Abstract
Background
The cost and complexity of current approaches to therapeutic drug monitoring during tuberculosis (TB) therapy limits widespread use in areas of greatest need. We sought to determine whether urine colorimetry could have a novel application as a form of therapeutic drug monitoring during anti-TB therapy.
Methods
Among healthy volunteers, we evaluated 3 dose sizes of rifampin (150 mg, 300 mg, and 600 mg), performed intensive pharmacokinetic sampling, and collected a timed urine void at 4 h post-dosing. The absorbance peak at 475 nm was measured after rifamycin extraction. The optimal cutoff was evaluated in a study of 39 HIV/TB patients undergoing TB treatment in Botswana.
Results
In the derivation study, a urine colorimetric assay value of 4.0 × 10−2 Abs, using a timed void 4 h after dosing, demonstrated a sensitivity of 92 % and specificity of 60 % to detect a peak rifampin concentration (Cmax) under 8 mg/L, with an area under the ROC curve of 0.92. In the validation study, this cutoff was specific (100 %) but insensitive (28 %). We observed similar test characteristics for a target Cmax target of 6.6 mg/L, and a target area under the drug concentration-versus-time curve (AUC0–8) target of 24.1 mg•hour/L.
Conclusions
The urine colorimetric assay was specific but insensitive to detect low rifampin serum concentrations among HIV/TB patients. In future work we will attempt to optimize sampling times and assay performance, with the goal of delivering a method that can translate into a point-of-care assessment of rifampin exposure during anti-TB therapy.
Similar content being viewed by others
Standardized anti-tuberculosis (TB) drug regimens that include rifampin, isoniazid, pyrazinamide, and ethambutol are the foundation of the global public health response to the TB epidemic [1]. Yet there is wide variability in absorption and metabolism of the anti-TB drugs, and low drug concentrations in blood are associated with inferior TB treatment outcomes, including treatment failure and relapse [2–5]. Pharmacokinetic variability has been identified as a key mediator of the rate of sterilizing effect and the emergence of new drug resistance mutations during anti-TB therapy [6, 7].
The TB clinician must consider the totality of the clinical information when making therapeutic decisions regarding dose adjustment. When therapeutic drug monitoring is performed, the result (a concentration above or below a threshold value) would support a clinical decision to increase the dose size of one or more of the drugs. The impact of this decision is evaluated during the clinical course, sometimes with repeated monitoring to ensure that the therapeutic target has been reached [8]. While the output from therapeutic drug monitoring provides a continuous measure of drug concentration, the practical use of that information is to classify the patient as having adequate or inadequate drug exposure.
In resource-rich settings, therapeutic drug monitoring can be performed during anti-TB therapy by measuring plasma or serum drug concentrations. The clinical decision to perform therapeutic drug monitoring may be motivated by factors such as a slow treatment response, the need for second-line drugs during the treatment of multi-drug resistant infection, or the presence of co-morbidities associated with inferior treatment outcomes (HIV co-infection, diabetes mellitus) [9]. The performance of therapeutic drug monitoring requires specialty laboratory capabilities, such as high performance liquid chromatography (HPLC) or gas chromatography (GC). Technical expertise is also required to collect, process, and ship samples to the specialty laboratory.
In many high-burden settings, the complexity and cost of these laboratory methods may seem to preclude the use of therapeutic drug monitoring in the clinical care of TB patients. Consequently, patients with inadequate drug exposure cannot be identified early during anti-TB therapy, at a time when dosing adjustments could be expected to improve treatment outcomes. Although dried blood spots have been proposed as a means to facilitate sample collection in the field [10], the measurement of drug concentrations in whole blood using dried blood spot techniques requires the same laboratory capabilities as traditional methods, and the interpretation of drug concentrations in whole blood (versus serum or plasma) is uncertain [11].
A simple, inexpensive test to classify TB patients based on an estimate of drug exposure, available at the point-of-care at the time of the patient encounter, could supplement other clinical information in support of treatment decisions. Urine colorimetry was first evaluated in the 1970’s as a method to assess the bioequivalence of different fixed-dose combinations of anti-TB drugs [12–14]. More recently, a urine colorimetric approach to detect isoniazid in urine (the “Arkansas method”) has been commercialized as a tool to monitor isoniazid adherence (IsoScreen, GFC Diagnostics LTD, Oxfordshire, UK) [15]. Potential advantages of urine colorimetric methods include a non-invasive sampling approach, improved patient acceptability, and the low cost and stability of chemical reagents.
