tended to have longer patient delays and a higher proportion of patients with patient delay> 14 days (although without statistical significance). This may be due to poor health awareness and health behavior among TB-affected migrants because of their poor socioeconomic characteristics, such as job instability, low income, poor living and working conditions [30,31,32,33,34]. In addition, our univariate analysis suggests that migrant TB patients with DM tended to have longer health system delays, total delay and a higher risk of health system delay> 14 days. This may be because migrants are mostly uninsured and floating and are less likely to follow the recommendation of timely referral [24]. Our study highlights the need to strengthen health education and referral and address financial barriers among migrant patients with DM, especially those with typical TB symptoms.
Similar to a previous study reporting delay in TB patients in China [35], our study showed that TB patients with DM who received initial TB diagnosis at a higher-level hospital (i.e. prefectural-level hospital) tended to have longer health system delays based on the multivariate regression analysis, and longer total delay and higher risk of health system delay> 14 days based on the univariate analysis. This suggests the challenge of timely referral of TB suspects or patients from the general especially tertiary hospitals to the TB program [36]. Co-morbidity with DM adds to this challenge as diabetic patients often seek care in tertiary hospitals. As a previous study in China showed, many patients made repeat visits to the prefectural-level hospital before being classified as having presumptive TB and referred to a TB-designated hospital for confirmed TB diagnosis [37]. It is, thus, important to improve monitoring and referral of persons with presumptive TB, especially among diabetic patients from higher-level health services to the TB designated hospital.
Our univariate and multivariate regression analysis suggests that TB patients with DM who were aged < 60 years endured longer total delay and have higher risk of total delay> 14 days, while other studies found older age is a risk factor for total delay [3]. One plausible reason is that patients < 60 years are of working age, and so it is difficult for them to take time away from work to visit health services [4].
Limitations
Our study has several limitations. First, as a case study, generalizability of our findings is limited but it provides the basis to undertake a larger-scale cohort study, which would help us better understand the multiple factors influencing delays of patients with TB- DM co-morbidity. Second, due to constraints of the routine practice database, we could not include further basic social-economic indicators like education and income level in the analysis. These factors could also have influence on TB patients’ health-seeking behavior [4,5,6]. Third, under-detection of DM among TB patients is possible, since we did not conduct blood sugar screening among TB patients but mainly based on patients’ self-report of the DM conditions in the routine TB practices. Finally, the comparison may be biased as we have a low proportion of TB patients with DM, but this case study provides an initial understanding of the delay characteristics of TB patients with DM and associated factors.
Conclusions
Our study suggests that DM does not contribute to further diagnostic delay as expected. Instead we observed significantly improved health system delay among TB patients with DM, although we did not find significantly reduced patient and total diagnostic delay among this patient group as compared to those without DM. Findings indicate the importance of early screening and diagnosis for TB among diabetic patients and of strengthening the integrated control and management of TB and diabetic programs.
Availability of data and materials
Information in our database is confidential, however, data used for the analysis is available upon reasonable request from corresponding author.
Abbreviations
- TB:
-
Tuberculosis
- DM:
-
Diabetes Mellitus
- IUATLD:
-
The International Union against Tuberculosis and Lung Disease
- WHO:
-
World Health Organization
- TBIMS:
-
China's Tuberculosis information management system
- IQR:
-
Interquartile Range
- ORs:
-
Odds ratio
- CIs:
-
Confidence intervals
- AOR:
-
Adjusted odds ratio
- CDCs:
-
Center for Disease Prevention and Control
- HbA1C:
-
Glycated hemoglobin
References
World Health Organization. Global tuberculosis report 2019. https://apps.who.int/iris/bitstream/handle/10665/329368/9789241565714-eng.pdf?ua=1. Accessed 17 May 2020.
Sreeramareddy CT, Qin ZZ, Satyanarayana S, Subbaraman R, Pai M. Delays in diagnosis and treatment of pulmonary tuberculosis in India: a systematic review. Int J Tuberc Lung Dis. 2014;18(3):255–66.
Wang Q, Ma A, Han X, Zhao S, Cai J, Kok FJ, et al. Hyperglycemia is associated with increased risk of patient delay in pulmonary tuberculosis in rural areas. J Diabetes. 2017;9(7):648–55.
