Abstract
Background
The etiology and epidemiology of acute otitis media (AOM) are poorly understood in China. This study aimed to describe the etiology of AOM and the phenotypic and molecular characteristics of AOM-causing Streptococcus pneumoniae (S.pneumoniae) recovered from Chinese children.
Methods
A retrospective study was conducted to enrol patients younger than 18 years diagnosed as AOM. Middle ear fluid specimens were collected then cultured for bacterial pathogens. All S.pneumoniae isolates were tested for antibiotic susceptibility, serotypes, virulence genes, antibiotic resistant determinants and sequence types.
Results
The dominant otopathogen among AOM children was S.pneumoniae (54.4%). Among S.pneumoniae isolates, there were 97.3, 97.3 and 75.7% isolates resistant to erythromycin, tetracycline and trimethoprim-sulfamethoxazole, respectively. There was 72.8% S.pneumoniae with multidrug resistance. The dominant sequence types (STs) were ST271 and ST320, whereas the prevailing serotypes were 19F and 19A. The 7-valent and 13-valent pneumococcal conjugate vaccine (PCV) coverage among AOM children were 73.0 and 94.6%, respectively. Additionally, we found that CC271 expressed more of mef(A/E) (P < 0.001), pspA (P = 0.022) and sipA (P < 0.001) than non-CC271 isolates.
Conclusion
The high prevalence of international multidrug-resistant clone (Taiwan19F-14) in China necessitates continued dedication to expand PCV13 immunization and better control of antibiotic use in China.
Similar content being viewed by others
Background
Acute otitis media (AOM) is one of the most frequently encountered bacterial infections and the leading cause of medical visits and antibiotic prescription in children worldwide [1, 2]. Among the pathogens involved in this infection, Streptococcus pneumoniae (S.pneumoniae) and Haemophilus influenzae (H.influenzae) are the principal bacterial otopathogens that account for up to 60–80% of microbiologically confirmed AOM [3, 4]. Concerns have been rising about the major role of S.pneumoniae in AOM etiology, which coincides with growing antibiotic resistance rates among S.pneumoniae isolates. In China, the rate of penicillin-nonsusceptible S.pneumoniae (PNSP) ranges from 11.5–39.4% [5, 6]. The Asian Network for Surveillance of Resistant Pathogens (ANSORP) reported that, in China, the rate of resistance to erythromycin was as high as 96.4%, whilst multidrug resistance was 83.3%, which rank China highest among the 11 enrolled Asian countries surveyed [7].
Increasing rates of resistance of S.pneumoniae to commonly used antibiotics highlights the increased urgency for use of vaccines for controlling pneumococcal diseases. The heptavalent pneumococcal polysaccharide conjugate vaccine (PCV7) has had a dramatic effect on reducing the disease burden of pneumococcal diseases globally, including AOM [8, 9]. The increased prevalence of pneumococcal diseases caused by non-vaccine serotypes, especially serotype 19A from an emerging clonal complex (CC) 320, has been well documented in the post PCV7 era [10, 11]. PCV7 has been available as a self-paid vaccine in mainland China since August 2008, but immunization rates were less than 10% in published studies [12, 13].
Despite a well-developed knowledge base of S.pneumoniae worldwide, in China, little has been reported on the microbial etiology of AOM and phenotypic-molecular characteristics of S.pneumoniae, the predominant otopathogen. Therefore, we conducted this study in China to investigate the bacterial etiology and characterize pneumococcal isolates obtained from children with AOM. Phenotypic and genotypic characteristics of the pneumococcal isolates from AOM patients, including antimicrobial susceptibility, serotypes, multilocus sequence typing (MLST) profiles, and virulence genes of S. pneumoniae were also investigated.
Methods
Patients and isolates
This study was approved by the Ethics Committee of Liuzhou Maternity and Child Healthcare Hospital, and was performed in accordance with the approved guidelines. Before enrolment of a child into this study, written informed consent was obtained from parents or legal guardians on behalf of the children involved in the study for collection of information and samples.
This retrospective study was conducted from January 1st 2016 to June 30th 2016 in the otolaryngology clinic of Liuzhou Maternity and Child Health Care Hospital. Children aged younger than 18 years old, with no prior AOM at the time of enrolment, were enrolled in this study. AOM was diagnosed by the otolaryngologist or pediatrician after otoscopic examination of the ear drum, confirmation of acute onset of symptoms lasting ≤7 days consistent with AOM and accompanied by one or more of the following symptoms: substantial bulging of the tympanic membrane, marked redness of the tympanic membrane, and a cloudy or purulent effusion was observed.