We sought to determine whether urine colorimetry could have a novel application as a measure of systemic rifampin exposure during anti-TB therapy, as defined by the maximum serum concentration (Cmax) or the area under the concentration-versus-time curve (AUC0–8). Our approach was first to develop the urine colorimetric assay among healthy volunteers, and then to validate the assay among HIV/TB patients from a high-burden setting.
Methods
Derivation study
Study design
We performed a non-randomized, open-label, cross-over study of the first-line anti-TB drugs (rifampin, isoniazid, ethambutol, pyrazinamide) in 6 healthy volunteers. We sequentially evaluated 3 dose sizes in separate study visits, with rifampin dosed at 150 mg, 300 mg, and 600 mg. Each study visit was separated by a wash-out period of at least 1 week. Blood samples were collected prior to oral administration of the study drugs, and then at 1, 2, 4, 6, and 8 h following oral administration of the study drugs. Frozen samples were shipped to the Infectious Disease Pharmacokinetics Laboratory at the University of Florida for measurement of serum drug concentrations. All urine was collected during the study visit, and the volume and time of collection were noted. Timed voids were obtained at 4 h and 8 h post-dosing. Urine was aliquoted into single-use 3 mL conical vials and stored at -70C until ready for analysis.
Urine colorimetric assay
We followed the Sunahara method to extract total rifamycins from urine samples [16]. In brief, 50 μl of 100 mM phosphate buffer (pH 7) was added to 100 μl of urine sample followed by 100 μl of isoamylalcohol. Each sample was mixed by vortexing for 20 s at max speed. Samples were centrifuged at 14,000 rpms for 5 min at room temperature. The aqueous phase (upper) was carefully removed and transferred to a clear 96-well plate and optical density was measured at 475 nm in a Multiskan™ GO Microplate Spectrophotometer (Thermo Fisher Scientific). A calibration curve was determined for 10 serial dilutions (1:2) starting at 1000 mg/L of pure rifampin (Sigma Aldrich) that had been extracted with isoamylalcohol.
Statistical analysis
The goal of the statistical analysis for the development cohort was to define the accuracy of the urine colorimetric assay to detect low rifampin serum Cmax or AUC0–8, across a range of possible cutoff values, and to identify the optimal cutoff value for subsequent evaluation in the validation cohort. The area under the receiver-operating-characteristic (ROC) curve provides a summary measure of the ability of the diagnostic test to distinguish between adequate and inadequate rifampin exposure. An area under the ROC curve equal to 1 demonstrates perfect discrimination, whereas an area of 0.5 demonstrates that the diagnostic test performs no better than chance alone [17].
For our primary analysis, we evaluated the ROC curve for the urine colorimetric assay corresponding to a serum rifampin Cmax target of 8 mg/L, which is the standard-of-care target for therapeutic drug monitoring during anti-TB treatment [8]. The 95 % confidence interval for the area under the ROC curve was calculated using 2000 bootstrap replicates [18]. In an a priori decision, we defined the optimal cutoff for the urine colorimetric assay as the value corresponding to 90 % sensitivity for each drug exposure target. Statistical significance was declared for p-values less than 0.05. All statistical analysis was performed in R, with non-compartmental pharmacokinetic (PK) analysis performed using the PK package, and ROC analysis performed using the pROC package [19].
Validation study
Setting and participants
The validation study was nested within a prospective cohort study of anti-TB drug PK in HIV/TB patients at 22 public clinics and Princess Marina Hospital in Gaborone, Botswana. HIV-infected adults (21 years of age and older) were eligible for enrollment in the parent study if they were citizens of Botswana, naïve to antiretroviral therapy (ART), and newly diagnosed with pulmonary TB. Patients must have been initiated on a standard first-line TB treatment regimen, following WHO guidelines for weight-based dosing bands. The diagnosis of pulmonary TB must have been established by either a positive sputum smear, a positive GeneXpert MTB/RIF assay (Cepheid, Sunnyvale, CA, USA), or the presence of WHO criteria for smear-negative pulmonary TB. Exclusion criteria included pregnancy, renal insufficiency (defined as a creatinine clearance less than 50 mL/min, and hepatic dysfunction (defined as either an alanine transaminase or aspartate transaminase greater than 3 times the upper limit of normal).
