Abstract
Background
Clostridioides difficile is considered the main pathogen responsible for hospital-acquired infections. This prospective study determined the prevalence, molecular epidemiological characteristics, and risk factors for C. difficile infection (CDI) and C. difficile colonization (CDC) among patients in the intensive care unit (ICU) of a large-scale tertiary hospital in China, with the aim of providing strategies for efficient CDI and CDC prevention and control.
Methods
Stool samples were collected and anaerobically cultured for C. difficile detection. The identified isolates were examined for toxin genes and subjected to multilocus sequence typing. Patients were classified into CDI, CDC, and control groups, and their medical records were analyzed to determine the risk factors for CDI and CDC.
Results
Of the 800 patients included in the study, 33 (4.12%) and 25 (3.12%) were identified to have CDI and CDC, respectively. Associations with CDI were found for fever (OR = 13.993), metabolic disorder (OR = 7.972), and treatment with fluoroquinolone (OR = 42.696) or combined antibiotics (OR = 2.856). CDC patients were characterized by prolonged hospital stay (OR = 1.137), increased number of comorbidities (OR = 36.509), respiratory diseases (OR = 0.043), and treatment with vancomycin (OR = 18.168). Notably, treatment with metronidazole was found to be a protective factor in both groups (CDI: OR = 0.042; CDC: OR = 0.013). Eighteen sequence types (STs) were identified. In the CDI group, the isolated strains were predominantly toxin A and toxin B positive (A + B+) and the epidemic clone was genotype ST2. In the CDC group, the dominant strains were A + B+ and the epidemic clone was ST81.
Conclusions
The prevalences of CDC and CDI in our ICU were relatively high, suggesting the importance of routine screening for acquisition of C. difficile. Future prevention and treatment strategies for CDC and CDI should consider hospital stay, enteral nutrition, underlying comorbidities, and use of combined antibiotics. Moreover, metronidazole may be a protective factor for both CDI and CDC, and could be used empirically.
Similar content being viewed by others
Background
Clostridioides difficile is a Gram-positive spore-forming anaerobic bacterium listed as the leading cause of hospital-acquired diarrhea in many developed countries [1]. The pathogen secretes two main toxins, toxin A and toxin B, that mediate C. difficile-associated colitis and diarrhea [2]. The incidence of C. difficile infection (CDI) is steadily increasing worldwide and its mortality rate has risen accordingly [3, 4]. A previous report stated that the number of hospitalized patients with CDI in the United States has more than doubled during the last decade [5]. A similar situation is present in some Asian countries [6, 7], leading to prolonged hospital stays and higher costs for intensive care units (ICUs) and bringing significant economic burdens.
C. difficile can colonize individuals without causing detectable symptoms of infection. Such asymptomatic C. difficile-colonized patients may present a potential risk to other susceptible individuals by acting as infection reservoirs [8, 9]. Thus, it is considered that asymptomatic C. difficile-colonized patients may serve as potential vehicles for C. difficile transmission in medical settings [10], where there is a significantly higher risk of CDI [11]. The global spread of emerging hypervirulent toxigenic strains is of particular concern [12].
In a previous study, patients in intensive care units (ICUs) mainly received antimicrobial therapy and had comorbidities [13]. CDI patients in ICUs were reported to have prolonged hospital stays [14, 15], high hospital costs [16], and high mortality rates [17]. The current prevalence of CDI among ICU patients was estimated at 0.4–4% [18]. Furthermore, about 10–20% of ICU patients were colonized with C. difficile without any symptoms of infection [18]. Therefore, the presence of C. difficile may have a particular impact on the morbidity and mortality of patients in ICUs.
The incidence of toxigenic CDI or C. difficile colonization (CDC) among ICU patients in China remains largely uninvestigated. In addition, little is known about the epidemiology of strains in terms of typing, or about the in-depth risk factors. Therefore, this prospective study aimed to provide a better understanding of the prevalence, molecular epidemiological characteristics, and risk factors for CDI and CDC among patients in the ICU of a large-scale teaching hospital in China.