Middle ear fluid specimens were obtained by either tympanocentesis or collection of pus from draining ears and were immediately delivered to the clinical microbiology laboratory for culture. The specimens were plated on Colombia blood agar with 5% sheep blood and Chocolate agar and placed in 35 °C, 5% CO2 incubated for 24 h to 48 h. All agars were purchased from Autobio (Henan, China). S.pneumoniae was identified by typical alpha haemolysis and draftsman colonies on the blood agar plate together with optochin test and bile solubility test. H.influenzae was identified by growth requirements for factors V and X. The disk of optochin, X, V and X + V were purchased from OXOID (Hampshire, England). The suspected isolates were confirmed by the VITEK 2 Gram Positive Card (Streptococcus pneumoniae, Staphylococcus aureus, Streptococcus pyogenes), Gram Negative Card (Pseudomonas aeruginosa), and Neisseria-Haemophilus Card (Haemophilus influenzae) using VITEK 2 compact automatic microbial analysis system (Biomérieux, Marcyl’ Etoile, France). Chromogenic Agar medium was used for Candida species identification (Biomérieux) and API 20C AuX (Biomérieux) was used for confirmation. In this study, we only focused on the epidemiology and phenotypic-molecular characterization of S. pneumoniae. The pneumococcal isolates were stored at − 80 °C in 10% glycerol-brain heart infusion broth with 50% sheep blood.
Antibiotic susceptibility test
The antibiotic susceptibility of S.pneumoniae isolates were determined by the minimum inhibitory concentration with the VITEK 2 Gram Positive Susceptibility Card-AST-GP68 using VITEK2 systems. Definitions of antibiotic susceptibility were based on the Clinical and Laboratory Antimicrobial Standards Institute (CLSI) 2016 criteria [14]. Current CLSI susceptible, intermediate, resistant (respectively) breakpoints for parenteral penicillin (2 μg/ml, 4 μg/ml, 8 μg/ml; nonmeningitis), oral penicillin (0.06 μg/ml, 1 μg/ml, 2 μg/ml), cefotaxime (1 μg/ml, 2 μg/ml, 4 μg/ml; nonmeningitis), erythromycin (0.25 μg/ml, 0.5 μg/ml, 1 μg/ml), and levofloxacin (2 μg/ml, 4 μg/ml, 8 μg/ml), tetracycline (1 μg/ml, 2 μg/ml, 4 μg/ml), trimethoprim -sulfamethoxazole (0.5/0.95 μg/ml, 2/38 μg/ml, 4/76 μg/ml) were considered. The CLSI QA and QC guidelines were followed for antibiotic susceptibility testing together with S. pneumoniae ATCC49619 as the quality control strain. Isolates non-susceptible to at least 3 antibiotic classes were defined as multidrug-resistant S. pneumoniae.
DNA extraction
Chromosomal DNA was extracted from the overnight subculture of S.pneumoniae isolates by using Biospin Bacteria Genomic DNA Extraction Kit (Bioer Ltd., Hangzhou, China) according to the manufacturer’s instructions.
Serotyping
Pneumococcal serotyping was performed using multiplex polymerase chain reaction (m-PCR) methods as described in a previous study [15]. Thirty-six different serotypes were determined by 8 sequential m-PCR reactions and the method used to distinguish serotypes 6A/6B was as reported previously [6].
Detection of antibiotic-resistant genes
The macrolide-resistant genes erm(A), erm(B) and mef(A/E) were amplified by PCR methods for all erythromycin-resistant isolates [16]. The tet(K), tet(L) and tet(O) genes were amplified by PCR for all tetracycline-resistant isolates using the primers and PCR conditions as previously described [17]. All the PCR products were visualized by 1.5% agarose gel electrophoresis and gold-view staining.
Detection of virulence genes
Detection of virulence genes (ply, pasA, lytA, pspA) and pili genes (rlrA for PI-1and sipA for PI-2) of pneumococcal isolates were amplified using PCR and all primers, PCR reactions and conditions used were as previously described [17]. The 100 bp plus DNA ladder marker (Takara, China) was used for molecular weight reference.
Multilocus sequence typing
Sequence types (STs) of pneumococcal isolates were determined using the multilocus sequence typing (MLST) technique. Internal fragments of 7 housekeeping genes (aroE, gdh, gki, recP, spi, xpt, ddl) were amplified from chromosomal DNA by PCR [18]. Allelic profiles and STs were assigned by comparison with pneumococcal MLST database (https://pubmlst.org/spneumoniae), and clonal complexes (CCs) were determined using the eBURST algorithm. The definition of a clonal complex is a group in which every strain shares at least five identical alleles out of seven with at least one other genotype in the group [19].
