Abstract
Background
Antiretroviral therapy (ART) was rolled-out in Ethiopia in 2005, but there are no reports on outcome of ART and human immunodeficiency virus drug resistance (HIVDR) at national level. We described acquired drug resistance mutations in pol gene and performed a viral genome wide association study in virologic treatment failure patients who started first line ART during 2009–2011 in the first large countrywide HIV cohort in Ethiopia.
Methods
The outcome of tenofovir (TDF)- and zidovudine (ZDV)-based ART was defined in 874 ART naïve patients using the on-treatment (OT) and intention-to-treat (ITT) analyses. Genotypic resistance testing was done in patients failing ART (> 1000 copies/ml) at month 6 and 12. Near full-length genome sequencing (NFLG) was used to assess amino acid changes in HIV-1 gag, pol, vif, vpr, tat, vpu, and nef genes between paired baseline and month 6 samples.
Results
High failure rates were found in ITT analysis at month 6 and 12 (23.3%; 33.9% respectively). Major nucleoside and non-nucleoside reverse transcriptase (NRTI/NNRTI) drug resistance mutations were detected in most failure patients at month 6 (36/47; 77%) and month 12 (20/30; 67%). A high rate of K65R was identified only in TDF treated patients (35.7%; 50.0%, respectively). No significant difference was found in failure rate or extent of HIVDR between TDF- and ZDV- treated patients. All target regions of interest for HIVDR were described by NFLG in 16 patients tested before initiation of ART and at month 6.
Conclusion
In this first Ethiopian national cohort, a high degree of HIVDR was seen among ART failure patients, independent on whether TDF- or ZDV was given. However, the major reason to ART failure was lost-to-follow-up rather than virologic failure. Our NFLG assay covered all relevant target genes for antiretrovirals and is an attractive alternative for HIVDR surveillance.
Similar content being viewed by others
Background
In Ethiopia, over 700,000 people are currently estimated to live with human immunodeficiency virus (HIV), corresponding to an adult prevalence of 1.15% [1]. Since 2005, antiretroviral therapy (ART) has been widely accessible through the WHO public health approach [2, 3]; the first-line regimen consists of fixed-dose combinations (FDC) of two nucleoside/nucleotide reverse transcriptase inhibitors (NRTI; zidovudine (ZDV) or tenofovir (TDF) plus lamivudine (3TC) or emtricitabine (FTC)), and a non-nucleoside RTI (NNRTI; efavirenz (EFV) or nevirapine (NVP) [4]. About 420,000 people living with HIV (PLHIV) were on ART by 2016 (nearly 60% coverage) [5]. However, the absence of viral load monitoring in Ethiopia and the high proportion of lost-to-follow-up (LTFU) [6, 7] are predicted to lead to a high rate of treatment failure and emergence of drug resistance, as seen in other sub-Saharan African countries (sSA) [8].
Only a few studies with relatively small number of patients from limited geographical regions in Ethiopia have reported ART failure rates, including acquired HIV drug resistance (HIVDR) [9,10,11,12]. Although ART has been rapidly scaled up throughout the country, to the best of our knowledge, there is no data at the national level. Hence, using a large nationwide HIV cohort, we assessed treatment failure, including acquired HIVDR by genotypic resistance testing and performed viral genome wide association studies by near-full length genome (NFLG) sequencing. In addition, we evaluated our NFLG assay for its capacity to amplify all HIVDR target regions of interest since it is an attractive alternative for HIV drug resistance mutation (DRM) surveillance.
Methods
Patients
A total of 874 ART naïve patients were enrolled into the Advanced Clinical Monitoring (ACM) of ART in Ethiopia from seven university hospitals during 2009–2011 as we described elsewhere [13, 14]. As per the national guidelines [4], patients were given fixed dose combinations (FDC): TDF + 3TC + EFV (n = 389), TDF + 3TC + NVP (n = 78), ZDV + 3TC + EFV (n = 104), ZDV + 3TC + NVP (n = 258), stavudine (d4T) + 3TC + EFV (n = 23), d4T + 3TC+ NVP (n = 21), and abacavir (ABC) + 3TC + EFV (n = 1). Altogether TDF was given to 467 (53.4%), ZDV to 362 (41.4%), d4T to 44 (5%) and ABC to one patient (Table 1).