Methods
Study design, case definitions, and data collection
We conducted a prospective study on adult patients admitted to our ICU, an 18-bed department in Shanghai Ruijin Hospital, from January 2015 to June 2017. Patients were screened for C. difficile within 48 h of admission [19], and subsequently tested every week or at onset of diarrhea. The surveillance continued until patients died or were discharged from hospital. The study was approved by the Ethics Committee of Ruijin Hospital in Shanghai, China.
According to European guidelines [20], CDI was defined as the symptom of diarrhea and laboratory findings for toxigenic C. difficile, while CDC was defined as positivity for toxigenic C. difficile but no diarrhea [21]. To reduce the influence of confounding factors, we chose C. difficile-negative patients with diarrhea as controls for CDI and those without diarrhea as controls for CDC. The control groups were randomly selected from ICU patients who were admitted to the hospital during the same time period but had no history of CDI or CDC in the previous 8 weeks.
For all patients in the study, we recorded their demographic data and clinical characteristics, including duration of hospital stay, mortality, surgery (in previous 6 months), history of antibiotic use or gastric acid suppressants (for 1 month before onset of diarrhea in CDI patients and their controls, and 1 month before CDC development in CDC patients and index hospital stay in their controls), and enteral nutrition. Primary disease diagnoses were divided into six major categories: gastrointestinal disease, respiratory disease, cardiovascular disease, renal disease, neurological disease, and metabolic disorders including diabetes, hypertension, or hyperlipidemia. For laboratory test indices, body temperature, leukocyte count, serum albumin level, and serum creatinine level were measured. All laboratory indicators were recorded when patients were diagnosed with CDI or CDC. For patients in the two control groups, related laboratory indicators were tested on admission to hospital.
C. difficile strain isolation and collection
Stool samples were collected from ICU patients within a set time period, plated onto C. difficile agar base supplemented with norfloxacin and moxalactam (Oxoid Ltd., Basingstoke, UK), and cultured anaerobically at 37 °C for 48–72 h. Colonies were identified by morphological features, latex agglutination test (C. difficile Agglutination Test Kit; Oxoid Ltd.), and gluD gene detection. Feces and C. difficile isolates were also subjected to toxin A/B detection by enzyme-linked fluorescence assay with a VIDAS automatic analyzer (Biomerieux, Marcy-l’Etoile, France) [22,23,24].
Multilocus sequence typing (MLST)
MLST was performed for genotyping of C. difficile strains. For this, DNA was isolated using a DNA extraction kit (Sangon Biotech, Shanghai, China). Next, seven housekeeping genes (adk, atpA, dxr, glyA, recA, sodA, tpi) were amplified from all strains and sequenced as described previously [25]. The obtained sequences were aligned with sequences in the MLST database (http://pubmlst.org/clostridium difficile).
Data analysis
Continuous variables were expressed as median and standard deviation, and compared by Student’s t-test. Categorical variables were presented as frequency or percentage, and compared by the chi-square test or Fisher’s exact test. Univariate analyses were performed to evaluate the potential risk factors relevant to the cases. The statistically significant variables from the univariate analyses were then included in a multivariate logistic regression model. The results of the logistic regression analysis were presented as odd ratio (OR) and 95% confidence interval (95% CI).
All analyses were performed with Statistical Program for Social Sciences (SPSS) version 22.0 for Windows software. Values of P < 0.05 were considered statistically significant.
Results
Patient population
As shown in Figs. 1, 800 adult patients were admitted to the ICU during the study period. Of these, 115 developed diarrhea and 33 (28.70%) were identified to have CDI. Another 25 toxigenic C. difficile strains were isolated from patients without diarrhea, and defined as CDC cases. The overall prevalence of CDI and CDC was 4.12 and 3.12%, respectively, and all cases were healthcare facility-associated. One patient had recurrence of infection, one patient transitioned from CDC to CDI, and two patients had infections with two different types. The CDI and CDC patients had median age of 54.15 and 62 years, male proportion of 66.7 and 68%, and period from admission until positive test result of 17.06 ± 12.97 and 31.16 ± 33.85 days, respectively. Neither age nor sex showed any significant difference between the groups. To assess the potential risk factors and clinical characteristics, 66 non-CDI and 50 non-CDC patients were included as control groups.