Statistical analysis
Data were analyzed using descriptive statistics and Pearson’s chi-squared (χ2) test or Fisher exact test. A two-sided P-value of less than 0.05 was considered as being of statistical significance. All statistical analyses were conducted using SPSS version 20.0 (SPSS Inc. Chicago, Il, USA). PHYLOViZ method (http://www.phyloviz.net) was used to demonstrate the relationship of serotypes and MLST.
Results
Microbiology of AOM
Seventy-nine patients were enrolled during the study period. Eighty-four samples were collected from 79 patients, 68 samples from 68 patients were culture positive. The predominant pathogen was S.pneumoniae (37 isolates), followed by H.influenzae (12 isolates), Staphylococcus aureus (7 isolates), Pseudomonas aeruginosa (5 isolates), Streptococcus pyogenes (4 isolates) and Candida albicans (3 isolates). The demographic information of children with AOM is shown in Table 1. Seventy-four of the 79 children with AOM (93.7%) were less than 2 years old, and the majority of the patients were male (58.2%). All 37 children experiencing pneumococcal AOM were less than 2 years old, whilst 62.2% were male.
Antibiotic susceptibility of S. pneumoniae
In pneumococcal isolates, no isolate was resistant to parenteral penicillin, but the intermediate and resistant rates reached 59.5% (22/37) and 27.0% (10/37), respectively, based on the oral breakpoints. The non-susceptibility rates were low for levofloxacin (2.7%) and chloramphenicol (8.1%), but high for erythromycin (100.0%), tetracycline (97.3%) and trimethoprim-sulfamethoxazole (83.8%) (Table 2). The multidrug-resistant rate of pneumococcal isolates was 72.8%. The most common multidrug-resistant patterns were non-susceptibility to erythromycin/tetracycline/trimethoprim-sulfamethoxazole (59.5%), erythromycin/tetracycline/trimethoprim-sulfamethoxazole/chloramphenicol (8.1%) and erythromycin/tetracycline/trimethoprim-sulfamethoxazole/cefotaxime (5.4%).
Serotyping, vaccine coverage and sequence types of S. pneumoniae
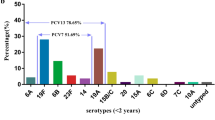
Among the 37 S.pneumoniae isolates, the predominant serotypes were 19F (62.2%) and 19A (18.9%), accounting for 81.1% of all the isolates. The pneumococcal vaccine coverage rate for the pneumococcal isolates was 73.0% for PCV7, but high at 94.6% for PCV13 (Fig. 1). All 36 S.pneumoniae isolates were successfully typed by MLST, only one isolate, serotype 19F, failed to sequence. Among the 8 STs identified, the most common STs were ST271 (n = 19, 51.4%) and ST320 (n = 9, 24.3%), with the majority of ST271 and ST320 isolates serotyped as 19F and 19A, respectively (Fig. 2). The eBURST analysis revealed 1 CC and 6 singletons containing 75.7 and 21.6% of the isolates, respectively, with the predominant CC271 including 28 isolates (75.7%).
Antibiotic resistance genes and virulence genes of S. pneumoniae
Among the 37 pneumococcal isolates (Table 3), 94.6% (35 isolates) carried erm(B) gene, 64.9% (24 isolates) carried mef(A/E) gene, 94.6% (35 isolates) carried tet(M) gene, and only 1 isolate carried tet(O) gene. Notably, about 94.6% of S.pneumoniae isolates were co-resistant to tetracycline and erythromycin, carrying both erm(B) and tet(M) genes, whilst none of the 37 isolates contained macrolide or tetracycline resistance genes, such as erm(A), tet(K) or tet(L). Tetracycline phenotypic resistance results showed that over 91.9% (34/37) of isolates carried tet(M) gene. In comparison, the macrolide phenotypic resistance results, showed 94.6% (35/37) of isolates carried erm(B) gene, while only 62.2% (23/37) carried mef(A/E) gene, indicating good correlation between phenotypic-genotypic results in terms of tetracycline and erythromycin resistance. In all of the isolates, 97.3% (36 isolates) carried both pneumococcal virulence genes lytA and ply, and 83.8% (31 isolates) carried pspA gene. The rlrA gene was identified in 22 of 37 (59.5%) isolates, and sipA was detected in 67.6% (25 isolates) of the pneumococcal isolates.