Treatment outcome measurements
The treatment outcomes at month 6 and 12 were determined by on-treatment (OT) and intention-to-treat (ITT) analyses. Two categories of virological treatment failures were defined as i) > 150 copies/ml (limit of detection of the assay); ii) > 1000 copies/ml (as per WHO definition). For ITT, treatment failure was defined as failure to attain viral suppression (as described for OT) or lost-to-follow-up (LTFU) including confirmed death, moved from study sites or similar reasons.
Clinical and laboratory tests
Clinical, routine laboratory and CD4 T-cells were analysed at the study sites [4]. Viral load was quantified by MT 2000 real time PCR (Abbott, USA) (detection limit 150 copies/ml).
Population-based sanger sequencing (PBSS)
PBSS was attempted on patients with viral load ≥1000 copies/ml at month 6 in 51 subjects and month 12 in 33 patients [14]. In brief, HIV RNA was extracted from 140 μl plasma. The first-round of PCR was done using JA203F-C (forward) and JA206R-C (reverse) primer pair, followed by the second-round PCR, using JA204F-C (forward) and JA205R-C (reverse) primer pair [15]. The amplified fragments were purified and sequenced with JA204F-C and JA205R-C primers plus PR2R (5′-GGATTTTCAGGCCCAATTTTTG-3′) and RT07 (5′-AAGCCAGGAATGGATGGCCCA-3′). Sequences were analysed using the BioEdit software version 7.2.6.1 (http://www.mbio.ncsu.edu/bioedit/bioedit.html). Acquired DRM were identified by the Stanford HIVdb Program (hivdb.stanford.edu). Genotypic drug resistance defined as the presence of ≥1 major amino acid substitution included in the 2015 International Antiviral Society–USA (IAS) mutation list [16] and the Stanford algorithm was used to predict drug susceptibility. Drug classes considered were NRTI, NNRTI, and protease inhibitors (PI).
Near-full length genomes (NFLG)
NFLG sequencing was performed on plasma from baseline and month 6 of 16 randomly selected patients among virologic failure patients with VL > 1000 copies/ml at month 6 of whom 12 were given TDF and four ZDV (Table 1), as described earlier [17, 18]. In brief, the NFLG (HXB2: 790 to 9554) was amplified in two primary fragments of 5.5 kb and 3.7 kb with an overlap of 400 bp and sequenced with up to 23 primers. CAP3 Sequence Assembly Program with default parameter was used to assemble the final NFLG [19]. The first NFLG HIV-1CET sequence (U46016) described by our group was used as a reference [20]. A multiple sequence alignment with our NFLG sequences was generated with the reference genome in AliView ver. 1.17.1 software [21] and analysed with an in-house Perl script that recognized the nucleotide changes from the reference sequence and created a corresponding number code as per HXB2 coordinates (790 to 9417). The resulting matrix was plotted using the TraMineR package [22] in R ver. 3.1.2 [23] to obtain a diversity plot. Maximum likelihood phylogenetic analysis was performed using Molecular Evolutionary Genetics Analysis version 7.0 (MEGA 7) software.
Identification of mutations
Using AliView ver 1.17.1 and BioEdit ver 7.2.6.1 softwares, we aligned nucleotides and amino acids generated for each gene from the paired samples and described the specific amino acid mutations, which had appeared at month 6. The protein alignments were manually reviewed to identify changed residues. As European guideline recommended we have used the Geno2pheno tools at FPR 10% cut-off (Geno2PhenoFPR10%) for prediction of tropism throughout the analysis [24].
Statistical analysis
Descriptive statistics (mean, median, standard deviation, and percentiles for numerical variables; frequencies and percentages for categorical variables) were used to summarize sociodemographic, clinical and virological parameters. Treatment outcomes were compared between patients with different NRTI regimens by Chi-square or Fisher’s exact test. The prevalence and type of DRM were compared between patients with TDF- or ZDV-based regimens by Chi-square or Fisher’s exact test. P-value < 0.05 was considered statistically significant. Data analysis was performed using STATA software 14 (Stata Corp. College Station, Texas, USA).
Results
Outcome of TDF- and ZDV-based ART
Among TDF treated patients, OT analysis identified virologic failure in 52/350 (15.0%) and 34/350 (9.7%) at month 6 using the 150 copies/ml and 1000 copies/ml cut-off values, respectively. ITT analysis identified treatment failure in 129/426 (30.2%) and 111/426 (26.0%) patients, respectively (Table 2). For ZDV, the figures were 34/288 (11.8%), 19/288 (6.6%) in the OT; 83/337 (24.6%), and 68/337 (20.2%) in the ITT analysis, respectively. For d4T treated patients, OT and ITT identified 4/38 (10.5%) and 9/43 (20.9%) virologic failure in both viral load cut-off values, respectively. There was no statistically significant difference in outcome across the NRTI regimens.