Clinical characteristics and risk factors for ICU patients with CDI
As illustrated in Table 1, univariate analyses were conducted to determine the differences between the CDI group and the non-CDI control group in terms of clinical characteristics, diagnosis, and treatment. The CDI patients were more likely to suffer from fever (OR, 6.786; 95% CI, 2.634–17.483; P < 0.001) and metabolic disorders (OR, 3.28; 95% CI, 1.363–7.893; P < 0.05) than the non-CDI patients. The CDI patients also had a larger number of comorbidities (P < 0.05). Compared with the non-CDI patients, the CDI patients more frequently received enteral feeding (78.8% vs. 50%; OR, 3.714; 95% CI, 1.416–9.74), antiviral drugs (15.2% vs. 1.52%; OR, 11.607; 95% CI, 1.296–103.948), and fluoroquinolone (21.2% vs. 3%; OR, 8.615; 95% CI, 1.678–44.247) during their hospitalization (P < 0.05). Furthermore, a larger proportion of CDI patients were administered more than one type of antibiotic (P < 0.05). To further assess the potential risk factors for CDI, a multivariable logistic regression analysis was performed. The results showed that fever, metabolic disorder, and treatment with fluoroquinolone or combined antibiotics were risk factors associated with development of CDI among ICU patients. However, treatment with metronidazole was found to be a protective factor (OR, 0.042; 95% CI, 0.006–0.288; P = 0.001).
Clinical characteristics and risk factors for ICU patients with CDC
The median hospital stay for CDC patients was 62 days and significantly longer than that for non-CDC patients (P < 0.05). This difference was further verified by the multivariable logistic regression model. Colonization of C. difficile did not cause any significant differences in laboratory test indices, including leukocyte count and serum albumin or creatinine levels. However, patients with respiratory or neurological disease were more likely to acquire asymptomatic CDC. Number of comorbidities was a potential risk factor for CDC patients (OR, 36.509; 95% CI, 2.602–512.183; P = 0.08). For treatment procedures, surgical intervention, enteral feeding, antifungal agent usage, and carbapenem medication were more frequently found in CDC patients than in non-CDC patients (P < 0.05). The multivariable model analysis showed that vancomycin was an independent risk factor (OR, 18.168; 95% CI, 1.036–318.503; P = 0.047), while metronidazole was a protective factor (OR, 0.013; 95% CI, 0–0.512; P = 0.021) for CDC (Table 2).
Molecular characteristics of C. difficile
The toxin types were detected for the 58 C. difficile strains isolated from the CDI and CDC patients. In total, 34 (58.6%) were A + B+ (positive for both tcdA and tcdB) and 24 (41.3%) were A − B+ (negative for tcdA and positive for tcdB). Specifically, 20 (60.6%) were A + B+ and 13 (39.4%) were A − B+ in the CDI group, while 14 (56%) were A + B+ and 11 (44%) were A − B+ in the CDC group.
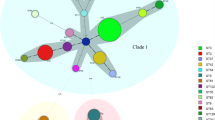
MLST was also performed on the strains, and 18 sequence types (STs) were identified (Fig. 2). In the CDI group, ST2, ST81, ST54, and ST3 were the major STs, constituting 19, 15, 12, and 12% of the strains, respectively. In the CDC group, ST81, ST35, ST37, and ST54 were the dominant types, accounting for 20, 12, 12, and 12% of the strains, respectively.
Based on the STs of the strains, a map was constructed to compare the temporospatial relationships of the same STs in the two groups during the study period (Fig. 3). Two overlaps were detected within the CDI group for ST2 and one overlap was detected between the CDI and CDC groups for ST103. No overlaps were detected among the other STs.