Relations between ST types, serotypes, and resistance-virulence characteristics
All the serotype 19A isolates belonged to ST320 (Table 3). Overall, 73.3% of serogroup 19 harbored mef(A/E), while 14.3% of the non-serotype 19 harbored mef(A/E) (P = 0.007). The same difference was observed in sipA (P < 0.001). Up to 80% (22 isolates) of CC271 clones expressed mef(A/E), and only 11.1% of other isolates expressed mef(A/E), indicating significant difference between two groups (P < 0.001). Similar significant differences were observed in the carriage of pspA (P = 0.022) and sipA (P < 0.001) genes expressed in CC271 clones and other isolates. As to antibiotic resistance genes and pneumococcal virulence genes, we observed that ST271 expressed more of mef(A/E) (P = 0.004) and sipA (P = 0.001) than non-ST271 isolates.
Discussion
AOM is a common upper respiratory tract infection among children younger than 5 years old. The monitoring of etiology of AOM, antibiotic resistance and the molecular background of AOM-causing S.pneumoniae provides opportunities to identify changes in the predominant bacteria and the impact of changing antibiotic resistance and prevalence of virulence genes within these bacteria that may result from environmental selection pressures such as pneumococcal immunization and antibiotic use. In this retrospective study, we found that S.pneumoniae was the most prevalent pathogen causing for AOM in the recruited children and was isolated from 46.8% of the cohort. The high proportion of the pneumococcal isolates resistant to erythromycin, tetracycline and trimethoprim-sulfamethoxazole (59.5%) is consistent with the Suzhou study of nasopharyngral isolates [20]. Interestingly, the proportion of pneumococcal isolates that were resistant to penicillin, was inconsistent with previous reports from China that demonstrated a high prevalence of resistance to penicillin [6, 20]. This inconsistency may be explained by the prescribing pattern in Liuzhou, which uses macrolide antibiotics such as erythromycin and azithromycin, not penicillin, as the first option prescription for pediatric patients. The second reason may be that the isolates we collected were from middle ear fluid of pediatric patients with AOM, which were reported more frequently in the community. There was report that the S.pneumoniae isolates were more susceptible to penicillin within the community than in the medical places [21], which may explain the inconsistency of our results from other reports collected isolates from hospitalized children with a high prevalence of resistance to penicillin [6].
It was reported that the resistance of S.pneumoniae to the third-generation cephalosporin was increasing in some area of China. For example, in Suzhou, among the S.pneumoniae isolates collected from the children with AOM, the penicillin resistant S.pneumoniae (PRSP) was 10.2%, while the resistance rate of cefotaxime (CTX) was 34.3% [20]. Among the 58 S.pneumoniae CC271 clones of the serotype 19F collected from children with non-meningitis in Beijing, the non-susceptible rate to ceftriaxone was 96.4%, while only 8.6% isolates was penicillin non-susceptible S.pneumoniae (PNSP) [22]. In our study, only 1 of the S.pneumoniae isolates was determined as PNSP, but as many as 16.2% (6/37) of isolates were determined as CTX non-susceptible S.pneumoniae (using non-meningitis breakpoints based on the CLSI criteria [14]), which was consistent with the previous reports [5, 6, 20, 22]. Interestingly, all 7 of these isolates are serotype 19F, ST271clone. These findings are supported by a report from Ip M et al. [23] which observed that in Hongkong 2011, the rates of PNSP and CTX non-susceptibility were 23.3 and 30.3% respectively but that all of the CTX resistant isolates were attributed to the single serotype 19F, CC271, with only 25.3% of these isolates was penicillin resistant [23]. Resistance of S.pneumoniae to the third-generation cephalosporin CTX was increasing in the above areas due to the acquisition of an additional 19 amino acid substitutions in penicillin binding protein 2b (PBP2b) and PBP2x through genome recombination events [23]. Furthermore, the pbp2b gene of S.pneumoniae has very low affinity for cephalosporins and inactivation of this gene seems not to be working on the CTX [24] thus cause the high rate of non-susceptible CTX but low rate of PNSP in our study.
Macrolide resistance rates of pneumococcal isolates vary greatly throughout the world however the pneumococci resistance rate to erythromycin was 72.7% in Asia, with the highest rate in China (96.4%) as reported by ANSORP [25]. It is noteworthy that pneumococci harboring both erm(B) and mef(E) genes continue to be reported, with a global prevalence of 16.4% among macrolide-resistant pneumococcal isolates [26]. Overall in this study, 94.6 and 62% of the macrolide-resistant pneumococci were positive for erm(B) and mef(A/E), respectively. The present study also showed that 78.6% of the Taiwan19F-14 clone harbored dual positive genes, while only 11.1% of other STs harbored both genes which is consistent with a previous report that erm(B) and mef genes have been related to the Taiwan19F-14 clone [265]. As a result of the insertion of erm(B) into conjugative transposons of the Tn916 family, most of pneumococcal isolates carried erm(B) genes are also resistant to tetracycline, typically carrying tet(M) [22]. In our study, most of Taiwan19F-14 isolates harbored erm(B), mef(A/E) and tet(M) genes, while only 41.2% of other isolates expressed these three genes. These findings suggest that the widespread of highly resistant CC271 maybe driven by the selective pressure of the antibiotic use, which poses a serious public health concern for increasing treatment failure with the empirical usage of macrolide antibiotics.