At month 12, for TDF patients OT analysis showed virologic failure in 30/219 (13.7%) and 19/219 (8.7%) patients using the 150 copies/ml and 1000 copies/ml cut-off values, respectively. In ITT analysis treatment failure was found in 133/322 (41.3%) and 122/322 (37.9%) patients, respectively (Table 2). For ZDV, the figures were 27/207 (13.0%), 13/207 (6.3%), 100/280 (35.7%), and 86/280 (30.7%), respectively. For d4T, the figures were 4/33 (12.1%), 2/33 (6.1%), 11/40 (27.5%), and 9/40 (22.5%), respectively. There was no statistically significant difference in treatment outcome across the NRTI regimens. No statistically significant difference was found in treatment outcome at month 12 as well when patients failing TDF were compared with those failing ZDV.
Acquired DRM detected by PBSS at month 6 and 12
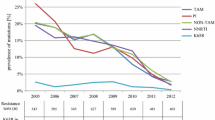
At month 6, a total of 47 sequences were obtained from 28/34 (82.4%), 15/19 (78.9%), and 4/4 (100%) of the TDF-, ZDV-, d4T-failing patient samples with VL > 1000 copies/ml, respectively (Table 3). One to seven (median: three) major NRTI and/or NNRTI DRM (but no major PI DRM) were found in 36/47 (76.6%) samples: NRTI+NNRTI: n = 24 (66.7%); only NRTI: n = 2 (5.6%); only NNRTI: n = 10 (27.8%) (detailed description of the DRM presented on Additional file 1: Table S1). Twenty-one of the 28 (75.0%) TDF failure patients had DRM (NNRTI+NRTI: 17; NNRTI only: 4). K65R was found in 10 (35.7%) subjects. Six patients had TAM (TAM-1: 1; TAM-2: 5; TAM-1 + TAM-2: 1). No significant difference was found in viral load at month 6 for patients failing TDF with or without K65R. In ZDV patients, 12/15 (80.0%) had DRM (NNRTI+NRTI: 5; NNRTI only: 6, NRTI only: 1). No K65R was found in this group.
At month 12, a total of 30 sequences were obtained from 16/17 (94.1%), 10/12 (83.3%) and 4/4 (100%) of the TDF-, ZDV-, d4T-failing patients, respectively. One to eight (median: four) major NRTI and/or NNRTI DRM were found in 20 (66.7%) samples: NRTI+NNRTI: n = 16 (80.0%); only NRTI: n = 1 (5.0%); only NNRTI: n = 3 (15.0%). No significant difference was found in viral load at month 6 or month 12 for patients failing TDF with or without DRM and for patients failing ZDV with or without DRM. Thirteen of 16 (81.3%) TDF failure patients had DRM (NNRTI+NRTI: 12; NNRTI only: 1). K65R was found in eight (50%) patients and TAM-2 in five patients. In ZDV patients, 5/10 (50%) had DRM (NNRTI+NRTI: 2; NNRTI only: 2; NRTI only: 1). With regard to the number of DRM in sequences harboring such mutations, no difference was found between patients failing TDF or ZDV at month 6. However, at month 12 sequences harboring mutations from patients failing TDF had more DRM as compared to those failing ZDV (p = 0.017), NNRTI DRM (p = 0.037), and NRTI DRM (p = 0.040).
Amino acid changes identified by NFLG
NFLG sequences including gag, pol, vif, vpr, tat, vpu, and nef were successfully generated in all 32 (16 paired) samples, except for the nef gene at month 6. Maximum likelihood phylogenetic analysis revealed proper matching of the paired NFLG sequences with 100% bootstrap support (Fig. 1). No sample had hypermutations and the analysis predicted only functional viruses. Analysis of the 16 baseline pol sequences showed no major DRM. No patient had PI- or integrase strand inhibitor (INSTI) DRM.
