Abstract
Background
In Yemen, the underlying causes of infectious vaginitis have been neglected. Therefore, this study aimed to determine the prevalence and risk factors associated with bacterial vaginosis (BV), vulvovaginal candidiasis (VVC) and trichomonal vaginitis (TV) among non-pregnant reproductive-aged women.
Methods
A cross-sectional study was conducted among 347 non-pregnant reproductive-aged women seeking primary healthcare in Sana’a city, Yemen. Data about sociodemographic characteristics, lifestyle-related behaviors, routine hygienic practices, menstrual care and history and type of contraceptive intake were collected using a structured questionnaire. Vaginal discharge samples were collected and examined for discharge characteristics and pH by a gynecologist. Then, samples were examined for BV, VVC and TV. Data were analyzed using suitable statistical tests.
Results
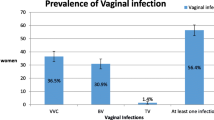
Vaginal infections were prevalent among 37.6% of reproductive-aged women, where BV was the most prevalent (27.2%). VVC was significantly higher among symptomatic women and significantly associated with itching (P = 0.005). Using bivariate analysis, the age of < 25 years (odds ratio [OR] = 1.9, 95% confidence interval [CI]: 1.16–3.10; P = 0.010) and using intrauterine contraceptive devices (IUCDs) (OR = 1.8, 95% CI: 1.09–2.89; P = 0.020) were significantly associated with BV, while history of miscarriage was significantly associated with a lower risk of BV (OR = 0.5, 95% CI: 0.31–0.85, P = 0.009). However, polygyny was significantly associated with VVC (OR = 3.4, 95% CI: 1.33–8.66; P = 0.007). Multivariable analysis confirmed that age of < 25 years and using IUCD were the independent predictors of BV, while history of miscarriage was an independent protective factor against BV. On the other hand, marriage to a polygamous husband was the independent predictor of VVC.
Conclusions
More than a third of non-pregnant reproductive-aged women seeking PHC in Sana’a have single or mixed infections with BV, VVC or TV. BV is the most frequent cause of vaginitis and is significantly associated with the age of < 25 years and using IUCDs, while VVC is significantly higher among women with polygamous husbands. Health education of polygamous husbands and their wives, regular monitoring of BV among IUCD users and screening women for vaginitis before treatment are recommended.
Similar content being viewed by others
Background
Inflammation of the vagina, or vaginitis, is caused by various infectious and non-infectious factors [1]. The most common causes of infectious vaginitis are bacterial vaginosis (BV), vulvovaginal candidiasis (VVC) and trichomonal vaginitis (TV) [2]. The healthy vaginal tract of reproductive-aged women is colonized by normal microbiota dominated by lactobacilli, which protect against pathogenic bacterial species when present in sufficient numbers [3]. Therefore, depletion of lactobacilli distorts the balance of the vaginal microbiota and leads to an increase in anaerobic organisms, contributing to BV [4, 5]. Although BV is most commonly asymptomatic, it can be characterized by the discharge of homogeneous grayish-white smelly secretions, fishy smell after intercourse or during menstruation and an elevation of vaginal pH to above 4.5 [6,7,8,9]. The prevalence of BV ranges from 8 to 51%, depending on geographical location, socioeconomic status and ethnicity [10].
VVC is caused by the overgrowth of yeasts, mainly Candida albicans, which are essentially part of the vaginal flora [11]. Symptoms of VVC include vaginal discharge, itching, pain, and swelling. In addition, vulvar erythema and edema with excoriations are common findings. The typical vaginal discharge in VVC is described as cottage cheese-like in character [9]. It has been suggested that 75.0% of women may experience VVC during their lifetimes [12].
Trichomonas vaginalis is a flagellate protozoan parasite that causes trichomoniasis, which is mainly characterized by severe vaginitis among symptomatic females. The global incidence of trichomoniasis cases was estimated at 140.8 million [95% Uncertainty interval (UI): 121.2–163.2 million] in 2015, with a percentage change of 15.4 (14.5 to 16.5) between 2005 and 2015 [13]. Its transmission is usually sexual, and frequent recurrences often occur if the male partner is not simultaneously treated. Women with TV may complain of yellowish-green, foul-smelling, frothy vaginal discharge. Additionally, dysuria, dyspareunia, vulvar itching and pain may be found. The vulva may be erythematous, edematous and excoriated, and subepithelial hemorrhages or “strawberry spots” may be observed on the vagina and cervix [9].