Discussion
During recent decades, there has been a continuous increase in CDI and CDC cases among hospitalized patients in many medical settings [1, 6, 7, 21]. Patients in ICUs often suffer from various comorbidities, which greatly increase the risk of developing CDI and lead to difficulties in treatment of the underlying medical conditions [26]. A review described that approximately 2% of ICU patients suffered from CDI, which was significantly higher than the 0.9% of patients on general wards [27]. In our study, we found that the prevalence of CDI was 4.12%, and much higher than that in most studies reported from European countries [27]. Furthermore, 28.7% of ICU patients with diarrhea developed CDI, which was much higher than the 8% reported in another Chinese study [28]. Meanwhile, the detection rate of CDC in our study was 3.12%, and relatively lower than the 7% reported in a retrospective study from Kuwait [29]. Above all, the prevalences of CDI and CDC varied geographically. The high acquisition of toxigenic C. difficile may result from increased screening and the highly sensitive detection methodology used, as well as enhanced awareness for prevention of C. difficile-related diseases. Moreover, it is possible that the incidence of C. difficile changed distinctly, especially in ICU patients.
The main reported risk factors for CDI included antibiotic exposure, age > 60 years, longer hospital stay, severe dyspepsia, history of gastric acid inhibitor use [30], enteral feeding, and proton pump inhibitor (PPI) medication [31]. ICU admission was also a common pathogenic factor [32]. In the present study, we found that medication with multiple antibiotics significantly increased the risk of developing CDI. Specifically, increased use of fluoroquinolones contributed to the incidence of CDI, as previously suggested [32, 33]. Routine interventions especially relevant for patients in ICUs, such as surgery, enteral feeding, and PPI medication, doubled the risk of CDI infection [16, 30, 34,35,36]. PPIs caused a change in the gastrointestinal flora, thereby creating a niche for CDC [37]. In the present study, neither history of surgery nor PPI medication were found to differ among CDI patients. Meanwhile, CDI patients were more likely to receive enteral feeding in our univariable analyses, but not in the multivariable logistic regression analysis. Regarding underlying conditions, the study revealed a significant association between occurrence of CDI and metabolic diseases. However, the mechanisms involved remain unclear and require further investigations.
For patients with CDC in our study, large differences in number of comorbidities and duration of hospital stay were detected, consistent with a previous study [38]. However, CDC rarely occurred in patients with respiratory diseases, and the underlying reason for this finding remains to be clarified. Exposure to a variety of antibiotics was a risk factor for CDI, but not for CDC. The significant discrepancy between these findings may indicate that destruction of the intestinal microbiota by antibiotic exposure is not a key feature of CDC.
For decades, metronidazole and vancomycin have been the main antimicrobial agents for treatment of CDI [39]. In treatment analyses of three randomized controlled trials comparing metronidazole and vancomycin [40,41,42], no significant differences were found [43, 44]. In the present study, metronidazole was identified as a protective factor against CDI, consistent with previous studies [30, 45]. However, oral vancomycin was a risk factor for CDC, in accordance with the findings of Johnson et al. [46]. These results suggest that preventive use of metronidazole may contribute to prevention of CDI and CDC, while caution is required for medication with vancomycin in the clinic.
ST2 and ST81 were the most common strain types in the CDI group and CDC group, respectively. These findings differed from the identification of ST54 as the most common genotype in a previous study [47]. In addition, neither ST1 nor ST11, which were epidemic in Western countries [48], was detected in the present study. Our map showing the temporospatial relationships among the strains revealed that C. difficile dispersed among normal colonized patients could be a potential source of infection, but there was still no definitive evidence demonstrating its transmission from colonized patients to other patients.