Two serotypes (19F and 19A) were the most prevalent serotypes (81.1% of these pneumococcal isolates) in our study, which was consistent with a report from children with AOM from Suzhou (80.5%) [20]. Reports for pneumococcal isolates identified from other specimens such as respiratory and nasopharynx revealed that 19F, 14, 23F, 6B and 19A were the most common serotypes [6]. In our study, the serotype coverage rate of PCV7 and PCV13 was 73.0 and 94.6%, respectively. Together with the reports from Suzhou and Shenzhen, China [5, 6], these results support that the increasing coverage of PCV13 was mainly because of the high occurrence of serotype 19A and suggest that expanded use of PCV13 in China, may significantly reduce the prevalence of pneumococcal diseases (especially the invasive pneumococcal diseases) resulting from high prevalence of serotype 19A, although serotype replacement always remains a concern.
Using the eBURST analysis both ST271 and ST320 are members of CC271 which accounts for 75.7% of the pneumococcal isolates in this study. CC271 is a multidrug resistant pneumococcal clone (Taiwan19F-14) which has been described internationally [27]. The clone described in this study is classified as multi drug resistant as it is resistant to penicillin, erythromycin and tetracycline which also carry the Tn2010 genetic element encoded on the erm(B), mef(E) and tet(M) genes [28]. Although not examined in the present study, previous studies have shown the increase of CC271 from 14.3% in 1997 in Beijing, China which rose to 92.0% in 2010 [29]. CC271 has also previously been described as the most prevalent clone causing invasive pneumococcal disease [30]. This clone appears to have become successful due to a number of factors, for example increasing international movements, selective pressure due to antibiotic use [29], this clone is now distributed globally and is widespread having been described in Colombia, Spain, USA and China [31,32,33,34]. Increased resistance to antibacterial medications continue to pose great challenges for treating the pneumococcal diseases.
Recent evidence has revealed that the pneumococcal serotype 19A/ST320 clone, driven from Taiwan19F-14 clone (ST236), switched serotype in response to the selective pressures of the immunization of PCV7 and has become prevalent in many countries, within Asia including China [33, 34]. This clone afflicted Chinese children before the introduction of PCV7 in 2008 [27] and that serotype 19A/ST320 has become resistant to multiple antibiotics [34]. Notably, serotype 19A/ST320 was also found in our study, and most of the serotype 19A/ST320 isolates were multidrug-resistant, and harbored erm(B), mef(A/E) and tet(M) genes. These findings suggest that antibiotic prescribing patterns in China may play a significant role in the switching serotype from 19F to 19A and their homogeneous genetic background. The high prevalence of serotype 19A/ST320 clone suggests that PCV13 should be included into the Chinese Expanded Program on Immunizations, which could further reduce the burden of pneumococcal diseases.
Vaccine candidates such as pspA, a protein-based vaccine candidate has been demonstrated to be independent of capsular serotypes [35]. This high identical capacity made pspA as a good vaccine candidate to improve the protecting efficacy of the pneumococcal isolates compared with the current usage of serotype-based vaccines, since pspA could avoid the serotype replacement which has been observed in the current usage of pneumococcal vaccines [36]. As serogroups 6 and 19 seem to be associated with pspA families, this suggests that vaccines covering the pspA family might be a better option than the current pneumococcal vaccines which are dependent on the presence of certain capsules. In line with a previous study [35], the current results confirmed that 86.5% of the pneumococci harbored pspA gene and was associated with serotypes 6B, 15B/C, 14, 23A, 19A and 19F, further supporting the consideration of pspA as a leading candidate for future vaccine development.
Pilus islet has recently been implicated in its virulence for assisting the pneumococci with adherence to host tissue [37] and its capacity of initiating mucosal damage and triggering mucosal inflammation means the pilus islet is able to invade the tissue [37]. A prior study emphasized the high prevalence of the pilus islet among capsular serotypes included in PCV7 such as 19F [38], and the re-emergence of pilus islet among non-vaccine serotype of pneumococcal isolates also has been reported recently [39]. Both the PI-1 (rlrA) and PI-2 (sipA) genes have previously been associated with the Taiwan19F-14 clone (CC271), we describe a genetic relatedness to CC271 together with serotypes 19F and 19A and found the strains also carried the erm(B), mef(A/E) and tet(M) genes. This suggests there may be a clinical relevance between the presence of these genes and AOM in Chinese children. Our work suggests that there is a clonal relationship between the virulence gene, serotype and presence of the pilus islet within CC271 [40].