Procedures
The PK study visit was scheduled prior to the completion of the intensive phase of anti-TB therapy. All PK visits were conducted at the Infectious Disease Care Clinic at Princess Marina Hospital. Oral doses of the anti-TB drugs were obtained from the Gaborone City Clinic and directly administered to the participant on the morning of the PK visit. A baseline blood sample was drawn prior to dosing, and then at 0.3, 0.9, 2.2, 4.5, and 8 h post-dosing. These sampling times were selected based on the objectives of the parent study to evaluate the population PK of isoniazid. At each time point, 10 mL of blood was drawn and transported to the Botswana Harvard Partnership Laboratory. After centrifugation, serum was stored at -70C. Serum drug concentrations were measured at the Gumbo Laboratory at the Baylor Research Institute (Dallas, TX) using liquid chromatography-tandem mass spectrometry methods. For the performance of the urine colorimetric assay, a single urine sample was obtained 4 h after dosing, based on the diagnostic accuracy of this time point to identify healthy subjects with Cmax less than 8 mg/L in the derivation study. Urine samples were frozen and shipped to the Infectious Disease Clinical Research Laboratory at Drexel University College of Medicine (Philadelphia, PA). The urine assay steps were performed as described for the healthy volunteers.
Statistical analysis
We evaluated the overall distribution of the urine colorimetric assay in the validation sample, along with potentially relevant covariates (age, body weight, renal function). We plotted individual rifampin concentration-versus-time profiles for each patient, and identified the corresponding rifampin Cmax for each patient. Non-compartmental analysis was performed to estimate rifampin AUC0–8 for each patient that completed the study visit.
Validation of the urine colorimetric assay was performed for 3 separate targets. In the primary analysis, we validated the optimal cutoff previously identified from the healthy subjects to detect Cmax less than 8 mg/L [8]. In secondary analyses, we evaluated two additional drug exposure targets. A Cmax target of 6.6 mg/L was recently shown to predict delayed sputum conversion in a South African cohort [3]. Based on pre-clinical rifampin pharmacodynamic data, we also evaluated a rifampin AUC0–8 target of 24.1 mg•hour/L [20]. For each target, bootstrapping (2000 replicates) was performed to estimate the 95 % confidence interval for the area under the ROC curve [18]. We assessed sensitivity, specificity, positive and negative predictive values, and positive and negative likelihood ratios at the cutoff identified in the derivation study. We also calculated 90 % confidence intervals for sensitivity and specificity with 2000 bootstrap replicates [18].
Results
Derivation study
Calibration of the urine colorimetric assay with known standards is shown in Fig. 1. The extraction of rifampin via Sunahara method demonstrated a linear relationship between the absorbance peak at 475 nm and standard rifampin concentrations in a range from 2.0 to 1000 mg/L (R2 greater than 0.99), which is similar to the original report.
We enrolled 6 healthy volunteers in the derivation study, and each volunteer completed all study visits. Delayed oral absorption of rifampin is a common phenomenon [8], and in 3 of 6 healthy volunteers the time to Cmax was greater than 2 h. We observed a reasonable correlation between Cmax and the urine colorimetric assay (r = 0.83), as shown in Fig. 2. For the Cmax target of 8 mg/L, the area under the ROC curve (Fig. 3) was 0.92 (95 % CI 0.74–1.0). A cutoff of 4 × 10−2 absorbance units (AU) had a sensitivity of 92 % (90 % CI 77–100 %) and specificity of 60 % (90 % CI 20–100 %) to detect rifampin serum Cmax less than 8 mg/L.
Validation study
Thirty-nine HIV/TB patients completed the intensive PK study and provided a 4-h urine sample for analysis, and all of these patients were included in the validation sample. Baseline demographic and clinical characteristics for these 39 patients are shown in Table 1. Following WHO weight-based dosing guidelines, a single patient was dosed with 300 mg of rifampin, 18 patients were dosed with 450 mg, 17 patients were dosed with 600 mg, and 3 patients were dosed with 750 mg. Individual serum rifampin concentrations versus time are shown in Fig. 4. Based on the observed concentration data, rifampin Cmax concentrations were below the target of 8 mg/L in 25 of 39 patients (64 %).
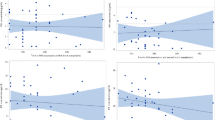
The urine colorimetric assay was poorly correlated with serum Cmax in the validation cohort (r = 0.24). The distribution of the urine colorimetric assay grouped according to serum target attainment is shown in Fig. 5. For the Cmax target of 8 mg/L (Fig. 5a), the difference in urine colorimetric assay values between groups did not reach statistical significance (p = 0.18 by Kruskal-Wallis test). The difference in urine colorimetric assay values between groups was statistically significant (p = 0.049) for the secondary Cmax target of 6.6 mg/L (Fig. 5b), and also reached statistical significance (p = 0.02) for the AUC0–8 target of 24.1 mg•hour/L (Fig. 5c).