There are several limitations to our study. First, the samples were collected from a single center and may not be representative of all healthcare institutions. However, to our knowledge, this research is one of the limited studies to report the clinical and molecular characteristics of C. difficile among ICU patients in China. To overcome this limitation, long-term multicenter studies should be carried out in the future. Second, because ICU wards are always isolated and strictly disinfected rooms, environmental samples were not obtained and we could not fully assess C. difficile transmission. To identify the risk factors for development of CDI and CDC, most previous studies compared C. difficile-positive cases with C. difficile-negative cases [49,50,51]. However, because most negative cases had no diarrhea, the risk factors identified for CDI in these cases were unlikely to be specific. To overcome this shortcoming, we used two sets of patients with and without diarrhea as negative controls.
Conclusions
Our study provided prospective independent comparisons of patients in an ICU and characterized the molecular epidemiology. The overall prevalence of CDI and CDC was 4.12 and 3.12%, respectively. Fever, metabolic disorders, use of fluoroquinolone, and exposure to multiple antibiotics were significantly associated with CDI. Longer hospital stay, number of comorbidities, and use of vancomycin were associated with acquisition of CDC. Regarding metronidazole, protective effects were detected for both groups. The most common epidemic strain was ST2 and ST81 in the CDI group and CDC group, respectively. The present results highlight the importance of antimicrobial stewardship and pathogen isolation for the prevention and treatment of C. difficile-related diseases. The role of asymptomatic carriers in the transmission of C. difficile requires further investigation. In conclusion, it is essential for medical staff to emphasize the importance of C. difficile-related diseases, especially for ICU patients.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CDC:
-
Clostridioides difficile colonization
- CDI:
-
Clostridioides difficile infection
- ICU:
-
Intensive care unit
- MLST:
-
Multilocus sequence typing
- ST:
-
Sequence type
References
Freeman J, Bauer MP, Baines SD, Corver J, Fawley WN, Goorhuis B, et al. The changing epidemiology of Clostridium difficile infections. Clin Microbiol Rev. 2010;23(3):529–49.
Soes LM, Brock I, Persson S, Simonsen J, Pribil Olsen KE, Kemp M. Clinical features of Clostridium difficile infection and molecular characterization of the isolated strains in a cohort of Danish hospitalized patients. Eur J Clin Microbiol Infect Dis. 2012;31(2):185–92.
Lo Vecchio A, Zacur GM. Clostridium difficile infection: an update on epidemiology, risk factors, and therapeutic options. Curr Opin Gastroenterol. 2012;28(1):1–9.
Tattevin P, Buffet-Bataillon S, Donnio PY, Revest M, Michelet C. Clostridium difficile infections: do we know the real dimensions of the problem? Int J Antimicrob Agents. 2013;42(Suppl):S36–40.
Kuy S, Jenkins P, Romero RA, Samra N, Kuy S. Increasing incidence of and increased mortality associated with Clostridium difficile-associated Megacolon. JAMA surgery. 2016;151(1):85–6.
Lim PL, Barkham TM, Ling LM, Dimatatac F, Alfred T, Ang B. Increasing incidence of Clostridium difficile-associated disease, Singapore. Emerg Infect Dis. 2008;14(9):1487–9.
Shin BM, Kuak EY, Yoo HM, Kim EC, Lee K, Kang JO, et al. Multicentre study of the prevalence of toxigenic Clostridium difficile in Korea: results of a retrospective study 2000-2005. J Med Microbiol. 2008;57(Pt 6):697–701.
Riggs MM, Sethi AK, Zabarsky TF, Eckstein EC, Jump RL, Donskey CJ. Asymptomatic carriers are a potential source for transmission of epidemic and nonepidemic Clostridium difficile strains among long-term care facility residents. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2007;45(8):992–998.
McFarland LV, Mulligan ME, Kwok RY, Stamm WE. Nosocomial acquisition of Clostridium difficile infection. N Engl J Med. 1989;320(4):204–10.
Curry SR, Muto CA, Schlackman JL, Pasculle AW, Shutt KA, Marsh JW, et al. Use of multilocus variable number of tandem repeats analysis genotyping to determine the role of asymptomatic carriers in Clostridium difficile transmission. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2013;57(8):1094–1102.