Maximum likelihood phylogenetic analysis of the baseline and month 6 NFLG sequences showing proper matching. A Neighbor-Joining tree was generated in MEGA with the Kimura 2-parameter method and full-length sequences of all successfully assembled samples. All final branches display a full bootstrap support of 100% confirming proper sample matching without cross-contamination and therefore all samples could be used for longitudinal analysis. The scale bar corresponds to 0.01 change per nucleotide
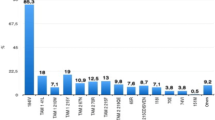
After six months of ART, eleven (68.8%) patients had acquired one to five (median: three) major DRM in pol (NRTI+NNRTI: 7; NNRTI: 4). The predicted sensitivities to NRTI and NNRTI are given on Fig. 2. Although none had known PI- or INSTI-associated DRMs, several other mutations were identified in the protease and integrase regions (A9P, E15K, A42E/T, K258 N, N278S/D, Y336C, G345A/S, K462R, and M532R/L) in two or three samples each. Four amino acid (GTIP/GALN/GTLV/GTLQ) insertions were displayed in the protease region at positions 48–55 (Fig. 3).
For gag, amino acid changes mostly clustered in the p6 and p17 regions. In the Gag-p6 region, A5G/E/P/P5S/A/R5A and K6S/P6A/S6K/E6K/V6E mutations were displayed in seven and five samples, respectively. PYKE tetra-peptide motif was found both at baseline and month 6 in nine (56.3%) of the 16 sample pairs on the C-terminal position of the p6 region. Duplicate tetra-peptide motif PTAP was identified in two samples at baseline, where the respective month 6 samples had single motif (deletion of one of the double motifs). A triple PTAP tetra-peptide motif was found in paired samples of a patient. The remaining 13 patients (81.3%) had only single P(T/S)AP motif in their paired samples. In the C-terminal of p17, 14 (87.5%) of the 16 patients displayed the R4S mutation. On the other hand, only one patient had a mutation (T375 M) in the p2/NC gag cleavage site.
Mutations were also identified in the vif (N36K/S n = 4; G101S/D n = 4, V31I n = 3, K33R, R34K, K63R, R92G, G101D n = 2) and in the nef gene (A15E/S n = 3, L20I/A/R n = 3; W5R/Q, K7N, C8S, V11P/G, R19A/P, D24A/G, K105 N/T, K118E/R n = 2). The tat, vpu and vpr showed very few amino acid changes (Fig. 3).
Co-receptor tropism and long-terminal repeat (LTR)
The V3 loop was successfully sequenced in 13 baseline and 14 month 6 samples (eleven paired). The Geno2PhenoFPR10% tool predicted 10/13 baseline viruses to be CCR5, two as CXCR4 tropic and one as CXCR4/CCR5 dual tropic. Of the 14 viruses at month 6, 12 were predicted as CCR5 and two as CXCR4 tropic. The CXCR4/CCR5 dual tropic virus at baseline switched to CXCR4 and the CXCR4 virus to CCR5 tropic.
Of the 16 baseline samples 15 (93.8%) displayed three nuclear factor kappa B (NF-kB) each in the enhancer region of the LTR of HIV-1C and the remaining one sample shows two NF-kB (which has short nucleotide sequence). Again 15 of the 16 (93.8%) month 6 samples displayed triple NF-kB, but one sample displayed large insertions in the LTR and showed four NF-kB instead.
Discussion
In this first large countrywide study of ART outcome in Ethiopia, a high failure rate was identified in the ITT-analysis; around one-fourth at month 6 and one-third at month 12, whereas the OT-analysis revealed less than 10% of the participants failed virologically (> 1000 copies/ml) at month 6 and 12. Viral load is a gold standard for monitoring ART response and is a marker of the treatment outcome [25]. However, the optimal threshold for defining virologic failure and for switching ART regimens has not been well established in the setting of LMIC, and WHO recommended a threshold of 1000 copies/ml [26]. Below 1000 copies/ml, viral blips or intermittent low level viremia (50–1000 copies/ml) can occur during effective treatment, but their relevance in the LMIC setting has not been proven [27]. In our cohort, the baseline HIV RNA levels were high (mean 5.2, SD 0.8 Log10 copies/ml) although the levels were in line with what has been earlier reported from real-life cohorts in sub-Saharan Africa [28]. Thus, in view of a possible slower decay of viremia for very high viral load values the treatment failure rate due to viral rebound at month 6 may have been overestimated in our study. In addition, although some studies suggest that drug resistance present in patients with low level viremia could impact the long term treatment outcomes [29], others highlighted standard drug resistance testing may be unreliable and difficult to obtain among such patients [30].