Lin Y, Enarson DA, Chiang CY, Rusen ID, Qiu LX, Kan XH, et al. Patient delay in the diagnosis and treatment of tuberculosis in China: findings of case detection projects. Public Health Action. 2015;5(1):65–9.
Cai J, Wang X, Ma A, Wang Q, Han X, Li Y. Factors associated with patient and provider delays for tuberculosis diagnosis and treatment in Asia: a systematic review and meta-analysis. PLoS One. 2015;10(3):e0120088.
Storla DG, Yimer S, Bjune GA. A systematic review of delay in the diagnosis and treatment of tuberculosis. BMC Public Health. 2008;8:15.
Chen HG, Liu M, Jiang SW, Gu FH, Huang SP, Gao TJ, et al. Impact of diabetes on diagnostic delay for pulmonary tuberculosis in Beijing. Int J Tuberc Lung Dis. 2014;18(3):267–71.
Li T, Zhang H, Shewade HD, Soe KT, Wang L, Du X. Patient and health system delays before registration among migrant patients with tuberculosis who were transferred out in China. BMC Health Serv Res. 2018;18(1):786.
International Diabetes Federation. IDF Diabetes Atlas 9th (2019). https://www.diabetesatlas.org. Accessed 17 May 2020.
Jeon CY, Murray MB. Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. PLoS Med. 2008;5:e152.
Zheng C, Hu M, Gao F. Diabetes and pulmonary tuberculosis: a global overview with special focus on the situation in Asian countries with high TB-DM burden. Glob Health Action. 2017;10(1):1–11.
Workneh MH, Bjune GA, Yimer SA. Prevalence and associated factors of tuberculosis and diabetes mellitus co-morbidity: a systematic review. PLoS One. 2017;12(4):e0175925.
Harries AD, Kumar AM, Satyanarayana S, Lin Y, Zachariah R, Lonnroth K, et al. Diabetes mellitus and tuberculosis: programmatic management issues. Int J Tuberc Lung Dis. 2015;19(8):879–86. https://doi.org/10.5588/ijtld.15.0069.
The International Union against Tuberculosis and Lung Disease. Management of diabetes mellitus- tuberculosis: a guide to the essential practice 2019. https://www.theunion.org/what-we- do/publications/technical/english/TheUnion_DMTB_Guide.pdf. Accessed 17 May 2020.
World Health Organization and the International Union against Tuberculosis and Lung Disease. Collaborative Framework for Care and Control of Tuberculosis and Diabetes 2011. https://www.theunion.org/what-we-do/publications/technical/english/collaborative framework tb-diabetes.pdf. Accessed 17 May 2020.
Li L, Lin Y, Mi F, Tan S, Liang B, Guo C, et al. Screening of patients with tuberculosis for diabetes mellitus in China. Tropical Med Int Health. 2012;17(10):1294–301.
Wang Q, Ma A, Han X, Zhao S, Cai J, Ma Y, et al. Prevalence of type 2 diabetes among newly detected pulmonary tuberculosis patients in China: a community based cohort study. PLoS One. 2013;8(12):e82660.
Zhong J, Yin J, Zou G, Hu Y, Walley J, Wang X, et al. Experience of implementing the integrated TB model in Zhejiang, China: a retrospective observational study. Trans R Soc Trop Med Hyg. 2016;110(4):246–51.
Wenzhou Prefectural Bureau of Statistics. 2018 Wenzhou Statistical Yearbook. http://wztjj.wenzhou.gov.cn/art/2018/11/14/art_1468704_24611681.html. Accessed 17 May 2020.
Zhejiang Provincial Bureau of Statistics. 2018 Zhejiang Statistical Yearbook. http://tjj.zj.gov.cn/col/col1525563/index.html. Accessed 17 May 2020.
TB Control Office in Ministry of Health in China. Implementation manual of the World Bank tuberculosis control program in China. 2nd ed. Beijing: Ministry of Health; 1992.
Li Y, Ehiri J, Tang S, Li D, Bian Y, Lin H, et al. Factors associated with patient, and diagnostic delays in Chinese TB patients: a systematic review and meta-analysis. BMC Med. 2013;11:156.
Department of Disease Control, Department of Medical Administration, Chinese Center for Disease Control and Prevention. Guidelines for implementing the National Tuberculosis Control Program in China (version2008) (in Chinese).
