Abstract
Background
In 2014 the Swedish government assigned to The Public Health Agency of Sweden to conduct studies to evaluate optimal use of existing antibiotic agents. The aim is to optimize drug use and dosing regimens to improve the clinical efficacy. The present study was selected following a structured prioritizing process by independent experts.
Methods
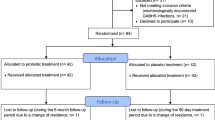
This phase IV study is a randomized, open-label, multicenter study with non-inferiority design regarding the therapeutic use of penicillin V with two parallel groups. The overall aim is to study if the total exposure with penicillin V can be reduced from 1000 mg three times daily for 10 days to 800 mg four times daily for 5 days when treating Streptococcus pyogenes (Lancefield group A) pharyngotonsillitis. Patients will be recruited from 17 primary health care centers in Sweden. Adult men and women, youth and children ≥6 years of age who consult for sore throat and is judged to have a pharyngotonsillitis, with 3–4 Centor criteria and a positive rapid test for group A streptococci, will be included in the study. The primary outcome is clinical cure 5–7 days after discontinuation of antibiotic treatment. Follow-up controls will be done by telephone after 1 and 3 months. Throat symptoms, potential relapses and complications will be monitored, as well as adverse events. Patients (n = 432) will be included during 2 years.
Discussion
In the era of increasing antimicrobial resistance and the shortage of new antimicrobial agents it is necessary to revisit optimal usage of old antibiotics. Old antimicrobial drugs are often associated with inadequate knowledge on pharmacokinetics and pharmacodynamics and lack of optimized dosing regimens based on randomized controlled clinical trials. If a shorter and more potent treatment regimen is shown to be equivalent with the normal 10 day regimen this can imply great advantages for both patients (adherence, adverse events, resistance) and the community (resistance, drug costs).
Trial registration
EudraCT number 2015-001752-30. Protocol FoHM/Tonsillit2015 date 22 June 2015, version 2. Approved by MPA of Sweden 3 July 2015, Approved by Regional Ethical Review Board in Lund, 25 June 2015.
Similar content being viewed by others
Background
In 2014 the Swedish government assigned to The Public Health Agency of Sweden to conduct studies to evaluate existing antibiotic agents from new perspectives. The aim is to optimise drug use and dosage regimens to improve the clinical efficacy of existing antibiotics, and to reduce ecological disturbances in the normal microbiota. The clinical trial described below is one of the projects prioritized by independent experts within this assignment.
Pharyngotonsillitis is one of the most common infections in primary health care (PHC) in Sweden [1] and accounts for about 20 % of all antibiotic prescriptions within PHC [2]. The estimated number of patients in Sweden treated for pharyngotonsillitis is about 400,000 each year [3]. Streptococcus group A (GAS) is the most common bacterial etiology and also the dominating reason for antibiotic treatment [3]. According to the Swedish guidelines the recommendation is to treat pharyngotonsillitis with phenoxymethylpenicillin (penicillin V) if 3–4 Centor criteria [2] and GAS is present [3]. The recommended dose for adults is 1000 mg penicillin V three times daily for 10 days which corresponds to about 12 t of penicillin V each year in Sweden, calculated on the basis of adult doses. The infection is most frequent among school children and young adults, resulting in a somewhat lower total amount of consumption [4].
According to present European and American guidelines, 10 days penicillin treatment is generally recommended as first line treatment of streptococcal pharyngotonsillitis [5, 6]. The historical reason for 10 days treatment duration with penicillin is mainly to avoid serious complications like acute rheumatic fever and glomerulonephritis. These conditions are currently extremely rare in western countries [7]. According to a Cochrane report from 2012 patients recover without antibiotic therapy but treatment is sometimes advised to hasten clinical resolution and prevent suppurative sequelae, like peritonsillitis, acute otitis media or sinusitis. Clinical studies on shorter treatment duration with penicillin for streptococcal pharyngotonsillitis are encouraged [8].
There are few published studies comparing 10 days treatment duration with shorter treatment durations with penicillin V. Three studies which were published in the 1980s have been identified. Five versus 10 days (250 mg three times daily and 800 mg twice daily respectively) [9, 10] and 7 versus 10 days (500 mg three times daily) [11] were studied. All of the studies concluded that 10 days of treatment was preferable but in the light of current knowledge of pharmacokinetics and pharmacodynamics (PK/PD) the doses used in these studies were suboptimal. The effect of beta-lactam antibiotics is dependent on time above the minimum inhibitory concentration (MIC) of the unbound drug concentration in serum (free drug time above MIC; fT > MIC). Usually, the target is that 95–99 % of the population should achieve a fT > MIC of around 30–40 % for non-severe infections [12], also expressed as the population target attainment for a given target. For beta-lactams a more frequent dosing is preferred and the dosing interval is more critical than the size of the dose [12]. The dosing regimen 800 mg four times daily provides better target attainment compared to 1000 mg three times daily (33 % fT > MIC compared to 25 % fT > MIC at the breakpoint MIC 0.25 mg/L). Our hypothesis is that the experimental dosage regimen 800 mg four times daily gives at least the same efficacy, possibly better patient adherence and a significantly lower total exposure.
