Abstract
Background
Pneumocystis jirovecii pneumonia (PJP) can be challenging to diagnose, often requiring bronchoscopy. Since most patients suspected of PJP undergo imaging, we hypothesized that the findings of these studies could help estimate the probability of disease prior to invasive testing.
Methods
We created a cohort of patients who underwent bronchoscopy specifically to diagnose PJP and conducted a nested case-control study to compare the radiographic features between patients with (n = 72) and without (n = 288) pathologically proven PJP. We used multivariable logistic regression to identify radiographic features independently associated with PJP.
Results
Chest x-ray findings poorly predicted the diagnosis of PJP. However, multivariable analysis of CT scan findings found that “increased interstitial markings” (OR 4.3; 95%CI 2.2–8.2), “ground glass opacities” (OR 3.3; 95%CI 1.2–9.1) and the radiologist’s impression of PJP being “possible” (OR 2.0; 95%CI 1.0–4.1) or “likely” (OR 9.3; 95%CI 3.4–25.3) were independently associated with the final diagnosis (c-statistic 0.75).
Conclusions
Where there is clinical suspicion of PJP, the use of CT scan can help determine the probability of PJP. Identifying patients at low risk of PJP may enable better use of non-invasive testing to avoid bronchoscopy while higher probability patients could be prioritized.
Similar content being viewed by others
Background
Pneumocystis jirovecii is a fungal organism that is a frequent cause of opportunistic pneumonia (PJP) in immunocompromised patients with and without HIV [1, 2]. Making the diagnosis can be challenging, requiring a respiratory specimen from induced sputum or bronchoalveolar lavage (BAL) be obtained for direct immunofluorescent or cytologic staining [1]. Unfortunately, induced sputum is not always possible, and bronchoscopy carries a risk of complications. Nonetheless, establishing a definitive diagnosis of PJP remains important because treatment based on clinical presentation alone is associated with a worse prognosis [3]. Consequently, better use of empiric therapy and improved case selection for bronchoscopy can both improve the quality of care. Such an improvement begins with a better estimate of the likelihood of PJP compared to other diagnoses.
Fortunately, most patients who are suspected of PJP will have imaging of the lungs. Previous studies have suggested that computed tomography (CT) of the chest is more sensitive than chest x-ray (CXR) for the detection of PJP and furthermore, that the most common CT finding is the presence of ground-glass opacities [1, 2, 4,5,6]. We sought to determine if the differences in imaging findings between those with and without pathologically confirmed PJP could help better estimate the probability of PJP.
Methods
The McGill University Health Centre (Montréal, Canada) is a 770-bed tertiary care hospital which serves as a referral center for: HIV/AIDS; rheumatologic disease; solid organ transplantation (liver, kidney, pancreas, and heart); and autologous and allogeneic stem cell transplantation. We identified all patients who had a BAL and/or transbronchial biopsy which was tested for pneumocystis using Calcofluor staining between January 2015 and January 2018. We then manually reviewed each patient’s bronchoscopy documentation and removed those where diagnosing PJP was not the reason for the bronchoscopy. From the remaining cohort, we selected all cases with pathologically confirmed PJP. For each case we randomly selected 4 controls who underwent bronchoscopy during the same timeframe to investigate for PJP but who tested negative. Controls were not matched on any other variables. We extracted readily available demographic information. We then extracted the full text radiology reports for the X-Rays and CT scans performed closest (but prior) to bronchoscopy and coded them based on categories of pertinent findings. The radiologist’s final impression was classified as: PJP not mentioned or unlikely, PJP possible, or PJP likely.
Univariable analyses were performed for both CXR and CT scan reports to evaluate for associations between the radiographic features and the final diagnosis of PJP. Multivariable analysis used logistic regression and a stepwise backwards elimination method beginning with features identified in the univariate analysis as potentially associated (p ≤ 0.2) while attempting to optimize the Akaike Information Criterion [7]. Independently associated variables (p < .05) were retained in the final models. For CT scan, we created a separate model without the radiologist’s impression to allow for an assessment of the radiographic features alone. Internal validation of each final multivariable model was performed using bootstrapping with 200 repetitions and the estimate of model optimism was used to generate a bootstrap corrected c-statistic [8]. A nomogram to estimate the probability of PJP based on CT scan features was created using the STATA program “nomolog”. All analyses used STATA version 15 (StataCorp LP, USA).