The value for the urine colorimetric assay was below the cutoff of 4.0 × 10−2 Abs in 7 of 39 patients (18 %), identified among healthy volunteers as predictive of Cmax less than 8 mg/L. The 2 × 2 table corresponding to this cutoff among HIV/TB patients is shown in Table 2, and the ROC curve is shown in Fig. 6. This cutoff was 100 % specific but poorly sensitive (28 %, 90 % CI 16–44 %). Given that the prevalence of rifampin Cmax less than 8 mg/L was 64 % in the validation cohort, these diagnostic test characteristics correspond to a positive predictive value of 100 % and a negative predictive value of 44 %. Overall, the urine colorimetric assay demonstrated low diagnostic accuracy for the detection of rifampin Cmax less than 8 mg/L in the validation sample, with an area under the ROC curve of 0.63 (95 % CI 0.45–0.82).
In secondary analyses, a urine colorimetric assay cutoff of 4.0 x 10−2 Abs was 28 % sensitive (90 % CI 11–44 %) and 90 % specific (90 % CI 81–100 %) for identifying patients with a rifampin Cmax less than 6.6 mg/L, which was the threshold identified as predictive of delayed sputum sterilization among South African TB patients [3]. The area under the ROC curve (Fig. 7) was 0.69 (95 % CI 0.52–0.86). At the optimal threshold identified by Youden’s J statistic, a cutoff of 11.1 × 10−2 Abs identified patients with rifampin Cmax less than 6.6 mg/L with 94 % sensitivity (90 % CI 83–100 %) and 43 % specificity (90 % CI 24–62 %).
The ROC curve corresponding to an AUC0–8 target of 24.1 mg•hour/L is shown in Fig. 8. At the cutoff of 4.0 × 10−2 Abs, the urine colorimetric assay was 40 % sensitive (90 % CI 20–70 %) and 89 % specific (90 % CI 78–96 %). The summary diagnostic accuracy of the urine colorimetric assay at a target AUC0–8 of 24.1 mg•hour/L (area under the ROC curve of 0.71, 95 % CI 0.52–0.90) was similar to targets of 20 mg•hour/L (area under the ROC curve of 0.78, 95 % CI 0.56–1.0) and 30 mg•hour/L (area under the ROC curve of 0.68, 95 % CI 0.50–0.85).
Discussion
The cost and complexity of current approaches to therapeutic drug monitoring during TB therapy limit widespread use in areas of greatest need, despite the increasing recognition of the role of pharmacokinetic variability in driving treatment outcomes such as delayed sputum sterilization, treatment failure, and the acquisition of new drug resistance [2–6]. The extent of tradeoff between complexity and diagnostic accuracy will determine whether a point-of-care test to estimate anti-TB drug exposure can be a useful addition to the tools of TB clinicians in high-burden settings. There could be additional roles for non-invasive measures of anti-TB drug exposure among pediatric TB populations, where wide pharmacokinetic variability is typically observed, and blood sampling presents an even greater challenge [21].
A simple colorimetric assay of a single urine sample collected 4 h post-dosing performed well in the derivation study with healthy volunteers, but performed less accurately in the validation cohort of HIV/TB patients. The urine colorimetric assay demonstrated similar accuracy for classifying patients based on a Cmax target of 6.6 mg/L and an AUC0–8 target of 24.1 mg•hour; the former has been shown to be predictive of 2-month sputum conversion in TB patients. Nevertheless, test performance characteristics still did not reach the threshold of other commonly used diagnostic tools during the treatment of TB, such as chest radiograph or sputum smear [22, 23].
We selected urine sampling times based on previously published urinary excretion data for rifampin, and chose a time point for evaluation in the validation study based on diagnostic accuracy in healthy subjects. In future work we will employ optimal sampling theory to identify urine sampling schemes that predict serum pharmacokinetics better than a single 4-h timed void [24].
Another limitation of this study is that the development and validation samples arose from different populations. There is important pharmacogenetic variability related to the metabolism and excretion of rifampin, and this variability may contribute to observed differences in the urine assay results between the two groups [25]. Additionally, although the healthy volunteers were dosed with the standard combination regimen (rifampin, isoniazid, pyrazinamide, ethambutol), many of the HIV/TB patients also received additional medications (e.g., cotrimoxazole, fluconazole) as part of their HIV care. The interference of these and other potentially co-administered medications with the urine colorimetric assay for rifampin will require further evaluation.
