Abstract
Aim
We systematically reviewed and meta-analyzed the predictors of major adverse cardiac and cerebrovascular events (MACE/MACCE) in older adults who underwent PCI.
Methods
Three databases, PubMed, Embase, and Scopus, were searched for observational studies considering the out-of-hospital MACE/MACCE in adults ≥ 60 years old with coronary artery disease (acute or chronic) who underwent PCI. Studies were eligible if they had determined at least two statistically significant predictors of MACE/MACCE by multivariable analysis. We used the QUIPS tool to evaluate the risk of bias in the studies. Random-effects meta-analysis was utilized to pool the hazard ratios (HRs) of the most reported predictors.
Results
A total of 34 studies were included in the review. Older age (HR = 1.04, 95% Confidence Interval (CI): 1.03–1.06, P-value < 0.001), diabetes (HR = 1.36, 95% CI: 1.22–1.53, P < 0.001), history of myocardial infarction (MI) (HR = 1.88, 95% CI: 1.37–2.57, P < 0.001), ST-elevation MI (STEMI) at presentation (HR = 1.72, 95% CI: 1.37–2.18, P < 0.001), reduced left ventricular ejection fraction (LVEF) (HR = 2.01, 95% CI: 1.52–2.65, P < 0.001), successful PCI (HR = 0.35, 95% CI: 0.27–0.47, P < 0.001), eGFR (HR = 0.99, 95% CI: 0.97-1.00; P-value = 0.04) and left main coronary artery (LMCA) disease (HR = 2.07, 95% CI: 1.52–2.84, P < 0.001) were identified as predictors of MACE.
Conclusion
We identified older age, diabetes, history of MI, STEMI presentation, lower LVEF, and LMCA disease increased the risk of MACE/MACCE after PCI in older adults. Meanwhile, higher eGFR and successful PCI predicted lower adverse events risk. Future studies should focus on a more robust methodology and a precise definition of MACE.
Registration
PROSPERO (CRD42023480332).
Similar content being viewed by others
Introduction
Cardiovascular diseases, particularly coronary artery disease (CAD), are the most prevalent cause of mortality worldwide and represent a major health challenge [1,2,3,4,5]. With the improvements in the health care system and, thereby, the increase in life expectancy, the population of older people has become a noticeable component of society globally. This increase in the aging population means a dramatic incline in patients suffering from non-communicable diseases, and CAD is not an exception [6]. The burden of CAD on the older population necessitates more worldwide dedication to geriatric studies, especially in developing countries [7].
Atherosclerosis might progress more rapidly in older individuals and form more complex and calcified plaques associated with a higher risk of CAD [8]. Moreover, older people may not only be more prone to CAD, but their comorbidities also result in more complications and undesirable outcomes [9]. Making decisions about the appropriate therapeutic approach following CAD is a complex challenge for physicians, as older people and their families often prefer to choose a less invasive approach and conservative drug treatment. Although older age is a significant predictor of increased risk of major adverse cardiac events (MACE)/ major adverse cardiac and cerebrovascular events (MACCE) following percutaneous coronary intervention (PCI), other predictors, such as clinical or procedural characteristics, are also important [10].
Studies discussing the predictors of MACE/MACCE following PCI in older individuals are few, and the suggested predictors differ between these studies due to variations in population, sampling methods, and the definition of endpoints. Furthermore, no prior studies have examined these predictors systematically. Thus, The main objective of the present systematic review was to identify the main determinants of MACE/MACCE after PCI in the older population.
Methods
The review protocol was registered at the International Prospective Register of Systematic Reviews (PROSPERO) with the identification code CRD42023480332. The present study followed the updated Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement for conducting systematic reviews and meta-analyses [11]..