There were several limitations within the present study. An obvious limitation of the study is the retrospective design and the small sample size, which may limit the generalizability of our study results.. Second, this study mainly focused on children with AOM obtained by tympanocentesis and these isolates may not be representative of the whole spectrum of otitis media, however, the PCR detection of the whole genetic background of pneumococcal isolates including serotype, MLST, antibiotic determinants and virulence factors represent important strengths of the study design. The third one was that we didn’t use controls in the PCRs. However, the 100 bp plus DNA ladder marker was used for molecular weight reference as the references described to make sure we get the right results.
Conclusions
In conclusion, the emergence of multidrug-resistant S.pneumoniae 19A/ST320 harboring multiple antibiotic resistance genes and virulence genes is both concerning and a great challenge to treat the pneumococcal diseases which warrants the continued monitoring of pneumococcal diseases in Chinese children. The high prevalence of international multidrug-resistant clone (Taiwan19F-14) in western China necessitates the continued dedication to expand PCV13 immunization in national vaccination programs along with efforts to curb and control the overuse of antibiotics in Chinese children.
Abbreviations
- ANSORP:
-
Asian Network for Surveillance of Resistant Pathogens
- AOM:
-
Acute otitis media
- CC:
-
Clonal complex
- CTX:
-
Cefotaxime
- H.influenzae :
-
Haemophilus influenzae
- MLST:
-
Multilocus sequence typing
- PCV:
-
Pneumococcal conjugate vaccine
- PNSP:
-
Penicillin-nonsusceptible S.pneumoniae
- PRSP:
-
Penicillin resistant S.pneumoniae
- S.pneumoniae :
-
Streptococcus pneumoniae
- STs:
-
Sequence types
References
Pichichero ME. Ten-year study of the stringently defined otitis-prone child in Rochester. NY Pediatr Infect Dis J. 2016 Sep;35(9):1033–9.
Ngo CC, Massa HM, Thornton RB, Cripps AW. Predominant Bacteria detected from the middle ear fluid of children experiencing otitis media: a systematic review. PLoS One. 2016;11(3):e0150949.
Coker TR, Chan LS, Newberry SJ, Limbos MA, Suttorp MJ, Shekelle PG, et al. Diagnosis, microbial epidemiology, and antibiotic treatment of acute otitis media in children: a systematic review. JAMA. 2010;304(19):2161–9.
Shekelle PG, Takata G, Newberry SJ, Coker T, Limbos MA, Chan LS, et al. Management of Acute Otitis Media: update. Evid Rep Technol Assess (Full Rep). 2010 Nov;(198):1–426.
Ma X, Zhao R, Ma Z, Yao K, Yu S, Zheng Y, et al. Serotype distribution and antimicrobial resistance of Streptococcus pneumoniae isolates causing invasive diseases from Shenzhen Children's hospital. PLoS One. 2013;8(6):e67507.
Geng Q, Zhang T, Ding Y, Tao Y, Lin Y, Wang Y, et al. Molecular characterization and antimicrobial susceptibility of Streptococcus pneumoniae isolated from children hospitalized with respiratory infections in Suzhou. China PLoS One. 2014;9(4):e93752.
Kim SH, Song JH, Chung DR, Thamlikitkul V, Yang Y, Wang H, ANSORP Study Group, et al. Changing trends in antimicrobial resistance and serotypes of Streptococcus pneumoniae isolates in Asian countries: an Asian network for surveillance of resistant pathogens (ANSORP) study. Antimicrob Agents Chemother. 2012;56(3):1418–26.
Rodgers GL, Arguedas A, Cohen R, Dagan R. Global serotype distribution among Streptococcus pneumoniae isolates causing otitis media in children: potential implications for pneumococcal conjugate vaccines. Vaccine. 2009;27(29):3802–10.
Cohen R, Varon E, Doit C, Schlemmer C, Romain O, Thollot F, et al. A 13-year survey of pneumococcal nasopharyngeal carriage in children with acute otitis media following PCV7 and PCV13 implementation. Vaccine. 2015;33(39):5118–26.
Balsells E, Guillot L, Nair H, Kyaw MH. Serotype distribution of Streptococcus pneumoniae causing invasive disease in children in the post-PCV era: a systematic review and meta-analysis. PLoS One. 2017;12(5):e0177113.