One strength of our approach was that a priori decisions were made regarding the diagnostic criteria of the optimal cutoff, and the target Cmax and AUC values to be evaluated. Post-hoc analyses demonstrated that the urine colorimetric assay performed better at lower targets of drug exposure, as defined by Cmax. The use of a borderline or re-testing zone in assay development is likely to improve the positive and negative predictive values for the assay and will be evaluated in subsequent work [26].
The standard rifampin dosing guidelines during anti-TB treatment are increasingly questioned, and a recent dose-finding study of rifampin among TB patients has confirmed the non-linear pharmacokinetics characteristic of rifampin, with disproportionate increases in AUC with increasing dose sizes [27]. The utility of any novel, point-of-care approach will depend on its flexibility to adapt to new evidence for the underlying relationship between drug concentrations and treatment outcomes.
Conclusion
A urine colorimetric assay for rifampin, conducted on a single urine sample collected 4 h post-dosing, was only modestly accurate in the identification of HIV/TB patients with a rifampin Cmax below 8 mg/L. Future work will focus on refinement of the approach, with the goal of developing a simple, point-of-care, test that could be available for therapeutic drug monitoring during anti-TB therapy in high-burden settings.
Abbreviations
AU, absorbance unit; AUC, area under the curve; Cmax, maximum concentration; GC, gas chromatography; HIV, human immunodeficiency virus; HPLC, high performance liquid chromatography; PK, pharmacokinetic; ROC, receiver operating characteristic; TB, tuberculosis.
References
Treatment of tuberculosis. MMWR Recomm Rep 2003;52:1–77.
Pasipanodya J, Gumbo T. An oracle: antituberculosis pharmacokinetics-pharmacodynamics, clinical correlation, and clinical trial simulations to predict the future. Antimicrob Agent Chemother. 2011;55:24–34.
Pasipanodya JG, McIlleron H, Burger A, Wash PA, Smith P, Gumbo T. Serum drug concentrations predictive of pulmonary tuberculosis outcomes. J Infect Dis. 2014;208:1464–73.
Tappero JW, Bradford WZ, Agerton TB, Hopewell P, Reingold AL, Lockman S, et al. Serum concentrations of antimycobacterial drugs in patients with pulmonary tuberculosis in Botswana. Clin Infect Dis. 2005;41:461–9.
Dorman SE, Savic RM, Goldberg S, et al. Daily rifapentine for treatment of pulmonary tuberculosis. A randomized, dose-ranging trial. Am J Respir Crit Care Med. 2015;191:333–43.
Pasipanodya JG, Sristava S, Gumbo T. Meta-analysis of clinical studies supports the pharmacokinetic variability hypothesis for acquired drug resistance and failure of antituberculosis therapy. Clin Infect Dis. 2012;55:169–77.
Chigutsa E, Pasipanodya JG, Visser ME, van Helden PD, Smith PJ, Sirgel FA, et al. Impact of nonlinear interactions of pharmacokinetics and MICs on sputum bacillary kill rates as a marker of sterilizing effect in tuberculosis. Antimicrob Agents Chemother. 2015;59:38–45.
Alsultan A, Peloquin CA. Therapeutic drug monitoring in the treatment of tuberculosis: an update. Drugs. 2014;74:839–54.
Heysell SK, Moore JL, Peloquin CA, Ashkin D, Houpt ER. Outcomes and use of therapeutic drug monitoring in multidrug-resistant tuberculosis patients treated in Virginia, 2009–2014. Tuberc Respir Dis. 2015;78:78–84.
Vu DH, Koster RA, Bolhuis MS, Greijdanus B, Altena RV, Nguyen DH, et al. Simultaneous determination of rifampicin, clarithromycin and their metabolites in dried blood spots using LC-MS/MS. Talanta. 2014;121:9–17.
Allanson AL, Cotton MM, Tettey JN, Boyter AC. Therapeutic drug monitoring by dried blood spot: progress to date and future directions. J Pharm Biomed Anal. 2007;44:963–9.
Brechnuhler S, Fluehler H, Riess W, Theobald W. The renal elimination of rifampin as a function of the oral dose. A convenient way to assess relative bioavailability. Arzneimittelforschung. 1978;28:480–3.
Ellard GA, Fourie PB. Rifampin bioavailability: a review of its pharmacology and the chemotherapeutic necessity for ensuring optimal absorption. Int J Tuberc Lung Dis. 1999;11 Suppl 3:S301–8.