Eligibility criteria
This study included observational research that investigated the predictors of out-of-hospital outcomes (MACE or MACCE) in older adults (≥ 60 years old, according to the United Nations definition [12]) with coronary artery disease (acute or chronic) who underwent PCI. Studies were excluded according to the following criteria: (1) Conference abstracts, reviews, case reports/ series, and editorials; (2) The analyzed population consisted of other treatment approaches, e.g., coronary artery bypass grafting (CABG), thrombolytic, and medical treatment; (3) Comparison of outcomes between older adults and younger patients, with no separate report on older people; (4) No MACE/MACCE predictor identification by multivariable analysis; (5) Only one associated exposure with the outcome in the multivariable analysis; (6) No composite MACE/MACCE outcomes (including studies that defined only mortality as the endpoint); (7) In-hospital outcomes only; (8) Non-English articles. In the case of studies using the same database or with overlapping populations, the studies with a more complete recruitment period, overall number of PCI patients, follow-up, and measured outcomes were selected. Since the definition of MACE and MACCE varied markedly between the studies, no eligibility criterion was set based on the components of MACE/MACCE. Instead, we assessed the outcome definitions of the included studies for risk of bias.
Information sources and search strategy
We searched PubMed, EMBASE, and Scopus from January 1st, 2000, to November 2nd, 2023, with no study design or language filters. Databases were searched using keywords like “elderly,” “Primary Percutaneous Intervention,” “Major Adverse Cardiovascular Events,” and “Major Adverse Cardiac-Cerebrovascular Events.” The detailed search syntax is provided in the Supplementary File.
Selection process and data collection
Two independent groups (Group 1: A.S. and M.S.N., and Group 2: Z.K. and S.N.) screened the records for eligibility criteria in two stages (title/abstract and full text). Discrepancies were resolved by discussion with the review team. Two reviewers (A.H. and M.D.) reviewed the included articles and independently extracted the variables of interest. Disagreements were solved by consensus. Data items have been explained in detail in the Supplementary File.
Risk of bias assessment
We utilized the Quality In Prognosis Studies (QUIPS) tool to evaluate the quality and risk of bias among the included studies [13]. QUIPS tool consists of six major components: (1) study participation (7 items), (2) study attrition (5 items), (3) prognostic factor measurement (6 items), (4) outcome measurement (3 items), (5) study confounding (7 items), and (6) statistical analysis and reporting (4 items). Two independent authors (M.S.N and S.N.) performed the assessment. The reviewers rated each component as low, moderate, and high risk of bias. The findings were compared, and any disagreement was solved by discussion with A.J.
Synthesis methods
The provided effect sizes (i.e., hazard ratio (HR) or odds ratio (OR)) of each predictor from the multivariate analysis were extracted. Summary tables were then used to report the results qualitatively. Using a random-effects model, we pooled the most commonly reported effect sizes (HRs) for the quantitative synthesis demonstrated in forest plots. The choice of the model was made due to suspected heterogeneity among the included studies. Meta-analysis was performed if at least five studies reported HRs as a predictor. More details about the synthesis method are available in the Supplementary File.
The I2 test was used for assessing statistical heterogeneity. For publication bias assessment, funnel plots and Egger’s test were utilized. All analyses were performed with R V.4.2.1 (R Foundation for Statistical Computing, Vienna, Austria) and RStudio (RStudio, Boston, Massachusetts, USA), using “meta,” “metafor,” and “dmetar” packages [14].
Results
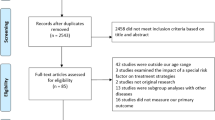
We reviewed the full texts of 226 studies from 6119 identified records. Finally, thirty-four studies of 25,550 individuals and 11 multicenter investigations were selected [15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48]. The PRISMA flowchart of the study selection process is shown in Fig. 1. The reasons for excluding the remaining studies can be found in the Supplementary File. The baseline characteristics of the eligible studies are summarized in Table 1. Eleven (31.4%) were multicenter studies, and the median follow-up time ranged from one to 120 months.