Malbasa M, Pesut D. Is there delay in diagnosis of pulmonary tuberculosis in an intermediate-to-low TB incidence setting? Pneumologia. 2011;60(3):138–42.
Wang W, Jiang Q, Abdullah ASM, Xu B. Barriers in accessing to tuberculosis care among non- residents in Shanghai: a descriptive study of delays in diagnosis. Eur J Pub Health. 2007;17(5):419–23.
Abarca Tomás B, Pell C, Bueno Cavanillas A, Guillén Solvas J, Pool R, Roura M. Tuberculosis in migrant populations. A systematic review of the qualitative literature. PLoS One. 2013;8(12):e82440.
Li X, Jiang S, Li X, Mei J, Zhong Q, Xu W, et al. Predictors on delay of initial health-seeking in new pulmonary tuberculosis cases among migrants population in East China. PLoS One. 2012;7(2):e31995.
Liang QF, Pang Y, Chen QY, Lin SF, Lin J, Zhao Y, et al. Genetic profile of tuberculosis among the migrant population in Fujian Province, China. Int J Tuberc Lung Dis. 2013;17(5):655–61.
Tobe RG, Xu L, Song P, Huang Y. The rural-to-urban migrant population in China: gloomy prospects for tuberculosis control. Biosci Trends. 2011;5(6):226–30.
Dang Y, Zou G, Peng B, Ling L. Health service seeking behavior among migrant Workers in Small and Medium-Sized Enterprises in Guangdong, China: does family migration matter? Biomed Res Int. 2018;2018:3620436.
Song X, Zou G, Chen W, Han S, Zou X, Ling L. Health service utilisation of rural-to-urban migrants in Guangzhou, China: does employment status matter? Tropical Med Int Health. 2017;22(1):82–91.
Zou G, Wei X, Deng S, Yin J, Ling L. Factors influencing the implementation of a pilot smoking cessation intervention among migrant workers in Chinese factories: a qualitative study. BMC Public Health. 2019;19(1):870.
Zou G, Zeng Z, Chen W, Ling L. Self-reported illnesses and service utilisation among migrants working in small-to medium sized enterprises in Guangdong, China. Public Health. 2015;129(7):970–8.
Wei X, Zou G, Yin J, Walley J, Yang H, Kliner M, et al. Providing financial incentives to rural-to- urban tuberculosis migrants in Shanghai: an intervention study. Infect Dis Poverty. 2012;1(1):9.
Wei X, Zou G, Walley J, Yin J, Lonnroth K, Uplekar M, et al. China tuberculosis policy at crucial crossroads: comparing the practice of different hospital and tuberculosis control collaboration models using survey data. PLoS One. 2014;9(3):e90596. https://doi.org/10.1371/journal.pone.0090596.
Zou G, King R, Walley J, Yin J, Sun Q, Wei X. Barriers to hospital and tuberculosis programme collaboration in China: context matters. Glob Health Action. 2015;8(1):27067.
Martinez L, Xu L, Chen C, Sekandi JN, Zhu Y, Zhang C, et al. Delays and pathways to final tuberculosis diagnosis in patients from a referral Hospital in Urban China. Am J Trop Med Hyg. 2017;96(5):1060.
Acknowledgements
The authors thank Dr. Keven Bermudez for his help in editing and improving English language of this manuscript.
Funding
This study was supported by the General Project of National Natural Science Foundation of China (71774049) and Zhejiang Provincial Science and Public Welfare Project (LGF19H260004). Funding source was not involved in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
GZ, WX and BC conceived and designed the study. DH and SL participated in data collection and analysis. GZ and WX wrote preliminary drafts of the report. GZ, XW and SZ revised manuscript greatly. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Ethical approval is exempted for this routine data analysis although the Center for Disease Prevention and Control (CDCs) of Cangnan County and Yongjia County, Zhejiang Province, have reviewed and approved the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Xiao, W., Huang, D., Li, S. et al. Delayed diagnosis of tuberculosis in patients with diabetes mellitus co-morbidity and its associated factors in Zhejiang Province, China. BMC Infect Dis 21, 272 (2021). https://doi.org/10.1186/s12879-021-05929-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-021-05929-8