Weil-Olivier C, van der Linden M, de Schutter I, Dagan R, Mantovani L. Prevention of pneumococcal diseases in the post-seven valent vaccine era: a European perspective. BMC Infect Dis. 2012;12:207.
Hu J, Sun X, Huang Z, Wagner AL, Carlson B, Yang J, et al. Streptococcus pneumoniae and Haemophilus influenzae type b carriage in Chinese children aged 12-18 months in Shanghai, China: a cross-sectional study. BMC Infect Dis. 2016;16:149.
Hu S, Shi Q, Chen CI, Caldwell R, Wang B, Du L, et al. Estimated public health impact of nationwide vaccination of infants with 7-valent pneumococcal conjugate vaccine (PCV7) in China. Int J Infect Dis. 2014;26:116–22.
Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing: twenty-sixth informational supplement M100-S19. Wayne, PA, USA: CLSI; 2016.
Ahn JG, Choi SY, Kim DS, Kim KH. Enhanced detection and serotyping of Streptococcus pneumoniae using multiplex polymerase chain reaction. Korean J Pediatr. 2012;55(11):424–9.
Malhotra-Kumar S, Lammens C, Piessens J, Goossens H. Multiplex PCR for simultaneous detection of macrolide and tetracycline resistance determinants in streptococci. Antimicrob Agents Chemother. 2005;49(11):4798–800.
Shakrin NN, Masri SN, Taib NM, Nordin SA, Jamal F, Desa MN. Genotypic characterization of Malaysian human isolates of Streptococcus pneumoniae from carriage and clinical sources. Comp Immunol Microbiol Infect Dis. 2014;37(5–6):347–54.
Enright MC, Spratt BG. A multilocus sequence typing scheme for Streptococcus pneumoniae: identification of clones associated with serious invasive disease. Microbiology. 1998;144(Pt 11):3049–60.
Scally M, Schuenzel EL, Stouthamer R, Nunney L. Multilocus sequence type system for the plant pathogen Xylella fastidiosa and relative contributions of recombination and point mutation to clonal diversity. Appl Environ Microbiol. 2005;71(12):8491–9.
Ding Y, Geng Q, Tao Y, Lin Y, Wang Y, Black S, et al. Etiology and epidemiology of children with acute otitis media and spontaneous otorrhea in Suzhou. China Pediatr Infect Dis J. 2015;34(5):e102–6.
Zhao C, Li Z, Zhang F, Zhang X, Ji P, Zeng J, et al. Serotype distribution and antibiotic resistance of Streptococcus pneumoniae isolates from 17 Chinese cities from 2011 to 2016. BMC Infect Dis. 2017;17(1):804.
Li QH, Yao KH, Yu SJ, Ma X, He MM, Shi W, et al. Spread of multidrug-resistant clonal complex 271 of serotype 19F Streptococcus pneumoniae in Beijing, China: characterization of serotype 19F. Epidemiol Infect. 2013;141(12):2492–6.
Ip M, Ang I, Liyanapathirana V, Ma H, Lai R. Genetic analyses of penicillin binding protein determinants in multidrug-resistant Streptococcus pneumoniae serogroup 19 CC320/271 clone with high-level resistance to third-generation cephalosporins. Antimicrob Agents Chemother. 2015;59(7):4040–5.
Coffey TJ, Daniels M, McDougal LK, Dowson CG, Tenover FC, Spratt BG. Genetic analysis of clinical isolates of Streptococcus pneumoniae with high-level resistance to expanded-spectrum cephalosporins. Antimicrob Agents Chemother. 1995 Jun;39(6):1306–13.
Del Grosso M, Northwood JG, Farrell DJ, Pantosti A. The macrolide resistance genes erm(B) and mef(E) are carried by Tn2010 in dual-gene Streptococcus pneumoniae isolates belonging to clonal complex CC271. Antimicrob Agents Chemother. 2007;51(11):4184–6.
Li Y, Tomita H, Lv Y, Liu J, Xue F, Zheng B, et al. Molecular characterization of erm(B)- and mef(E)-mediated erythromycin-resistant Streptococcus pneumoniae in China and complete DNA sequence of Tn2010. J Appl Microbiol. 2011;110(1):254–65.
Qian J, Yao K, Xue L, Xie G, Zheng Y, Wang C, et al. Diversity of pneumococcal surface protein a (PspA) and relation to sequence typing in Streptococcus pneumoniae causing invasive disease in Chinese children. Eur J Clin Microbiol Infect Dis. 2012;31(3):217–23.