This study confirms that early death and LTFU are major reasons to poor treatment outcome of ART in Ethiopia, as described from other sSA countries [31], although broad HIVDR to the first line regimens was common on those still on ART. In our study HIVDR was identified in 76.6 and 66.7% of virologic failure patient samples with VL > 1000 copies/ml at month 6 and 12, respectively as determined by PBSS. The treatment outcome of the different NRTI-based regimens did not differ, neither the extent of viral resistance at failure, although the K65R mutation was only found in TDF-treated patients. All relevant target regions for HIVDR were described in the subset of patients who were analysed by our NFLG.
In the WHO recommendations, TDF has since several years replaced thymidine analogues (ZDV and stavudine) in first-line regimens. As shown in our study, the introduction of TDF without virological monitoring may result in an extensive evolution of the K65R mutation, especially since it is preferentially selected by HIV-1C in ex vivo and in vitro analysis [32, 33]. Importantly, strains with the K65R may be transmitted further which may jeopardize future therapeutic and prophylactic use of TDF and of tenofovir alafenamide [34].
Because of its well-recognized toxicities and as per the WHO recommendation [3, 35], Ethiopia has amended its guidelines to initiate all new patients on non-d4T-based ART regiments. Accordingly, the use of d4T-based regimen has been observed reduced in our study into 5%. However, the retention rate observed among our patients who received this regimen was significantly higher than those who were on TDF-based and ZDV-based regimens (p < 0.05). In contrary to our observation, studies from resource-limited settings revealed that TDF-based regimens performed better than d4T, most notably with a significantly higher rate of LTFU for d4T patients [36].
Retention in care was low and undocumented mortality and self-transfer of patients are likely to have contributed. A possible way forward to improve treatment outcome could possibly be the use of long-acting drugs, such as the integrase inhibitor cabotegravir, which inhibits HIV-1C at least as efficient as HIV-1B [37], and the NNRTI rilpivirine. However, rilpivirine may not be an optimal drug in HIV-1C infected Ethiopians due to the high viral load in the majority of patients at diagnosis and the less binding efficacy to the HIV-1C reverse transcriptase [38]. Also, it has now become clear that the clearance of these two long-lasting drugs takes a very long time after cessation of therapy. Therefore the risk of development of resistance to both rilpivirine and cabotegravir is high if a patient is not adherent to the injection schedule [39].
Our HIV-NFLG sequencing assay was used to study the amino acid changes between paired samples from baseline and month 6 of virologic failure patients at several genes. It was found to efficiently amplify key HIV-1 drug target sites (PI, RTI, and IN) in all 32 tested samples, and the env and the LTR in the majority of patients. In addition, non-drug target sites like Gag and gp41 were sequenced which also can affect the drug efficiency [40, 41]. Thus, changes in the gag region may influence the efficacy of PI which is second line treatment option in Ethiopia. Gag mutations were found by NFLG in the majority of our patients at failure although the identified point mutations are not known to influence the response to PI. A PYKE tetra-peptide, which was found in the ALIX-binding motif of Gag-p6 at baseline in all patients, remained unaffected by the treatment in all subjects. In an earlier study, this tetra-peptide motif was observed among half of treatment naïve Ethiopian patients, but the status was not known among ART experienced patients [42]. In contrast, the PYKE motif was observed only in few sequences from South African and Indian ART naïve HIV-1C infected patients (1 and 3%, respectively). Therefore, it is important to elucidate the clinical relevance of the PYKE motif in terms of viral fitness and susceptibility to ART, especially to PI drugs with larger number of samples in HIV-1C subtypes.
Changes of the motif PTAP in Gag-p6 were also seen. Thus, a duplicate of the motif was found in baseline samples of two patients of which one motif was deleted from the virus of each patient at month 6. Also, a triple PTAP motif was detected in paired samples of one patient, which has not been described earlier. Subtype specific differences have earlier been observed in Gag-p6 with regard to the motif PTAP. In addition, a difference in ART outcome in relation to duplication of the PTAP motifs for HIV-1C has been reported [43]. The duplication probably restores the ALIX mediated virus release pathway, which is lacking in HIV-1C, as PTAP motif is thought to be a key player in viral budding [44]. After 6 months of ART, one of the duplicated motifs we initially observed in two baseline samples were deleted. This was not in line with a study that showed accumulations of long duplications within PTAP during ART in a high proportion of HIV-1C patients [43]. Therefore, a further study about the significance of this tetra-peptide motif on treatment outcome and its clinical relevance is recommended.