In the present study we will examine if penicillin V given four times daily for 5 days is non-inferior to the currently recommended dose regimen of 10 days to patients with pharyngotonsillitis caused by streptococcus group A. Reducing the treatment duration to 5 days will almost halve the consumption of penicillin V for this indication. A shorter treatment duration may potentially improve patient adherence [13], cause less effect on the human microbiota [14] as well as lower medicinal costs for patients and community.
Methods
Aim
The overall aim is to study if the total exposure with antibiotics can be reduced from 1000 mg three times daily for 10 days to 800 mg four times daily for 5 days when treating streptococcus group A pharyngotonsillitis with penicillin V.
Design
This Phase IV study is a randomized, open-label, multicenter study with non-inferiority design regarding the therapeutic use of penicillin V with two parallel groups.
Setting
Patients will be recruited from 17 primary health care centers (PHCC) in the counties of Skåne, Kronoberg and Västra Götaland in the southern of Sweden. The PHCCs are representing both urban and rural regions.
Characteristics of the patients
Inclusion criteria
Adult men and women, youth and children ≥6 years of age who are seeking a PHCC for sore throat with suspected tonsillitis and meeting the criteria in accordance with current treatment recommendations for streptococcal pharyngotonsillitis, i.e. 3–4 Centor criteria (fever ≥ 38.5, tender lymph nodes, coatings of the tonsils and absence of cough) and a positive rapid antigen detection test (RADT) for GAS will be included in the study [15].
Exclusion criteria
A patient is not eligible for inclusion if any of the following criteria apply: 1) Signs of serious illness according to pre-specified criteria, 2) hypersensitivity to penicillin, 3) chronic illness that significantly impair the immune system, 4) immunomodulating treatment, 5) treatment with antibiotics for pharyngotonsillitis during the last month (relapse), 6) any antibiotic treatment during the last 72 h before inclusion, 7) difficulty swallowing tablets, 8) any other cause which, according to the physician’s judgment, makes it unsuitable for the patient to participate in the study.
Intervention
Patients will be randomized to treatment with penicillin V four times daily for 5 days or three times daily for 10 days. The dosages for different weight groups are presented in Table 1.
Randomization
Randomization within blocks for each PHCC separately was performed by a statistician at The Public Health Agency of Sweden. Sealed envelopes with unique numbers and allocated treatment were distributed to each of the 17 participating PHCCs.
Context and procedure
Study procedures are presented in Table 2.
Visit one
Patients presenting with sore throat will be clinically examined by a physician. In case of suspected faryngotonsillitis and 3–4 Centor criteria a RADT for GAS will be performed. Patients with a positive RADT test for GAS who meet all the inclusion criteria, and none of the exclusion criteria, will be asked to participate in the trial. After signed informed consent, by patient or guardian, the patient will be asked about medical history, demographic data, previous antibiotic exposure and concomitant drug treatment.
A throat swab for semi quantitative cultures of streptococcus group A, C and G will be performed.
After randomization to intervention group 5 or 10 days the physician prescribes penicillin V which the patient will retrieve from the pharmacy. A patient diary is handed out which the patient or guardian is asked to fill in until the follow-up visit, five to seven days after treatment discontinuation.
Demographic data and clinical data will be recorded in a case report form (CRF) by study personnel. In addition, data regarding intake of penicillin V doses and analgesics, presence of sore throat and fever, adverse events etc. is filled in daily by each patient or guardian in the diary.
Test-of-cure visit
A test-of cure (TOC) visit will be performed at the PHCC 5 to 7 days after completion of antibiotic treatment. Clinical judgement of throat status, a new RADT and culture, and recording of adverse events will be performed.
Follow-up controls
Follow-up controls will be done by a telephone call 1 month (28–35 days) and 3 months after the last treatment day. Throat symptoms, potential relapses and complications will be monitored as well as adverse events ongoing at previous visits.
Outcomes
Primary outcomes
The primary outcome is clinical cure 5–7 days after discontinuation of antibiotic treatment (TOC). The patients will be asked about throat symptoms and a clinical judgement will be performed by the investigator. Clinical cure is defined as complete recovery without remaining/residual symptoms or clinical findings of pharyngotonsillitis or symptomatic relapse at TOC [15].