Ramos V, Parra EL, Duarte C, Moreno J. Characterization of Streptococcus pneumoniae invasive serotype 19A isolates recovered in Colombia. Vaccine. 2014;32(7):755–8.
Ardanuy C, Rolo D, Fenoll A, Tarrago D, Calatayud L, Liñares J. Emergence of a multidrug-resistant clone (ST320) among invasive serotype 19A pneumococci in Spain. J Antimicrob Chemother. 2009;64(3):507–10.
Isturiz R, Sings HL, Hilton B, Arguedas A, Reinert RR, Jodar L. Streptococcus pneumoniae serotype 19A: worldwide epidemiology. Expert Rev Vaccines. 2017;16(10):1007–27.
Shin J, Baek JY, Kim SH, Song JH, Ko KS. Predominance of ST320 among Streptococcus pneumoniae serotype 19A isolates from 10 Asian countries. J Antimicrob Chemother. 2011;66(5):1001–4.
Lyu S, Yao KH, Dong F, Xu BP, Liu G, Wang Q, et al. Vaccine Serotypes of Streptococcus pneumoniae with High-level Antibiotic Resistance Isolated More Frequently Seven Years After the Licensure of PCV7 in Beijing. Pediatr Infect Dis J. 2016;35(3):316-21.
Calatayud L, Ardanuy C, Cercenado E, Fenoll A, Bouza E, Pallares R, et al. Serotypes, clones, and mechanisms of resistance of erythromycin-resistant Streptococcus pneumoniae isolates collected in Spain. Antimicrob Agents Chemother. 2007;51(9):3240–6.
Rice LB. Tn916 family conjugative transposons and dissemination of antimicrobial resistance determinants. Antimicrob Agents Chemother. 1998;42(8):1871–7.
Yatim MM, Masri SN, Desa MN, Taib NM, Nordin SA, Jamal F. Determination of phenotypes and pneumococcal surface protein A family types of Streptococcus pneumoniae from Malaysian healthy children. J Microbiol Immunol Infect. 2013 Jun;46(3):180–6.
Hotomi M, Togawa A, Kono M, Ikeda Y, Takei S, Hollingshead SK, et al. PspA family distribution, antimicrobial resistance and serotype of Streptococcus pneumoniae isolated from upper respiratory tract infections in Japan. PLoS One. 2013;8(3):e58124.
Golden AR, Rosenthal M, Fultz B, Nichol KA, Adam HJ, Gilmour MW, et al. Characterization of MDR and XDR Streptococcus pneumoniae in Canada, 2007-13. J Antimicrob Chemother. 2015;70(8):2199–202.
Zähner D, Gudlavalleti A, Stephens DS. Increase in pilus islet 2-encoded pili among Streptococcus pneumoniae isolates, Atlanta, Georgia, USA. Emerg Infect Dis. 2010;16(6):955–62.
Wierzbowski AK, Karlowsky JA, Adam HJ, Nichol KA, Hoban DJ. Zhanel GG; Canadian antimicrobial resistance Alliance. Evolution and molecular characterization of macrolide-resistant Streptococcus pneumoniae in Canada between 1998 and 2008. J Antimicrob Chemother. 2014;69(1):59–66.
Moschioni M, Donati C, Muzzi A, Masignani V, Censini S, Hanage WP, et al. Streptococcus pneumoniae contains 3 rlrA pilus variants that are clonally related. J Infect Dis. 2008;197(6):888–96.
Acknowledgements
Not applicable.
Funding
This manuscript was funded by Guangxi Medical and Health Self-funding Project (No Z2014379, No Z2016531, Z20170509, Z20180022), Guangxi Nature Science Foundation (No. 2015GXSFBA139129), and Liuzhou Science and Technology Bureau Project (No 2014 J030422, 2017BD20201, 2018BJ10502, 2018BJ10504). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Availability of data and materials
We declare that the data supporting the conclusions of this article are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
J F and L L designed the study and drafted an outline. S X, Z L, N L and P Q participated in data analysis, JF draft of initial manuscript, L L, Z L and N L participated in diagnosed and collected the data, E M and X Y revised the manuscript and all of authors approved the final content off this manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of Liuzhou Maternity and Child Healthcare Hospital (No 2017003). A written informed consent was obtained from parents or legal guardians on behalf of the children involved in the study for collection of information and samples. The Ethics Committee also permitted the authors to use the patients records to write this article.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Fu, J., Li, L., Liang, Z. et al. Etiology of acute otitis media and phenotypic-molecular characterization of Streptococcus pneumoniae isolated from children in Liuzhou, China. BMC Infect Dis 19, 168 (2019). https://doi.org/10.1186/s12879-019-3795-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-019-3795-8