In the present study we have used our NFLG assay to analyse HIV drug resistance in an Ethiopian population and all relevant target regions for HIVDR were described. Although HIV resistance testing is presently not used clinically in most low- and middle income countries (LMIC), the method is an alternative for surveillance of HIV drug resistance. Earlier we have shown the negative impact of pretreatment drug resistance mutations on virologic outcome in our Ethiopian cohort [14]. Also, in view of the increased use of dolutegravir in the first line ART in LMIC and of boosted protease inhibitors in the second line ART it is important that the associated DRM can be surveilled.
It can also be noted that resistance to dolutegravir has been described outside the integrase, mainly in Nef and LTR overlapped region, in vitro and in patients failing monotherapy with dolutegravir [45]. In contrast to the findings in the gag gene, no common pattern of amino acid changes was seen in the other genes, with the exception of known RTI DRM in the pol gene. However, in all of the NFLG sequences we identified three NF-kB binding regions in the LTR which is unique for HIV-1C and described by us earlier [46]. Altogether the data suggest that our cost-effective NFLG assay has a potential for extended genotypic resistance testing, as compared to PBSS, and also for studies of the viral population dynamics in the HIV epidemic.
Conclusions
In conclusion, although the retention in care was low in this first countrywide Ethiopian cohort no differences was found between patients given TDF- or ZDV-based regimens with regard to treatment outcome or level of drug resistance. A broad RT-inhibitor resistance was found in three quarters of the patients who were on-treatment with virologic failure. Our NLFG assay was shown to efficiently amplify genes of known or potential relevance for HIV drug resistance and is an attractive alternative for such surveillance in low and middle income countries.
Availability of data and materials
All data generated during this study are included in this published article and its supplementary information file. The pol sequences generated and analysed during the current study are available in the GenBank (accession numbers: MH666276 – MH666351).
Abbreviations
- 3TC:
-
Lamivudine
- ACM:
-
Advanced Clinical Monitoring
- ART:
-
Antiretroviral therapy
- DRM:
-
Drug resistance mutations
- EFV:
-
Efavirenz
- FDC:
-
Fixed-dose combinations
- FTC:
-
Emtricitabine
- GRT:
-
Genotypic resistance testing
- HIV:
-
Human immunodeficiency virus
- HIV-1C:
-
HIV-1 subtype C
- HIVDR:
-
Human immunodeficiency virus drug resistance
- INSTI:
-
Integrase strand inhibitors
- ITT:
-
Intention-to-treat
- LTFU:
-
Lost-to-follow-up
- LTR:
-
Long-terminal repeat
- NFLG:
-
Near-full-length genome sequencing
- NNRTI:
-
Non-nucleoside reverse transcriptase
- NRTI:
-
Nucleoside reverse transcriptase
- NVP:
-
Nevirapine
- OT:
-
On-treatment
- PBSS:
-
Population-based Sanger sequencing
- PI:
-
Protease inhibitors
- TDF:
-
Tenofovir
- VL:
-
Viral load
References
EPHI, HIV Related Estimates and Projections for Ethiopia–2017. Ethiopian Public Health Institute. March 2017, Addis Ababa 2017.
Assefa Y, et al. Scaling up antiretroviral treatment and improving patient retention in care: lessons from Ethiopia, 2005-2013. Glob Health. 2014;10:43.
Gilks C, Vitoria M, And World Health Organization. Dept. of HIV/AIDS., Antiretroviral therapy for HIV infection in adults and adolescents: recommendations for a public health approach. 2006 rev. ed. Geneva: World Health Organization; 2006. p. 128.
HAPCO. Guidelines for Management of Opportunistic Infections and Anti-retroviral Treatment in Adolescents and Adults in Ethiopia. 2008. Available at: https://www.who.int/hiv/pub/guidelines/ethiopia_art.pdf. Accessed 31 May 2016.
UNAIDS. UNAIDS DATA 2017. Joint United Nations Programme on HIV/AIDS (UNAIDS) 2017.
HAPCO, Report on progress towards implementation of the UN declaration of commitment on HIV/AIDS, Federal Democratic Republic of Ethiopia, federal HIV/AIDS prevention and control office, Addis Ababa, Ethiopia. 2010.
HAPCO. Country progress report on HIV/AIDS response, Federal Democratic Republic of Ethiopia, Federal HIV/AIDS Prevention and Control Office, Addis Ababa, Ethiopia April 2012. Federal HAPCO; 2012.
Hamers RL, et al. Emerging HIV-1 drug resistance after roll-out of antiretroviral therapy in sub-Saharan Africa. Curr Opin HIV AIDS. 2013;8(1):19–26.