Zacharioudakis IM, Zervou FN, Pliakos EE, Ziakas PD, Mylonakis E. Colonization with toxinogenic C. difficile upon hospital admission, and risk of infection: a systematic review and meta-analysis. Am J Gastroenterol. 2015;110(3):381–90 quiz 91.
Clements AC, Magalhaes RJ, Tatem AJ, Paterson DL, Riley TV. Clostridium difficile PCR ribotype 027: assessing the risks of further worldwide spread. Lancet Infect Dis. 2010;10(6):395–404.
Bobo LD, Dubberke ER, Kollef M. Clostridium difficile in the ICU: the struggle continues. Chest. 2011;140(6):1643–53.
Micek ST, Schramm G, Morrow L, Frazee E, Personett H, Doherty JA, et al. Clostridium difficile infection: a multicenter study of epidemiology and outcomes in mechanically ventilated patients. Crit Care Med. 2013;41(8):1968–75.
Karanika S, Paudel S, Zervou FN, Grigoras C, Zacharioudakis IM, Mylonakis E. Prevalence and Clinical Outcomes of Clostridium difficile Infection in the Intensive Care Unit: A Systematic Review and Meta-Analysis. Open forum infectious diseases. 2016;3(1):ofv186.
Lawrence SJ, Puzniak LA, Shadel BN, Gillespie KN, Kollef MH, Mundy LM. Clostridium difficile in the intensive care unit: epidemiology, costs, and colonization pressure. Infect Control Hosp Epidemiol. 2007;28(2):123–30.
Gao T, He B, Pan Y, Deng Q, Sun H, Liu X, et al. Association of Clostridium difficile infection in hospital mortality: a systematic review and meta-analysis. Am J Infect Control. 2015;43(12):1316–20.
Prechter F, Katzer K, Bauer M, Stallmach A. Sleeping with the enemy: Clostridium difficile infection in the intensive care unit. Critical care (London, England). 2017;21(1):260.
Dubberke ER, Reske KA, Seiler S, Hink T, Kwon JH, Burnham CA. Risk factors for acquisition and loss of Clostridium difficile colonization in hospitalized patients. Antimicrob Agents Chemother. 2015;59(8):4533–43.
Debast SB, Bauer MP, Kuijper EJ. European Society of Clinical Microbiology and Infectious Diseases: update of the treatment guidance document for Clostridium difficile infection. Clin Microbiol Infect. 2014;20(Suppl 2):1–26.
Furuya-Kanamori L, Marquess J, Yakob L, Riley TV, Paterson DL, Foster NF, et al. Asymptomatic Clostridium difficile colonization: epidemiology and clinical implications. BMC Infect Dis. 2015;15:516.
Lemee L, Dhalluin A, Testelin S, Mattrat MA, Maillard K, Lemeland JF, et al. Multiplex PCR targeting tpi (triose phosphate isomerase), tcdA (toxin a), and tcdB (toxin B) genes for toxigenic culture of Clostridium difficile. J Clin Microbiol. 2004;42(12):5710–4.
Kato H, Kato N, Watanabe K, Iwai N, Nakamura H, Yamamoto T, et al. Identification of toxin A-negative, toxin B-positive Clostridium difficile by PCR. J Clin Microbiol. 1998;36(8):2178–82.
Pituch H, Kreft D, Obuch-Woszczatynski P, Wultanska D, Meisel-Mikolajczyk F, Luczak M, et al. Clonal spread of a Clostridium difficile strain with a complete set of toxin a, toxin B, and binary toxin genes among polish patients with Clostridium difficile-associated diarrhea. J Clin Microbiol. 2005;43(1):472–5.
Griffiths D, Fawley W, Kachrimanidou M, Bowden R, Crook DW, Fung R, et al. Multilocus sequence typing of Clostridium difficile. J Clin Microbiol. 2010;48(3):770–8.