Results
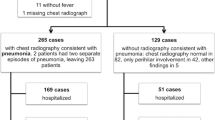
860 patients underwent testing for pneumocystis of whom 626 (72.8%) were deliberately investigated for PJP. From the latter group, 72 unique cases (11.5% positivity) were paired with 288 randomly chosen controls without PJP. The median age of cases and controls was 58 (IQR: 49.75–67.5) and 62 (51–69) respectively. 62.5% of cases and 61.2% of controls were male. HIV infection was present in 29.1% of cases and 8.6% of controls (p < 0.001).
The radiographic features of the CXRs (n = 69 cases/270 controls) and CT scans (n = 64 cases/231 controls) are shown in Table 1. Following multivariable analysis of CXR features, only the radiologist’s impression that PJP was possible or likely (OR 4.5; 95%CI 1.8–10.9) and the presence of “increased interstitial markings” (OR 2.9; 95%CI 1.6–5.1) remained independently associated with the diagnosis of PJP (adjusted c-statistic 0.64). Multivariable analysis of CT scan features demonstrated that “increased interstitial markings” (OR 4.3; 95%CI 2.2–8.2), “ground glass opacities” (OR 3.3; 95%CI 1.2–9.1) and the radiologist’s impression of PJP being “possible” (OR 2.0; 95%CI 1.0–4.1) or “likely” (OR 9.3; 95%CI 3.4–25.3) were independently associated with the diagnosis (adjusted c-statistic 0.75). In the model for CT scan without the radiologist’s impression, “increased interstitial markings” (OR 5.0; 95%CI 2.6–9.7) and “ground glass opacities” (OR 4.2; 95%CI 1.5–11.5) were positively associated with the diagnosis, whereas the presence of pleural effusion(s) (OR 0.44; 95%CI 0.22–0.91) and “nodular findings” (OR 0.41; 95%CI 0.21–0.81) were negatively associated (adjusted c-statistic 0.74). Nomograms to estimate the post-CT probability of PJP are presented in Table 2 and assume a prevalence of PJP similar to ours.
Discussion
We found that the chest x-ray was poor at differentiating PJP from other diagnoses (c-statistic 0.64, poor) within a cohort of patients with a clinical suspicion of PJP, whereas a CT scan could remain helpful (c-statistic 0.75; fair). Neither imaging modality performed well enough to preclude further testing; however, separating patients into lower and higher risk for PJP based on imaging could allow for better use of non-invasive testing (e.g. beta-D-glucan) in patients at low risk of disease and expedited invasive testing in those at higher risk.
While there have been other studies which describe the findings of CT thorax in patients with PJP, only a few small studies have compared them with a clinically relevant control group. Richards et al. found that ground glass opacities on CT were predictive of P. jirovecii diagnosed on bronchoscopy in the 4 cases of PJP out of 13 HIV positive patients tested [9]. Similarly, Hidalgo et al. evaluated 30 patients with HIV and presumed PJP and found that diffuse or upper lobe predominant ground glass opacifications were present in all 24 patients with PJP versus 2 of the 6 without [10]. Finally, Gruden et al. evaluated 33 patients with HIV who underwent bronchoscopy for PJP and found that nodular or patchy ground glass opacities were present in all 6 patients with PJP and 5/27 without [11]. The major strengths of our study are our inclusion of a mixed cohort of HIV positive and negative patients and our use of a comparator group who were clinically suspected of having PJP. Consequently, our cohort is a fairer representation of the population that clinicians will encounter when they clinically suspect PJP.
Nonetheless, our study has several limitations. First, this was a single centre study and we are an academic referral hospital. We see PJP frequently and suspected cases are reviewed by dedicated chest radiologists who may have more experience with PJP than other centres. To account for this, we also constructed a model for CT scan without the radiologist’s final impression. Furthermore, we do not use a structured means of recording the clinical details on our radiology requests. Hence, it is possible that requisitions which explicitly included the diagnosis of PJP or a more detailed clinical histories may have biased our radiologists towards reporting features positively associated with the diagnosis. We also cannot say for certain that all radiographic features included in Table 1 were systematically assessed by our radiologists as our institution does not use structured reporting templates. Secondly, while we present more cases than all previous studies, our sample size remains limited. Thirdly, patients with a false negative BAL may have been included in the control group and this would bias all estimates towards the null. Fourth, there could be misclassification bias due to false negative bronchoscopy results representing an imperfect gold standard; however, the BAL was considered the best possible reference. Finally, we were only able to abstract radiographic and not clinical data. A more refined clinical prediction model that includes clinical, radiographic, and non-invasive laboratory data would be expected to further improve the ability to estimate probability of PJP. Despite these limitations, we believe our results can help clinicians and be validated in other cohorts.