Mulu A, Maier M, Liebert UG. Low incidence of HIV-1C acquired drug resistance 10 years after roll-out of antiretroviral therapy in Ethiopia: a prospective cohort study. PLoS One. 2015;10(10):e0141318.
Abdissa A, et al. Drug resistance in HIV patients with virological failure or slow virological response to antiretroviral therapy in Ethiopia. BMC Infect Dis. 2014;14:181.
Mulu A, Maier M, Liebert UG. Upward trends of acquired drug resistances in Ethiopian HIV-1C isolates: a decade longitudinal study. PLoS One. 2017;12(10):e0186619.
Tadesse BT, et al. High levels of dual-class drug resistance in HIV-infected children failing first-line antiretroviral therapy in southern Ethiopia. Viruses. 2018;10(2):1-21.
Kalu AW, et al. Monophylogenetic HIV-1C epidemic in Ethiopia is dominated by CCR5-tropic viruses-an analysis of a prospective country-wide cohort. BMC Infect Dis, 2017;17(1):37.
Telele NF, et al. Pretreatment drug resistance in a large countrywide Ethiopian HIV-1C cohort: a comparison of sanger and high-throughput sequencing. Sci Rep. 2018;8(1):7556.
Lindstrom A, Albert J. A simple and sensitive 'in-house' method for determining genotypic drug resistance in HIV-1. J Virol Methods. 2003;107(1):45–51.
Wensing AM, et al. Update of the drug resistance mutations in HIV-1. Top Antivir Med, 2017. 2017;24(4):132–3.
Amogne W, et al. Phylogenetic analysis of Ethiopian HIV-1 subtype C near full-length genomes reveals high Intrasubtype diversity and a strong geographical cluster. AIDS Res Hum Retrovir. 2016;32(5):471–4.
Grossmann S, Nowak P, Neogi U. Subtype-independent near full-length HIV-1 genome sequencing and assembly to be used in large molecular epidemiological studies and clinical management. J Int AIDS Soc. 2015;18.
Huang X, Madan A. CAP3: a DNA sequence assembly program. Genome Res. 1999;9(9):868–77.
Salminen MO, et al. Full-length sequence of an ethiopian human immunodeficiency virus type 1 (HIV-1) isolate of genetic subtype C. AIDS Res Hum Retrovir. 1996;12(14):1329–39.
Larsson A. AliView: a fast and lightweight alignment viewer and editor for large datasets. Bioinformatics. 2014;30(22):3276–8.
Gabadinho A, et al. Analyzing and visualizing state sequences in R with TraMineR. J Stat Softw. 2011;40(4):1–37.
R Development Core Team, R. A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2014.
Vandekerckhove LP, et al. European guidelines on the clinical management of HIV-1 tropism testing. Lancet Infect Dis. 2011;11(5):394–407.
DHHS. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents Living with HIV. Laboratory testing; plasma HIV-1 RNA (viral load) and CD4 count monitoring. Last updated: may 1, 2014; last reviewed: may 1, 2014. U.S. Department of Health & Human Services; 2018. Available at: https://aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescent-arv/458/plasma-hiv-1-rna--viral-load--and-cd4-count-monitoring. Accessed 10 May 2018.
WHO. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection Recommendations for a public health approach - Second edition. 2016. Available at: http://www.who.int/hiv/pub/arv/arv-2016/en/. Accessed 10 June 2018.
Havlir DV, et al. Prevalence and predictive value of intermittent viremia with combination HIV therapy. JAMA. 2001;286(2):171–9.
Kityo C, et al. Raltegravir-intensified initial antiretroviral therapy in advanced HIV disease in Africa: a randomised controlled trial. PLoS Med. 2018;15(12).
Ryscavage P, et al. Significance and clinical management of persistent low-level viremia and very-low-level viremia in HIV-1-infected patients. Antimicrob Agents Chemother. 2014;58(7):3585–98.
Nettles RE, et al. Genotypic resistance in HIV-1-infected patients with persistently detectable low-level viremia while receiving highly active antiretroviral therapy. Clin Infect Dis. 2004;39(7):1030–7.
Haas AD, et al. Retention and mortality on antiretroviral therapy in sub-Saharan Africa: collaborative analyses of HIV treatment programmes. J Int AIDS Soc. 2018;21(2).
Brenner BG, et al. HIV-1 subtype C viruses rapidly develop K65R resistance to tenofovir in cell culture. AIDS. 2006;20(9):F9–13.