Risk of bias assessment
The risk of bias in study attrition, prognostic factor measurement, and study confounding domains was generally low (low-risk: 91.2%, 94.1%, and 94.1%, respectively). The risk of bias was higher in study participation and statistical analysis (moderate risk: 32.4% and 47.1%, respectively) domains. In the outcome measurement domain, 13 studies (38.2%) had low risk, four (11.8%) had high risk, and 17 (50%) had moderate risk of bias. The risk of bias among the included studies has been summarized in Fig. 2. A detailed version of the risk of bias assessment for each study is presented in Supplementary Table S1.
Qualitative synthesis
The most common independent demographic predictors of increased risk of MACE/MACCE were higher age [15, 16, 22, 27, 28, 31,32,33, 36, 40, 42, 45] and male sex [25, 37]. The main comorbidities which predicted escalated MACE/MACCE risk were as follows: history of myocardial infarction (MI) before the studied exposure [15, 17, 24, 28, 33, 42, 45], CABG [20], Stroke [23], cardiovascular disease [42], diabetes (DM) [17, 26, 28, 30, 37, 39, 43, 44], hypertension (HTN) [20, 26, 45, 47], and chronic kidney disease (CKD) [29]. One study presented that patients with a positive CAD family history had a higher risk of adverse events [37]. Two studies identified higher frailty scores as a clinical predictor of higher MACE/MACCE risk [19, 31]. ST-elevation MI (STEMI) diagnosis in patients also resulted in a significantly higher occurrence of endpoints [27, 33, 35]. Lower left ventricular ejection fraction (LVEF) [17, 18, 20, 24, 29, 30, 32, 34,35,36, 38, 41, 46, 49], lower estimated glomerular filtration rate (eGFR) [17, 21, 22, 38, 41, 42], and anemia [17, 18, 42] were reported more frequently among the paraclinical predictors of higher risk of adverse events.
Several procedural variables, including multivessel disease (MVD) [16, 19, 26] and left main coronary artery (LMCA) involvement [17, 19, 21], were also identified as predictors of increased MACE/MACCE risk. PCI through radial access [17, 24, 35] and either successful PCI (thrombolysis in myocardial infarction or TIMI grade III) or complete revascularization [21, 38, 43, 48] resulted in a lower risk of adverse events. The summaries of significant predictors and adjusted variables in each study are provided in Supplementary Table S2. Effect sizes of exposures on MACE/MACCE are summarized in Supplementary Table S3.
Quantitative synthesis
The findings of 27 studies were eligible for meta-analysis. Increasing age was associated with higher MACE/MACCE risk (HR = 1.04, 95% CI: 1.03–1.06; P-value < 0.001, I2 = 11.3%). However, sex did not significantly predict increased MACE/MACCE risk (Female HR = 0.86, 95% CI: 0.70–1.04; P-value = 0.12, I2 = 52.6%, Fig. 3). Among the clinical exposures, DM (HR = 1.36, 95% CI: 1.22–1.53; P-value < 0.001, I2 = 56.7%), history of MI (HR = 1.88, 95% CI: 1.37–2.57; P-value < 0.001, I2 = 37.8%), and STEMI presentation (HR = 1.72, 95% CI: 1.37–2.18; P-value < 0.001, I2 = 0%) were significant determinants of MACE/MACCE increased occurrence (Fig. 3). Incremental LVEF prevented adverse events (HR = 0.96, 95% CI: 0.93–0.98; P-value < 0.001, I2 = 79.2%). On the other hand, reduced LVEF increased the risk of MACE/MACCE (HR = 2.01, 95% CI: 1.52–2.65; P-value < 0.001, I2 = 42.4%, Fig. 4). Higher kidney function, measured by eGFR, caused a slight decrease in the MACE/MACCE risk (HR = 0.99, 95% CI: 0.97-1.00; P-value = 0.04, I2 = 70.7%).