Secondary outcomes
The secondary outcomes are bacteriological eradication according to the culture taken at the TOC, time to relief of symptoms according to the patient diary, frequency of relapses one month after first diagnosis, frequency of complications during the study period as well as pattern of adverse events.
Definitions
Definitions of outcome measures are presented in Table 3.
Statistical analysis
Sample size/power calculation
The power calculation is based on the assumption of 90 % clinical recovery in the reference group and no difference between the two groups. The non-inferiority limit is set to minus 10%, the confidence level (two-sided) to 95 %, the power to 85 % and the distribution between the groups to 1/1. Based on these presumptions, 324 evaluable patients (162 in each group) are needed to be able to demonstrate non-inferiority between treatment groups. Presuming that 25 % of the included patients will not be possible to evaluate 432 (324*(1/0.75)) patients are needed to be included.
All effect variables will be analyzed for per protocol population (PP) as well as for the modified intention to treat (MITT) population as sensitivity analysis.
Safety
Patterns of adverse events between the two treatment groups will be evaluated and analysed descriptively. Active surveillance of adverse events will be done by a physician at TOC and by self-reporting by the patient/guardian in the patient diary. At the follow-up controls 1 and 3 months after discontinuation of treatment, patients will be asked about adverse events that are still ongoing at the previous visit.
Monitoring
Monitoring according to GCP/ICH will be performed by trained personnel allocated by the sponsor, The Public Health Agency of Sweden.
Ethical issues
It is important to include children in clinical trials, especially since children often suffer from infections and therefore are treated with antibiotics more often than other age groups. The lower age limit 6 years was chosen based on presumed ability to swallow tablets, since exposure is easier to control with tablets than with oral suspension.
We consider that there are no risks to participate in the study, regardless of age. The sampling is simple and follow the normal routines at the PHCCs. Both treatment alternatives are expected to be effective against the infection. A shorter treatment regimen could include benefits in form of less adverse events and less disturbances in the human microbiota. In addition, patient adherence to the treatment may improve with shorter treatment duration.
Discussion
In the era of increasing antimicrobial resistance and the shortage of new antimicrobial agents it is necessary to revisit old antibiotics to ensure that they are used correctly and to their full potential. Important old drugs deserve to be rigorously tested according to today’s standards and requirements for clinical studies. Old antimicrobial drugs are often associated with inadequate knowledge on PK/PD and lack of optimized dosing regimens based on randomized controlled clinical trials.
Since old antibiotics are usually out of patent protection, financial incentives to perform the necessary studies are lacking. Thus, public funding is often the only option available [16]. For the current study, funded by the Swedish government, an initial systematic review to identify knowledge gaps was performed, followed by a structured prioritizing process by independent experts, in order to select the most needed non-clinical and clinical studies.
There is currently no substantial scientific evidence to recommend less than 10 days treatment of penicillin V against streptococcal pharyngotonsillitis. A shorter treatment period though, with lower total antibiotic exposure, is expected to reduce the risk of ecological disturbance on the human microbiota [17] and possibly improve patient adherence [13].
One possible disadvantage with a shorter treatment duration could be an increased risk for relapses, since the time with protection against a new infection is shorter. Furthermore, it could be argued that the daily dose for the 5-day treatment is slightly higher than the recommended daily dose, which may increase the risk for adverse events. However, there is extensive clinical experience of even higher doses in treatment of other infections like acute otitis media, sinusitis, and dental infection, justifying the safety profile of penicillin V also at high doses.
Overall we believe that this study, facilitated by public funding, may contribute with important knowledge and may have a substantial impact on public health. If a shorter and more potent treatment regimen is shown to be equivalent with the normal 10 day regimen this can imply great advantages for both patients (resistance, adverse events, adherence) and the community (resistance, drug costs).
Abbreviations
- CRF:
-
Case record form
- GAS:
-
Gtreptococcus group A
- GCP:
-
Good clinical practice
- ICH:
-
International conference on harmonization
- MIC:
-
Minimum inhibitory concentration
- MITT:
-
Modified intention to treat population. Patients who received at least one dose of the study drug
- PHC:
-
Primary health care
- PHCC:
-
Primary health care centre
- PK/PD:
-
Pharmacokinetics and pharmacodynamics
- PP:
-
Per protocol population. Patients without violations to the study protocol, receiving at least 80 % of the prescribed study drug and evaluable at least until the TOC visit.
- RADT:
-
Rapid antigen detection test
- RCT:
-
Randomized controlled trial
- TOC:
-
Test of cure
References
Andre M, Vernby A, Odenholt I, Lundborg CS, Axelsson I, Eriksson M, et al. Diagnosis-prescribing surveys in 2000, 2002 and 2005 in Swedish general practice: consultations, diagnosis, diagnostics and treatment choices. Scand J Infect Dis. 2008;40(8):648–54.