Vaginitis has been associated with serious sequelae. BV during pregnancy increases the risk of preterm birth and miscarriage [14, 15]. TV can increase the transmission of human immunodeficiency virus [16], while VVC during pregnancy may lead to preterm birth [17]. Over the past 10 years, several risk factors of vaginitis have been identified. Douching, multiple partners and intrauterine contraceptive devices (IUCDs) are risk factors of BV [18,19,20,21], while low socioeconomic status, low educational level, douching and poverty are related to TV [22], and immunodeficiency, diabetes and recent antibiotic use are risk factors of VVC [23,24,25].
In Yemen, vaginitis is one of the most common conditions behind seeking medical care (Personal communication, M. Alhaj, 2019). Recently, BV has been reported among 39.2% of pregnant women in Hadhramout governorate, east of Yemen [26], while TV has been reported among 11.1% of pregnant women seeking primary healthcare (PHC) in Sana’a city [27]. Yet, the prevalence and risk factors associated with the infectious causes of vaginitis among reproductive-aged women are still unclear. Therefore, the present study aimed to determine the prevalence and risk factors associated with the most common infectious causes of vaginitis among Yemeni women.
Methods
Study design, area and population
A cross-sectional study was conducted among reproductive-aged women seeking healthcare in PHC centers in Sana’a city, the capital of Yemen, in the period from February to December 2017. Women were excluded from participation if they were menstruating, pregnant, or if they had received antibiotic or antifungal therapies in the preceding week or vaginal douching within the previous 24 h.
Sample size and sampling strategy
Cluster sampling was adopted, where all PHC centers in Sana’a were listed and four centers were randomly selected. Then, all reproductive-aged women attending each center were invited to voluntarily participate until obtaining the sample size required. The minimum sample size calculated was 294 women at a 95% confidence interval (CI), a precision of 7.0%, an expected prevalence rate of 50.0% and a design effect of 1.5. Yet, 347 women were included in the study.
Data collection
Data about sociodemographic characteristics, lifestyle-related behaviors, routine hygienic practices, menstrual care and history of contraceptive intake were collected using a structured questionnaire through face-to-face interview.
Vaginal examination
The vagina of each woman was examined by a gynecologist for the characteristics of vaginal discharge (color, consistency and odor) using a dry sterile speculum. Then, vaginal pH was measured by applying a pH paper to its lateral wall.
Laboratory investigations
Clinical samples were collected from vaginal walls with two cotton-tipped swabs. The vaginal swabs were then inoculated into a tube containing approximately 2 ml of saline and transported to the Microbiology Laboratory of the University of Science and Technology Hospital. Gram-stained smears were prepared, examined and interpreted for the diagnosis of BV according to the Nugent scoring system [28]. A score of ≥7 was interpreted as positive for BV [29]. Ten percent potassium hydroxide (KOH) wet mounts were examined for C. albicans yeasts or pseudohyphae followed by colony identification after cultivation on Sabouraud dextrose agar for the diagnosis of VVC [30]. Saline wet mounts were examined for motile trophozoites of T. vaginalis followed by their morphological identification on Giemsa-stained smears for the diagnosis of TV [31].
Statistical analysis
Data were analyzed using IBM SPSS Statistics for Windows, version 23.0 (IBM Corp., Armonk, NY, USA). Frequencies and proportions were used to summarize and present the data. The association between independent and dependent variables was tested using Pearson’s chi-square or Fisher’s exact test, whichever suitable, and the odds ratio (OR) and its corresponding 95% CI were reported. A Multivariable logistic regression model was developed for all variables included in the bivariate analysis, and the adjusted OR and its corresponding 95% CI were reported. P-values < 0.05 were considered statistically significant.
Results
Characteristics of the study population
The age of reproductive-aged women in the present study ranged from 15 to 50 years, with a median age of 28.0 years (interquartile range: 10). Of 347 women, the majority of women were aged between 26 and 35 years (46.5%), of secondary level of education (38.6%), married (98.3%), urban residents (89.3%) and unemployed (90.5%). Approximately half of the women were living in rented houses (Table 1).
Prevalence and association of vaginal infections with symptomatic presentation
Table 2 shows an overall prevalence of 37.6% for any type of vaginal infections among reproductive-aged women. BV was the most frequent single infection (27.2%) followed by VVC among 6.6% of women. In contrast, TV was the least frequent vaginal infection, where only three (0.9%) women were found to be positive. Mixed infection with BV and VVC was observed among 2.6% of women, while mixed infection with TV and VVC was observed among 0.3% of women. Table 3 shows that BV was not significantly associated with the symptomatic presentation, characteristics of vaginal discharge or vulvovaginal itching. In contrast, VVC was significantly higher among symptomatic women (P = 0.006) and significantly associated with vulvovaginal itching (P = 0.005).