Conclusion
In patients for whom there is clinical suspicion of PJP, the use of CT scan can help estimate the probability of PJP. For low risk patients, non-invasive laboratory testing could be appropriately performed and interpreted whereas high risk patients could be considered for empiric therapy and more specific or invasive testing.
Availability of data and materials
The datasets generated and/or analysed during the current study are not publicly available due to Québec law but are available from the corresponding author on reasonable request and subject to a legal materials transfer agreement.
Abbreviations
- BAL :
-
Bronchoalveolar lavage
- CT :
-
Computed tomography
- HIV :
-
Human immunodeficiency virus
- PJP :
-
Pneumocystis jirovecii pneumonia
References
Catherinot E, Lanternier F, Bougnoux ME, Lecuit M, Couderc LJ, Lortholary O. Pneumocystis jirovecii Pneumonia. Infect Dis Clin N Am. 2010;24(1):107–38.
Kanne JP, Yandow DR, Meyer CA. Pneumocystis jiroveci pneumonia: high-resolution CT findings in patients with and without HIV infection. AJR Am J Roentgenol. 2012;198(6):W555–61.
Baughman RP, Liming JD. Diagnostic strategies in Pneumocystis carinii pneumonia. Frontiers in bioscience : a journal and virtual library. 1998;3:e1–12.
Salzer HJF, Schafer G, Hoenigl M, Gunther G, Hoffmann C, Kalsdorf B, et al. Clinical, diagnostic, and treatment disparities between HIV-infected and non-HIV-infected Immunocompromised patients with Pneumocystis jirovecii pneumonia. Respiration; international review of thoracic diseases. 2018;96(1):52–65.
Mu XD, Jia P, Gao L, Su L, Zhang C, Wang RG, et al. Relationship between radiological stages and prognoses of Pneumocystis pneumonia in non-AIDS Immunocompromised patients. Chin Med J. 2016;129(17):2020–5.
Hardak E, Brook O, Yigla M. Radiological features of Pneumocystis jirovecii pneumonia in immunocompromised patients with and without AIDS. Lung. 2010;188(2):159–63.
Sanchez-Pinto LN, Venable LR, Fahrenbach J, Churpek MM. Comparison of variable selection methods for clinical predictive modeling. Int J Med Inform. 2018;116:10–7.
Steyerberg EW, Harrell FE Jr, Borsboom GJ, Eijkemans MJ, Vergouwe Y, Habbema JD. Internal validation of predictive models: efficiency of some procedures for logistic regression analysis. J Clin Epidemiol. 2001;54(8):774–81.
Richards PJ, Riddell L, Reznek RH, Armstrong P, Pinching AJ, Parkin JM. High resolution computed tomography in HIV patients with suspected Pneumocystis carinii pneumonia and a normal chest radiograph. Clin Radiol. 1996;51(10):689–93.
Hidalgo A, Falco V, Mauleon S, Andreu J, Crespo M, Ribera E, et al. Accuracy of high-resolution CT in distinguishing between Pneumocystis carinii pneumonia and non- Pneumocystis carinii pneumonia in AIDS patients. Eur Radiol. 2003;13(5):1179–84.
Gruden JF, Huang L, Turner J, Webb WR, Merrifield C, Stansell JD, et al. High-resolution CT in the evaluation of clinically suspected Pneumocystis carinii pneumonia in AIDS patients with normal, equivocal, or nonspecific radiographic findings. AJR Am J Roentgenol. 1997;169(4):967–75.
Acknowledgements
The authors thank Heidi Kirshner from the Department of Pathology, McGill University Health Centre for her support.
Funding
This study was funded by the McGill Faculty of Medicine Student Research Bursary Program (Mr. Hsu and Mr. Hass). The funder did not participate in study design or analysis.
Author information
Authors and Affiliations
Contributions
The study was conceptualized and designed by CC, NE, EGM and TCL. Data was provided and organized by JC and RF. Data was abstracted and collated by JMH, AH and MAG. JMH and TCL performed the analysis. The first draft of the manuscript was written by JMH and TCL and subsequently revised by all other authors. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The McGill University Health Centre research ethics board approved this study with a waiver of informed consent.
Consent for publication
Not applicable.
Competing interests
Drs. Costiniuk, Ezer, Lee, and McDonald receive research salary support from the Fonds de Recherche du Québec – Santé.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Hsu, J.M., Hass, A., Gingras, MA. et al. Radiographic features in investigated for Pneumocystis jirovecii pneumonia: a nested case-control study. BMC Infect Dis 20, 492 (2020). https://doi.org/10.1186/s12879-020-05217-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-020-05217-x