Sabau L, Meybeck A, Gois J, Devos P, Patoz P, Boussekey N, et al. Clostridium difficile colitis acquired in the intensive care unit: outcome and prognostic factors. Infection. 2014;42(1):23–30.
Lucado J, Gould C, Elixhauser A. Clostridium difficile Infections (CDI) in Hospital Stays, 2009: Statistical Brief #124. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville (MD). Agency for Healthcare Research and Quality (US). 2006.
Li C, Li Y, Huai Y, Liu S, Meng X, Duan J, et al. Incidence and outbreak of healthcare-onset healthcare-associated Clostridioides difficile infections among intensive care patients in a large teaching Hospital in China. Front Microbiol. 2018;9.
Rotimi VO, Jamal WY, Mokaddas EM, Brazier JS, Johny M, Duerden BI. Prevalent PCR ribotypes of clinical and environmental strains of Clostridium difficile isolated from intensive-therapy unit patients in Kuwait. J Med Microbiol. 2003;52(Pt 8):705–9.
Dubberke ER, Reske KA, Yan Y, Olsen MA, McDonald LC, Fraser VJ. Clostridium difficile--associated disease in a setting of endemicity: identification of novel risk factors. Clin Infect Dis. 2007;45(12):1543–9.
Clabots CR, Johnson S, Olson MM, Peterson LR, Gerding DN. Acquisition of Clostridium difficile by hospitalized patients: evidence for colonized new admissions as a source of infection. J Infect Dis. 1992;166(3):561–7.
Bignardi GE. Risk factors for Clostridium difficile infection. J Hospital Infect. 1998;40(1):1–15.
Pepin J, Saheb N, Coulombe MA, Alary ME, Corriveau MP, Authier S, et al. Emergence of fluoroquinolones as the predominant risk factor for Clostridium difficile-associated diarrhea: a cohort study during an epidemic in Quebec. Clin Infect Dis. 2005;41(9):1254–60.
Cunningham R, Dale B, Undy B, Gaunt N. Proton pump inhibitors as a risk factor for Clostridium difficile diarrhoea. J Hospital Infect. 2003;54(3):243–5.
Bliss DZ, Johnson S, Savik K, Clabots CR, Willard K, Gerding DN. Acquisition of Clostridium difficile and Clostridium difficile-associated diarrhea in hospitalized patients receiving tube feeding. Ann Intern Med. 1998;129(12):1012–9.
Asha NJ, Tompkins D, Wilcox MH. Comparative analysis of prevalence, risk factors, and molecular epidemiology of antibiotic-associated diarrhea due to Clostridium difficile, Clostridium perfringens, and Staphylococcus aureus. J Clin Microbiol. 2006;44(8):2785–91.
Thorens J, Froehlich F, Schwizer W, Saraga E, Bille J, Gyr K, et al. Bacterial overgrowth during treatment with omeprazole compared with cimetidine: a prospective randomised double blind study. Gut. 1996;39(1):54–9.
Leekha S, Aronhalt KC, Sloan LM, Patel R, Orenstein R. Asymptomatic Clostridium difficile colonization in a tertiary care hospital: admission prevalence and risk factors. Am J Infect Control. 2013;41(5):390–3.
Cohen SH, Gerding DN, Johnson S, Kelly CP, Loo VG, McDonald LC, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31(5):431–55.
Teasley DG, Gerding DN, Olson MM, Peterson LR, Gebhard RL, Schwartz MJ, et al. Prospective randomised trial of metronidazole versus vancomycin for Clostridium-difficile-associated diarrhoea and colitis. Lancet (London, England). 1983;2(8358):1043–6.
Wenisch C, Parschalk B, Hasenhundl M, Hirschl AM, Graninger W. Comparison of vancomycin, teicoplanin, metronidazole, and fusidic acid for the treatment of Clostridium difficile-associated diarrhea. Clin Infect Dis. 1996;22(5):813–8.
Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45(3):302–7.