Association of certain sociodemographic factors, women’s practices and history of poor obstetric outcomes with vaginal infections among reproductive-aged women.
Bivariate analysis showed that women aged < 25 years (OR = 1.9, 95% CI: 1.16–3.10; P = 0.010) were at about two times higher risk of BV. In contrast, education, residence, employment status, husband’s employment status, polygyny and being married for the first time were not significantly associated with BV. On the other hand, polygyny was the only sociodemographic factor significantly associated with VVC, where those married to polygamous husbands were at about three and half times higher risk of being infected with VVC than those married to monogamous husbands (OR = 3.4, 95% CI: 1.33–8.66; P = 0.007). Although women married for more than once were 2.8 times more likely to be infected with VVC (OR = 2.8, 95% CI: 0.96–7.97 P = 0.007; P = 0.051), the significance of the association was on the borderline (Table 4).
Table 5 shows that using IUCD was the only practice significantly associated with BV among reproductive-aged women, where users were about two times more likely to be infected compared with their counterparts (OR = 1.8, 95% CI: 1.09–2.89; P = 0.020). In contrast, history of miscarriage was significantly associated with a half lower risk of BV among women (OR = 0.5, 95% CI: 0.31–0.85, P = 0.009). Multivariable analysis of the sociodemographic factors, practices and poor obstetric outcomes identified the age of < 25 years (AOR = 2.0, 95%CI: 1.10–3.62; P = 0.023) and using IUCD (AOR = 1.8, 95% CI: 1.04–3.08; P = 0.036) as the independent predictors of BV. However, history of miscarriage was identified as an independent protective factor (AOR = 0.5, 95% CI: 0.26–0.81; P = 0.006) against BV among reproductive-aged women seeking PHC in Sana’a. On the other hand, marriage to a polygamous husband was identified as an independent predictor of VVC, where women with polygamous husbands were four times more likely to get infected (AOR = 3.9, 95% CI: 1.15–13.29; P = 0.029) (Table 6).
Discussion
The present study revealed that 37.6% of Yemeni reproductive-aged women seeking PHC in Sana’a city have single or mixed vaginal infections with BV, VVC or TV. Such prevalence is almost comparable to those reported among women seeking medical care from Pakistan and Nepal, being 33.5 and 39.0%, respectively [32, 33]. On the contrary, it is lower than the prevalence (89.0%) reported among non-pregnant reproductive-aged women from Rajasthan in India but higher than the prevalence (15.4%) reported among Ethiopian reproductive-aged women seeking medical care [34, 35]. BV was the most common cause of vaginitis among Yemeni women seeking PHC in Sana’a, being more predominant than VVC. However, TV was the least frequent cause of vaginitis, being detected among less than 1.0% of women. The predominance of BV over the other two causes of vaginitis is consistent with the findings among reproductive-aged women from distantly separated countries worldwide, including Indonesia, southwestern Nigeria, Nepal, Iran, Turkey and Grenada [18, 25, 33, 36,37,38]. In contrast, VVC was the most prevalent cause of vaginitis among sexually active adolescents from Brazil [21] and reproductive-aged women seeking medical care in Ethiopia and northeastern/northwestern Nigeria [35, 39, 40].
Based on the Nugent scoring system as the “gold standard” for BV diagnosis [41], BV was found to be prevalent among 27.2% of Yemeni reproductive-aged women in the present study which is lower than that (39.2%) reported among pregnant women from Hadhramout, an eastern Yemeni governorate [26]. Compared to the finding of the present study, a similar prevalence of 27.0% was reported for BV among women from socioeconomically deprived communities in Peru [42]. However, lower BV prevalence of 15.2 and 19.5% were reported among reproductive-aged women attending hospitals in Ethiopia [35, 38], and non-pregnant women attending PHC centers in Iran (16.2%) [36]. Besides, higher prevalence of 48.6% was reported for BV among women with vaginitis attending hospital in Kochi, India [43]. It is noteworthy that the comparison between studies is difficult due to differences in study designs and populations, diagnostic techniques and technical variations. In addition to the negative impact of BV on the quality of life of Yemeni reproductive-aged women, BV could contribute to infertility [44, 45]. Given the high prevalence of BV among Yemeni women, screening or treatment of reproductive-aged women for BV should be undertaken to avoid its negative health impacts, particularly for women complaining of and seeking treatment for infertility.