Coutsinos D, et al. A template-dependent dislocation mechanism potentiates K65R reverse transcriptase mutation development in subtype C variants of HIV-1. PLoS One. 2011;6(5):e20208.
van Tienen C, et al. Letter to the editor: pre-exposure prophylaxis for HIV in Europe: the need for resistance surveillance. Euro Surveill. 2017;22(11).
World Health Organization. Antiretroviral therapy for HIV infection in adults and adolescents: recommendations for a public health approach - 2010 revision. 2010 rev. ed. Geneva: World Health Organization; 2010. p. 145.
Velen K, et al. Comparison of tenofovir, zidovudine, or stavudine as part of first-line antiretroviral therapy in a resource-limited-setting: a cohort study. PLoS One. 2013;8(5):e64459.
Neogi U, et al. Ex-vivo antiretroviral potency of newer integrase strand transfer inhibitors cabotegravir and bictegravir in HIV type 1 non-B subtypes. AIDS. 2018;32(4):469–76.
Neogi U, et al. Factors influencing the efficacy of rilpivirine in HIV-1 subtype C in low- and middle-income countries. J Antimicrob Chemother. 2016;71(2):367–71.
Gulick RM, Flexner C. Long-acting HIV drugs for treatment and prevention. Annu Rev Med. 2018.
Pillay SK, et al. Gag drug resistance mutations in HIV-1 subtype C patients, failing a protease inhibitor inclusive treatment regimen, with detectable lopinavir levels. J Int AIDS Soc. 2014;17(4 Suppl 3):19784.
Su Y, et al. Mechanism of HIV-1 resistance to short-peptide fusion inhibitors targeting the Gp41 pocket. J Virol. 2015;89(11):5801–11.
Neogi U, et al. Novel tetra-peptide insertion in gag-p6 ALIX-binding motif in HIV-1 subtype C associated with protease inhibitor failure in Indian patients. AIDS. 2014;28(15):2319–22.
Martins AN, et al. Accumulation of P(T/S)AP late domain duplications in HIV type 1 subtypes B, C, and F derived from individuals failing ARV therapy and ARV drug-naive patients. AIDS Res Hum Retrovir. 2011;27(6):687–92.
Sharma S, et al. The PTAP sequence duplication in HIV-1 subtype C gag p6 in drug-naive subjects of India and South Africa. BMC Infect Dis. 2017;17(1):95.
Wijting IEA, et al. HIV-1 resistance dynamics in patients failing dolutegravir maintenance monotherapy. J Infect Dis. 2018.
Johansson B, Sherefa K, Sonnerborg A. Multiple enhancer motifs in HIV type 1 strains from Ethiopia. AIDS Res Hum Retrovir. 1995;11(6):761–4.
Acknowledgements
We acknowledge Addis Ababa University (AAU) College of Health Sciences, and the ACM steering committee.
Funding
Samples and data were collected by the Advanced Clinical Monitoring of Antiretroviral Treatment in Ethiopia (ACM), funded by CDC Cooperative Agreement 5U2GPSOOO85 8. Part of this work has been funded by the Swedish Research Council, Swedish International Developing Agency, the Swedish Institute, the EDCTP and the EuResist consortium.
Funding bodies do not have any role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
NFT: designed the study, performed laboratory experiments, analysed sequences and data, and drafted and prepared the manuscript. AWK: designed parts of the study, performed laboratory experiments, and reviewed the manuscript. SGS, DF and BT: designed parts of the study, and reviewed the manuscript. SG: performed the NFLG analysis and reviewed the manuscript. GM: supervised the statistics and reviewed the manuscript. UN: designed and developed the NFLG assay and reviewed the manuscript. AS: designed the study, interpreted clinical data, supervised all aspects of the study, developed and reviewed the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approvals were obtained from the National Research Ethics Review Committee in Ethiopia (3.10|528|06) and the Institutional Review Board (IRB) of EHNRI (6.13/163). Written informed consent was also obtained from participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1:
Table S1. Drug resistance mutations (DRM) associated with reverse transcriptase inhibitors in patients failing at month 6 and/or 12 by the population-based Sanger sequencing (PBSS) assay. (DOCX 18 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Telele, N.F., Kalu, A.W., Gebre-Selassie, S. et al. A viral genome wide association study and genotypic resistance testing in patients failing first line antiretroviral therapy in the first large countrywide Ethiopian HIV cohort. BMC Infect Dis 19, 569 (2019). https://doi.org/10.1186/s12879-019-4196-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-019-4196-8