Molstad S, Andre M, Norman C, Hedin K, Engstrom S. [In common infections: to give or not to give antibiotics]. Lakartidningen. 2009;106(47):3162–4. 6.
Mölstad S, André M. Handläggning av faryngotonsilliter i öppenvården – Bakgrund. Information från Läkemedelsverket. 2012(6):27–9
Läkemedelsverket. Handläggning av faryngotonsilliter i öppenvård. Information från Läkemedelsverket. 2012(6):18–66
ESTG Group, Pelucchi C, Grigoryan L, Galeone C, Esposito S, Huovinen P, et al. Guideline for the management of acute sore throat. Clin Microbiol Infect. 2012;18 Suppl 1:1–28.
Shulman ST, Bisno AL, Clegg HW, Gerber MA, Kaplan EL, Lee G, et al. Clinical practice guideline for the diagnosis and management of group A streptococcal pharyngitis: 2012 update by the Infectious Diseases Society of America. Clin Infect Dis. 2012;55(10):1279–82.
Shulman ST, Tanz RR. Group A streptococcal pharyngitis and immune-mediated complications: from diagnosis to management. Expert Rev Anti-Infect Ther. 2010;8(2):137–50.
Altamimi S, Khalil A, Khalaiwi KA, Milner RA, Pusic MV, Al Othman MA. Short-term late-generation antibiotics versus longer term penicillin for acute streptococcal pharyngitis in children. Cochrane Database Syst Rev. 2012;8:CD004872.
Gerber MA, Randolph MF, Chanatry J, Wright LL, De Meo K, Kaplan EL. Five vs ten days of penicillin V therapy for streptococcal pharyngitis. Am J Dis Child. 1987;141(2):224–7.
Stromberg A, Schwan A, Cars O. Five versus ten days treatment of group A streptococcal pharyngotonsillitis: a randomized controlled clinical trial with phenoxymethylpenicillin and cefadroxil. Scand J Infect Dis. 1988;20(1):37–46.
Schwartz RH, Wientzen Jr RL, Pedreira F, Feroli EJ, Mella GW, Guandolo VL. Penicillin V for group A streptococcal pharyngotonsillitis. A randomized trial of seven vs ten days’ therapy. JAMA. 1981;246(16):1790–5.
Dagan R, Klugman KP, Craig WA, Baquero F. Evidence to support the rationale that bacterial eradication in respiratory tract infection is an important aim of antimicrobial therapy. J Antimicrob Chemother. 2001;47(2):129–40.
Eide TB, Hippe VC, Brekke M. The feasibility of antibiotic dosing four times per day: a prospective observational study in primary health care. Scand J Prim Health Care. 2012;30(1):16–20.
Sullivan A, Edlund C, Nord CE. Effect of antimicrobial agents on the ecological balance of human microflora. Lancet Infect Dis. 2001;1(2):101–14.
Centor RM, Witherspoon JM, Dalton HP, Brody CE, Link K. The diagnosis of strep throat in adults in the emergency room. Med Decis Making. 1981;1(3):239–46.
Theuretzbacher U, Van Bambeke F, Canton R, Giske CG, Mouton JW, Nation RL, et al. Reviving old antibiotics. J Antimicrob Chemother. 2015;70(8):2177–81.
Kouyos RD, Metcalf CJ, Birger R, Klein EY, Abel zur Wiesch P, Ankomah P, et al. The path of least resistance: aggressive or moderate treatment? Proceedings Biological sciences/The Royal Society. 2014;281(1794):20140566
Acknowledgements
Not applicable.
Funding
The study is funded by The Public Health Agency of Sweden which also acts as the sponsor of the study. Employees at The Public Health Agency is responsible for project management of study design, data collection, analysis and interpretation of data in cooperation with external experts.
Availability of data and material
Not applicable.
Authors’ contributions
GS, CE, CGG, SM, CN, P-DS and KH planned the study. SM is the principal investigator of the study and regional investigator for Region Skåne. KH and P-DS are regional investigators for Region Kronoberg and Region Västra Götaland respectively. GS, CE, SM and KH were major contributors in writing the manuscript. All authors read, commented and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests. CGG has received speaker’s honoraria from Pfizer, AstraZeneca and Meda.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The study was approved by the Regional Ethical Review Board in Lund, 25 June 2015 (reference number 2015/396). Written informed consent is obtained from each patient or accompanying guardian prior randomization.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Skoog, G., Edlund, C., Giske, C.G. et al. A randomized controlled study of 5 and 10 days treatment with phenoxymethylpenicillin for pharyngotonsillitis caused by streptococcus group A – a protocol study. BMC Infect Dis 16, 484 (2016). https://doi.org/10.1186/s12879-016-1813-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-016-1813-7