The significant association of VVC with vaginal itching among reproductive-aged women in the present study is consistent with a review evaluating vaginal complaints, which suggests more likelihood of vaginal itching among patients with candidiasis [46]. Furthermore, the lack of a significant difference between BV among asymptomatic and symptomatic women is a common observation [47]. It is noteworthy that even asymptomatic BV can contribute to a range of adverse outcomes [10, 48]. This, in turn, supports the need for screening reproductive-aged women irrespective of the symptomatic nature of vaginitis. The significant association between BV and young age is consistent with previous studies elsewhere [49, 50]. The risk of BV in younger women might be explained by the higher frequency of unprotected sexual intercourse among newly married adolescent and young ladies that can affect the vaginal environment in a way that increases the likelihood of BV [51]. This could be supported by the few women reporting the use of condoms (1.2%) in the present study. Frequent sexual intercourse prevents the restoration of the vaginal ecosystem after a coital act and, hence, sustains an ideal environment for the growth of anaerobic bacteria [52]. Frequent sexual intercourse can also increase the likelihood of transferring perianal and perivulvar bacteria to the vagina, leading to BV [53].
The identification of using IUCD as an independent predictor of BV among reproductive-aged women is in line with previous findings from Indonesia and Turkey [18, 54], which reported a significant association between long-term use of IUCD and BV. The significant association of IUCD with BV in the present study also agrees with the findings of earlier studies in Belgium and Sweden [55, 56]. In contrast, an earlier review on the association of IUCD and pelvic inflammatory diseases found no association between IUCD use and BV due to the lack of strong evidence [57]. According to the review, the lack of adequate adjustment for sexual behaviors and the use of inappropriate control groups were among the factors making the evidence of association not convincing [57]. The association between IUCD use and BV in the present study could be attributed to increased menstrual flow and irregular vaginal bleeding, where these can change the vaginal microbiome and decrease the ratio lactobacilli [18, 58]. In this study, vaginal bleeding was observed in 15.4% (16/104) of women using IUCD. Moreover, the IUCD may facilitate the ascent of cervicovaginal microorganisms into the uterus [59]. It has been suggested that about a half of IUCD users can have at least one episode of BV during the first 24 months [55]. Therefore, there is a need for continual monitoring of BV among women using IUCDs, with replacement if needed.
Although miscarriage has been suggested as a poor obstetric outcome of BV [15], history of miscarriage was a protective factor against BV among women in the present study. This finding could be partially explained by the higher awareness among aborted women of the risk of BV, which was translated into correct practices against BV. It is to be noted that a half of women in the present study were aware of vaginitis as a cause of miscarriage. BV has been suggested as a cause of preterm delivery irrespective of treatment [60]. Although the odds of preterm births were 1.7-fold higher among women with BV in the present study, the association did not attain statistical significance. Moreover, specific bacterial species in BV can be associated with preterm births [61], such an association can be influenced by the predominance of certain bacterial species.
In line with the low prevalence of VVC (6.6%) among reproductive-aged women in the present study, Yemen was identified as a country with the least frequent VVC infections on a global scale by a recent systematic review [62]. The present study, being married to a polygamous husband was an independent predictor of infection with VVC, suggesting that husbands may mechanically circulate this fungal infection among their multiple wives. Although the role of sexual transmission of Candida is still controversial, penile colonization with Candida has been reported [63,64,65]. This, in turn, highlights the importance of health education of polygamous husbands about sexually transmitted diseases (STDs). The prevalence of TV (0.9%) among reproductive-aged women in the present study is lower than the prevalence (11%) found among pregnant women attending PHC centers in Sana’a city [27]. The very low prevalence in the present study could be attributed to the low sensitivity of microscopic examination of wet mount preparations and Giemsa-stained smears in detecting all infections with TV [66,67,68].
The low prevalence of mixed vaginal infections (2.9%) among women in the present study entails the discontinuation of vaginitis management as a mixed infection, considering that this practice is thought to be common among physicians in Yemen (personal communication, Alhaj, M. 2019). Nevertheless, focusing on the management of BV as the most common cause of vaginitis by prescribing antibiotics may lead to the spread of VVC [25]. Therefore, routine examination of vaginal swabs is key to the proper clinical management of vaginitis among women in Yemen.