Pillai G, Ellard GA, Smith PJ, Fourie PB. The potential use of urinary excretion data for assessing the relative bioavailability of rifampin in fixed dose combination anti-tuberculosis formulations. Int J Tuberc Lung Dis. 2001;5:691–5.
Nicolau I, Tian L, Menzies D, Ostiguy G, Pai M. Point-of-care urine tests for smoking status and isoniazid treatment monitoring in adult patients. PLoS One. 2012;7, e45913.
Sunahara S, Nakagawa H. Metabolic study and controlled clinical trials of rifampin. Chest. 1972;61:526–32.
Pepe MS. The statistical evaluation of medical tests for classification and prediction. New York: Oxford University Press; 2003.
Carpenter J, Bithell J. Bootstrap confidence intervals: when, which, what? a practical guide for medical statisticians. Stat Med. 2000;19:1141–64.
Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez JC, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 2011. doi:10.1186/1471-2105-12-77.
Gumbo T et al. Concentration-dependent Mycobacterium tuberculosis killing and prevention of resistance by rifampin. Antimicrob Agents Chemother. 2007;51:3781–8.
Schaaf HS, Garcia-Prats AJ, Donald PR. Antituberculosis drugs in children. Clin Pharmacol Ther. 2015. doi:10.1002/cpt.164.
van Cleeff MR, Kivihya-Ndugga LE, Meme H, Odhiambo JA, Klatser PR. The role and performance of chest X-ray for the diagnosis of tuberculosis: a cost-effectiveness analysis in Nairobi. Kenya BMC Infect Dis. 2005;5:111.
Cattamanchi A, Dowdy DW, Davis JL, Worodria W, Yoo S, Joloba M, et al. Sensitivity of direct versus concentrated sputum smear microscopy in HIV-infected patients suspected of having pulmonary tuberculosis. BMC Infect Dis. 2009;9:53. doi:10.1186/1471-2334-9-53.
Chae JW, Seo JW, Mahat BM, Yun HY, Baek IH, Lee BY, et al. A simple pharmacokinetic model of alendronate developed using plasma concentration and urine excretion data from healthy men. Drug Dev Ind Pharm. 2014;40:1325–9.
Weiner M, Peloquin C, Burman W, Luo CC, Engle M, Prihoda TJ, et al. Effects of tuberculosis, race, and human gene SLCO1B1 polymorphisms on rifampin concentrations. Antimicrob Agents Chemother. 2010;54:4192–200.
Thanassi W, Noda A, Hernandez B, et al. Delineating a retesting zone using receiver operating characteristic analysis on serial QuantiFERON tuberculosis test results in US healthcare workers. Pulm Med. 2012;2012:291294.
Boeree MJ, Diacon AH, Dawson R, Newell J, Terpeluk P, Marder D, et al. A dose-ranging trial to optimize the dose of rifampin in the treatment of tuberculosis. Am J Respir Crit Care Med. 2015;191:1058–65.
Acknowledgments
We are grateful to the volunteers and patients who participated in the study.
Funding
This work was supported by a Grand Challenges and Explorations grant from the Bill & Melinda Gates Foundation (to Dr. Vinnard) and the National Institute of Allergy and Infectious Diseases (K23AI102639 to Dr. Vinnard, R21AI104441 to Dr. Bisson). The funding bodies had no role in the design of the study, the collection of data, the analysis and interpretation of data, or the manuscript preparation.
Availability of data and materials
The data analyzed in this study can be accessed by sending a request to the corresponding author, with approval of the institutional review boards.
Authors’ contribution
CV, GB, and HS conceived the study. LK, IZ, MK, NT, and CV contributed to study design. IZ, MK, and LK performed the urine assays. CP, JP, and TG performed the serum pharmacokinetic assays. HS, JP, TG, CP, GB, VI, and CV contributed to data analysis and interpretation. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent to publish
Not applicable.
Ethics and consent to participate
The derivation study was approved by the institutional review board at Drexel University College of Medicine, and all participants provided written informed consent. The validation study was approved by the institutional review boards of the University of Pennsylvania, Botswana Ministry of Health, and the Princess Marina Hospital, and all patients provided written informed consent.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Zentner, I., Schlecht, H.P., Khensouvann, L. et al. Urine colorimetry to detect Low rifampin exposure during tuberculosis therapy: a proof-of-concept study. BMC Infect Dis 16, 242 (2016). https://doi.org/10.1186/s12879-016-1576-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-016-1576-1