Nelson RL, Kelsey P, Leeman H, Meardon N, Patel H, Paul K, et al. Antibiotic treatment for Clostridium difficile-associated diarrhea in adults. Cochrane Database Syst Reviews. 2011;9:Cd004610.
Martinez FJ, Leffler DA, Kelly CP. Clostridium difficile outbreaks: prevention and treatment strategies. Risk Management Healthcare Policy. 2012;5:55–64.
Rodriguez S, Hernandez MB, Tarchini G, Zaleski M, Vatanchi M, Cardona L, et al. Risk of Clostridium difficile infection in hospitalized patients receiving metronidazole for a non-C difficile infection. Clin Gastroenterol Hepatol. 2014;12(11):1856–61.
Johnson S, Homann SR, Bettin KM, Quick JN, Clabots CR, Peterson LR, et al. Treatment of asymptomatic Clostridium difficile carriers (fecal excretors) with vancomycin or metronidazole. A randomized, placebo-controlled trial. Ann Intern Med. 1992;117(4):297–302.
Chen YB, Gu SL, Wei ZQ, Shen P, Kong HS, Yang Q, et al. Molecular epidemiology of Clostridium difficile in a tertiary hospital of China. J Med Microbiol. 2014;63(Pt 4):562–9.
Wiegand PN, Nathwani D, Wilcox MH, Stephens J, Shelbaya A, Haider S. Clinical and economic burden of Clostridium difficile infection in Europe: a systematic review of healthcare-facility-acquired infection. J Hospital Infect. 2012;81(1):1–14.
Miller M, Gravel D, Mulvey M, Taylor G, Boyd D, Simor A, et al. Health care-associated Clostridium difficile infection in Canada: patient age and infecting strain type are highly predictive of severe outcome and mortality. Clin Infect Dis. 2010;50(2):194–201.
Morrison RH, Hall NS, Said M, Rice T, Groff H, Brodine SK, et al. Risk factors associated with complications and mortality in patients with Clostridium difficile infection. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2011;53(12):1173–8.
Loo VG, Bourgault AM, Poirier L, Lamothe F, Michaud S, Turgeon N, et al. Host and pathogen factors for Clostridium difficile infection and colonization. N Engl J Med. 2011;365(18):1693–703.
Acknowledgements
The authors are grateful to all ICU teams, participants, laboratory staff, and professionals involved in the study for their valuable contributions. We thank Alison Sherwin, PhD, from Liwen Bianji, Edanz Group China (www.liwenbianji.cn/ac) for editing the English text of a draft of this manuscript.
Funding
This project was supported by the National Natural Science Foundation of China (grant nos. 81572053, 81902117, and 81971993), the Shanghai Sailing Program (grant no. 19YF1431300) and Research Project of Shanghai Municipal Health Commission (grant no.20194Y0318). The National Natural Science Foundation of China (grant no.81572053) and Shanghai Sailing Program (grant no.19YF1431300) funded in the design of the study, sample collection, related experiments and collection of case records. The National Natural Science Foundation of China (grant no.81902117, and 81971993) and Research Project of Shanghai Municipal Health Commission (grant no.20194Y0318) funded data analysis, interpretation of data and in writing and revision of the manuscript.
Author information
Authors and Affiliations
Contributions
YC, LZ, DD, and YP contributed to the study design. YC, LZ, EM, CJ, QN, CW, and DW contributed to the collection of clinical samples, related experiments, and case records. YC, LZ, and DD contributed to the data analysis. YC, LZ, DD, and YP drafted the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Ruijin Hospital Ethics Committee approved the study protocol and verbal informed consent because the study only involved stool samples and all data collected were anonymized. All participants provided verbal consent prior to participation.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Cui, Y., Dong, D., Zhang, L. et al. Risk factors for Clostridioides difficile infection and colonization among patients admitted to an intensive care unit in Shanghai, China. BMC Infect Dis 19, 961 (2019). https://doi.org/10.1186/s12879-019-4603-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-019-4603-1