Despite the suitability of the present cross-sectional design to determine the prevalence of vaginal infections, its value identifying the associated risk factors is limited. Therefore, case-control studies are recommended for a comprehensive analysis of the risk factors associated with vaginal infections among reproductive-aged women in Yemen. On the other hand, relying on microscopic examination for the diagnosis of TV could underestimate the prevalence of the infection, and the use of more sensitive techniques is recommended.
Conclusions
More than a third of non-pregnant reproductive-aged women seeking PHC in Sana’a city have single or mixed infections with BV, VVC or TV, with BV is the most frequent cause of vaginitis among 27.2% of women. BV is significantly associated with the age of < 25 years and using IUCDs. Second to BV as a cause of vaginitis, VVC is significantly higher among women with polygamous husbands. Health education interventions are recommended to raise women’s awareness of vaginitis and its prevention. In addition, regular monitoring of BV among women using IUCD, educating polygamous husbands and their wives about the transmission and prevention of STDs and screening women for the causes of vaginitis before treatment are recommended.
Availability of data and materials
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Abbreviations
- AOR:
-
Adjusted odds ratio
- BV:
-
Bacterial vaginosis
- CI:
-
Confidence interval
- IUCD:
-
Intrauterine contraceptive device
- OR:
-
Odds ratio
- PHC:
-
Primary healthcare
- SPSS:
-
Statistical Packages for Social Sciences
- STD:
-
Sexually transmitted disease
- TV:
-
Trichomonal vaginitis
- VVC:
-
Vulvovaginal candidiasis
References
Donders GG. Definition and classification of abnormal vaginal flora. Best Pract Res Clin Obstet Gynaecol. 2007;21(3):355–73.
Workowski KA, Bolan GA. Centers for Disease Control and Prevention (CDC). Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR–03):1–137.
Kumar N, Behera B, Sagiri SS, Pal K, Ray SS, Roy S. Bacterial vaginosis: etiology and modalities of treatment-a brief note. J Pharm Bioallied Sci. 2011;3(4):496–503.
Ventolini G. Progresses in vaginal microflora physiology and implications for bacterial vaginosis and candidiasis. Womens Health (Lond). 2016;12(3):283–91.
Borges S, Silva J, Teixeira P. The role of lactobacilli and probiotics in maintaining vaginal health. Arch Gynecol Obstet. 2014;289(3):479–89.
Amsel R, Totten PA, Spiegel CA, Chen KC, Eschenbach D, Holmes KK. Nonspecific vaginitis: diagnostic criteria and microbial and epidemiologic associations. Am J Med. 1983;74(1):14–22.
Verstraelen H, Verhelst R. Bacterial vaginosis: an update on diagnosis and treatment. Expert Rev Anti-Infect Ther. 2009;7(9):1109–24.
Hainer BL, Gibson MV. Vaginitis. Am Fam Physician. 2011;83(7):807–15.
Paladine HL, Desai UA. Vaginitis: diagnosis and treatment. Am Fam Physician. 2018;97(5):321–9.
Kenyon C, Colebunders R, Crucitti T. The global epidemiology of bacterial vaginosis: a systematic review. Am J Obstet Gynecol. 2013;209(6):505–23.
van Schalkwyk J, Yudin MH, Infectious Disease Committee. Vulvovaginitis: screening for and management of trichomoniasis, vulvovaginal candidiasis, and bacterial vaginosis. J Obstet Gynaecol Can. 2015;37(3):266–74.
Achkar JM, Fries BC. Candida infections of the genitourinary tract. Clin Microbiol Rev. 2010;23(2):253–73.
GBD 2017 Diseases and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789–858.
Riduan JM, Hillier SL, Utomo B, Wiknjosastro G, Linnan M, Kandun N. Bacterial vaginosis and prematurity in Indonesia: association in early and late pregnancy. Am J Obstet Gynecol. 1993;169(1):175–8.
Isik G, Demirezen S, Donmez HG, Beksac MS. Bacterial vaginosis in association with spontaneous abortion and recurrent pregnancy losses. J Cytol. 2016;33(3):135–40.
Bachmann LH, Hobbs MM, Sena AC, Sobel JD, Schwebke JR, Krieger JN, et al. Trichomonas vaginalis genital infections: progress and challenges. Clin Infect Dis. 2011;53(Suppl 3):S160–72.
Roberts CL, Algert CS, Rickard KL, Morris JM. Treatment of vaginal candidiasis for the prevention of preterm birth: a systematic review and meta-analysis. Syst Rev. 2015;4:31.
Joesoef MR, Karundeng A, Runtupalit C, Moran JS, Lewis JS, Ryan CA. High rate of bacterial vaginosis among women with intrauterine devices in Manado, Indonesia. Contraception. 2001;64(3):169–72.
Martinez F, Lopez-Arregui E. Infection risk and intrauterine devices. Acta Obstet Gynecol Scand. 2009;88(3):246–50.
Klebanoff MA, Nansel TR, Brotman RM, Zhang J, Yu KF, Schwebke JR, et al. Personal hygienic behaviors and bacterial vaginosis. Sex Transm Dis. 2010;37(2):94–9.
Mascarenhas RE, Machado MS, Costa e Silva BF, Pimentel RF, Ferreira TT, Leoni FM, et al. Prevalence and risk factors for bacterial vaginosis and other vulvovaginitis in a population of sexually active adolescents from Salvador, Bahia, Brazil. Infect Dis Obstet Gynecol. 2012;2012:378640.
Sutton M, Sternberg M, Koumans EH, McQuillan G, Berman S, Markowitz L. The prevalence of Trichomonas vaginal is infection among reproductive-age women in the United States, 2001–2004. Clin Infect Dis. 2007;45(10):1319–26.
Thoden J, Potthoff A, Bogner JR, Brockmeyer NH, Esser S, Grabmeier-Pfistershammer K, et al. Therapy and prophylaxis of opportunistic infections in HIV-infected patients: a guideline by the German and Austrian AIDS societies (DAIG/OAG) (AWMF 055/066). Infection. 2013;41(Suppl 2):S91–115.
Buchta V, Matula V, Kestranek J, Vejsova M, Krivcikova L, Spacek J. Is diabetes mellitus a risk factor in genital yeast infections? Ceska Gynekol. 2013;78(6):537–44.
Olowe OA, Makanjuola OB, Olowe R, Adekanle DA. Prevalence of vulvovaginal candidiasis, trichomoniasis and bacterial vaginosis among pregnant women receiving antenatal care in Southwestern Nigeria. Eur J Microbiol Immunol (Bp). 2014;4(4):193–7.
AL-Haik WM, Al-Haddad AM. Bacterial vaginosis among pregnant women in Hadhramout - Yemen. Alandalus J Appl Sci. 2017;7(16):23-33.
Al-Mekhlafi AM, El-Eryani SM. Prevalence and risk factors for Trichomonas vaginalis infection among pregnant women seeking primary health care in Sana'a city, Yemen. Yemeni J Med Sci. 2017;11:8–14.
Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol. 1991;29(2):297–301.
Rodrigues FS, Peixoto S, Adami F, Alves Bda C, Gehrke Fde S, Azzalis LA, et al. Proposal of a new cutoff for Nugent criteria in the diagnosis of bacterial vaginosis. J Microbiol Methods. 2015;115:144–6.
Bitew A, Abebaw Y. Vulvovaginal candidiasis: species distribution of Candida and their antifungal susceptibility pattern. BMC Womens Health. 2018;18(1):94.
Mason PR, Super H, Fripp PJ. Comparison of four techniques for the routine diagnosis of Trichomonas vaginalis infection. J Clin Pathol. 1976;29(2):154–7.
Sami S, Baloch SN. Vaginitis and sexually transmitted infections in a hospital based study. J Pak Med Assoc. 2005;55(6):242–4.
Shrestha S, Tuladhar NR, Basnyat S, Acharya GP, Shrestha P, Kumar P. Prevalence of vaginitis among pregnant women attending Paropakar maternity and Women’s hospital, Thapathali, Kathmandu, Nepal. Nepal Med Coll J. 2011;13(4):293–6.
Masand DL, Patel J, Gupta S. Utility of microbiological profile of symptomatic vaginal discharge in rural women of reproductive age group. J Clin Diagn Res. 2015;9(3):QC04–7.
Mulu W, Yimer M, Zenebe Y, Abera B. Common causes of vaginal infections and antibiotic susceptibility of aerobic bacterial isolates in women of reproductive age attending at Felegehiwot referral hospital, Ethiopia: a cross sectional study. BMC Womens Health. 2015;15:42.
Bahram A, Hamid B, Zohre T. Prevalence of bacterial vaginosis and impact of genital hygiene practices in non-pregnant women in Zanjan, Iran. Oman Med J. 2009;24(4):288–93.
Haltas H, Bayrak R, Yenidunya S. To determine of the prevalence of bacterial vaginosis, Candida sp, mixed infections (bacterial vaginosis + Candida sp), Trichomonas vaginalis, Actinomyces sp in Turkish women from Ankara, Turkey. Ginekol Pol. 2012;83(10):744–8.
Brooks-Smith-Lowe K, Rodrigo S. Prevalence of bacterial vaginosis in Grenadian women of reproductive age. West Indian Med J. 2013;62(7):599–603.
Nwosu CO, Djieyep NA. Candidiasis and trichomoniasis among pregnant women in a rural community in the semi-arid zone, North-Eastern Nigeria. West Afr J Med. 2007;26(1):17–9.
Ugwa EA. Vulvovaginal candidiasis in Aminu Kano teaching hospital, north-West Nigeria: hospital-based epidemiological study. Ann Med Health Sci Res. 2015;5(4):274–8.
Sha BE, Chen HY, Wang QJ, Zariffard MR, Cohen MH, Spear GT. Utility of Amsel criteria, Nugent score, and quantitative PCR for Gardnerella vaginalis, Mycoplasma hominis, and Lactobacillus spp. for diagnosis of bacterial vaginosis in human immunodeficiency virus-infected women. J Clin Microbiol. 2005;43(9):4607–12.
Jones FR, Miller G, Gadea N, Meza R, Leon S, Perez J, et al. Prevalence of bacterial vaginosis among young women in low-income populations of coastal Peru. Int J STD AIDS. 2007;18(3):188–92.
Bitew A, Abebaw Y, Bekele D, Mihret A. Prevalence of bacterial vaginosis and associated risk factors among women complaining of genital tract infection. Int J Microbiol. 2017;2017:4919404.
Gaudoin M, Dobbie R, Finlayson A, Chalmers J, Cameron IT, Fleming R. Ovulation induction/intrauterine insemination in infertile couples is associated with low-birth-weight infants. Am J Obstet Gynecol. 2003;188(3):611–6.
van Oostrum N, De Sutter P, Meys J, Verstraelen H. Risks associated with bacterial vaginosis in infertility patients: a systematic review and meta-analysis. Hum Reprod. 2013;28(7):1809–15.
Anderson MR, Klink K, Cohrssen A. Evaluation of vaginal complaints. JAMA. 2004;291(11):1368–79.
Klebanoff MA, Schwebke JR, Zhang J, Nansel TR, Yu KF, Andrews WW. Vulvovaginal symptoms in women with bacterial vaginosis. Obstet Gynecol. 2004;104(2):267–72.
Andrews WW, Hauth JC, Cliver SP, Conner MG, Goldenberg RL, Goepfert AR. Association of asymptomatic bacterial vaginosis with endometrial microbial colonization and plasma cell endometritis in nonpregnant women. Am J Obstet Gynecol. 2006;195(6):1611–6.
Lan PT, Lundborg CS, Phuc HD, Sihavong A, Unemo M, Chuc NT, et al. Reproductive tract infections including sexually transmitted infections: a population-based study of women of reproductive age in a rural district of Vietnam. Sex Transm Infect. 2008;84(2):126–32.
Vaca M, Guadalupe I, Erazo S, Tinizaray K, Chico ME, Cooper PJ, et al. High prevalence of bacterial vaginosis in adolescent girls in a tropical area of Ecuador. BJOG. 2010;117(2):225–8.
Vallor AC, Antonio MA, Hawes SE, Hillier SL. Factors associated with acquisition of, or persistent colonization by, vaginal lactobacilli: role of hydrogen peroxide production. J Infect Dis. 2001;184(11):1431–6.
Boskey ER, Telsch KM, Whaley KJ, Moench TR, Cone RA. Acid production by vaginal flora in vitro is consistent with the rate and extent of vaginal acidification. Infect Immun. 1999;67(10):5170–5.
Verstraelen H, Verhelst R, Vaneechoutte M, Temmerman M. The epidemiology of bacterial vaginosis in relation to sexual behaviour. BMC Infect Dis. 2010;10:81.
Demirezen S, Kucuk A, Beksac MS. The association between copper containing IUCD and bacterial vaginosis. Cent Eur J Public Health. 2006;14(3):138–40.
Avonts D, Sercu M, Heyerick P, Vandermeeren I, Meheus A, Piot P. Incidence of uncomplicated genital infections in women using oral contraception or an intrauterine device: a prospective study. Sex Transm Dis. 1990;17(1):23–9.
Moi H. Prevalence of bacterial vaginosis and its association with genital infections, inflammation, and contraceptive methods in women attending sexually transmitted disease and primary health clinics. Int J STD AIDS. 1990;1(2):86–94.
Meirik O. Intrauterine devices - upper and lower genital tract infections. Contraception. 2007;75(6 Suppl):S41–7.
Madden T, Grentzer JM, Secura GM, Allsworth JE, Peipert JF. Risk of bacterial vaginosis in users of the intrauterine device: a longitudinal study. Sex Transm Dis. 2012;39(3):217–22.
Ferraz do Lago R, Simoes JA, Bahamondes L, Camargo RP, Perrotti M, Monteiro I. Follow-up of users of intrauterine device with and without bacterial vaginosis and other cervicovaginal infections. Contraception. 2003;68(2):105–9.
Shimaoka M, Yo Y, Doh K, Kotani Y, Suzuki A, Tsuji I, Mandai M, Matsumura N. Association between preterm delivery and bacterial vaginosis with or without treatment. Sci Rep. 2019;9(1):509.
Manns-James L. Bacterial vaginosis and preterm birth. J Midwifery Womens Health. 2011;56(6):575–83.
Denning DW, Kneale M, Sobel JD, Rautemaa-Richardson R. Global burden of recurrent vulvovaginal candidiasis: a systematic review. Lancet Infect Dis. 2018;18(11):e339–47.
David LM, Walzman M, Rajamanoharan S. Genital colonisation and infection with candida in heterosexual and homosexual males. Genitourin Med. 1997;73(5):394–6.
Reed BD, Zazove P, Pierson CL, Gorenflo DW, Horrocks J. Candida transmission and sexual behaviors as risks for a repeat episode of Candida vulvovaginitis. J Womens Health (Larchmt). 2003;12(10):979–89.
Horowitz BJ, Edelstein SW, Lippman L. Sexual transmission of Candida. Obstet Gynecol. 1987;69(6):883–6.
Mahmoud A, Sherif NA, Abdella R, El-Genedy AR, El Kateb AY, Askalani AN. Prevalence of Trichomonas vaginalis infection among Egyptian women using culture and latex agglutination: cross-sectional study. BMC Womens Health. 2015;15:7.
Perazzi BE, Menghi CI, Coppolillo EF, Gatta C, Eliseth MC, de Torres RA, et al. Prevalence and comparison of diagnostic methods for Trichomonas vaginalis infection in pregnant women in Argentina. Korean J Parasitol. 2010;48(1):61–5.
Adjei C, Boateng R, Dompreh A, Okyere B, Owiredu EW. Prevalence and the evaluation of culture, wet mount, and ELISA methods for the diagnosis of Trichomonas vaginalis infection among Ghanaian women using urine and vaginal specimens. Trop Med Health. 2019;47:33.
Acknowledgments
Authors thank women participated in the study and the management of PHC centers where the study was conducted for cooperation. The authors acknowledge Dr. Salwa A. M. Alhelali, a gynecologist; Ebtisam A. Al-Absi, Medical Laboratory Sciences Program; Dr. Waleed Q. A. Farie, Laboratory of University of Science and Technology Hospital; and Dr. Mogahid Y. Nassar, Department of Clinical Pathology, Faculty of Medicine, University of Science and Technology, Yemen for their help during data collection and laboratory investigations.
Funding
This study received no fund.
Author information
Authors and Affiliations
Contributions
MAKM designed the study. NAA, LKAA, SAA, FAMA, NJMA, WB, and SJHA conducted the fieldwork and performed the laboratory investigations. MAKM analyzed the data. MAA drafted the manuscript. MAKM, RA, MA and AMAA revised the manuscript. All authors approved the final draft for submission.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocol was reviewed and approved by the Ethics Committee of the Faculty of Medicine, University of Science and Technology, Sana’a, Yemen. Informed written consent was obtained from each participant after a clear explanation of the study objectives. Drugs were prescribed for women positive for vaginal infections by the gynecologist in each PHC center.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Abdul-Aziz, M., Mahdy, M.A.K., Abdul-Ghani, R. et al. Bacterial vaginosis, vulvovaginal candidiasis and trichomonal vaginitis among reproductive-aged women seeking primary healthcare in Sana’a city, Yemen. BMC Infect Dis 19, 879 (2019). https://doi.org/10.1186/s12879-019-4549-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-019-4549-3