Drug-eluting stents (DES) were not associated with a statistically significant decrease in adverse events (HR = 0.68, 95% CI: 0.44–1.04; P-value = 0.08, I2 = 70.6%). LMCA disease was an important risk factor for MACE/MACCE (HR = 2.07, 95% CI: 1.52–1.2.84; P-value < 0.001, I2 = 0%), whereas procedural success (TIMI grade III) was protective against the endpoints (HR = 0.35, 95% CI: 0.27–0.47; P-value < 0.001, I2 = 0%). Other important predictors of a higher MACE risk after PCI were non-radial access [17, 24, 35], heart failure [15] and higher Killip class [23, 35, 36, 38], albumin [19], CONUT score [30], ACE inhibitors [15, 26], and GRACE score [34].
Funnel plots and Egger’s test measured publication bias. Except for the history of MI (Egger’s coefficient = 1.88, 95% CI: 0.63–3.14, P-value = 0.04), continuous LVEF (Egger’s coefficient=-3.38, 95% CI: -5.33,-1.42, P-value = 0.01), and DES (Egger’s coefficient=-2.45, 95% CI: -3.91,-0.98, P-value = 0.02), there was no significant publication bias among other predictors as described in Supplementary File. The funnel plots are presented in Supplementary Figure S1.
Discussion
To our knowledge, the present study was the first systematic review and meta-analysis investigating the predictors of MACE/MACCE among older adults who had undergone PCI. According to the meta-analysis results, older age, diabetes, history of MI, STEMI, reduced LVEF, and LMCA disease were significant predictors of escalated risk of adverse events. Meanwhile, higher eGFR and successful PCI predicted lower MACE/MACCE risk. However, the pooled estimates for hypertension, female sex, and DES showed no significant associations with the increased risk of MACE.
Advances in PCI technology and techniques have resulted in better outcomes and fewer adverse events, especially in vulnerable older individuals [50]. The landmark FIRE trial indicated that older adults who underwent physiology-guided complete revascularization had a significantly lower risk of 1-year MACE than culprit–lesion–only PCI [51]. Conversely, Hanna et al. showed that older adults with stable ischemic heart disease who underwent complex PCI had a lower risk of target lesion revascularization but a higher risk of all-cause death compared to those who underwent noncomplex PCI [52]. It is crucial to note that while older adults may benefit more from PCI, they also face a higher risk of post-procedural complications and adverse events compared to younger patients [53, 54].
Demographic and clinical predictors
Advanced age is a well-known risk factor for MACE/MACCE after PCI. Older adults often have more comorbidities, complex coronary lesions, and frailty that increase the procedural and post-procedural complications [55], which accounts for higher rates of MACCE [56]. Unlike age, the role of sex in the outcome of PCI is a topic of ongoing debate. Otawa et al. and Aghajani et al. were the only studies that found women to be a significant protective factor against one-year and five-year MACE, respectively [25, 37]. Contrariwise, in other studies not limited to older age, the female sex served as an independent predictor of a higher risk of one-year [57] and five-year [58] MACE after PCI. At the same time, it is essential to consider the interaction of age and sex, as studied by Alkhouli et al., in a large population of acute MI patients. They suggested that younger women generally have higher mortality compared to men, but older women have better outcomes compared to their male counterparts [59]. Similarly, Tonet et al. pointed out that in patients > 70 with acute coronary syndrome, the protective role of the female sex against higher mortality becomes evident when the model was adjusted for physical activity, and that female patients with preserved physical status had a better outcome compared to their male counterparts [60]. Therefore, identifying potential underappreciated confounding factors such as frailty, malnutrition [61], and physical activity and their interactions could clarify the complex role of age and sex in older adults on PCI outcomes. Alternatively, some studies proposed that the negative impact of female sex on PCI outcomes disappeared in older patients, and no significant difference was observed regarding the incidence of all-cause mortality and MACCE between men and women [62, 63]. The conflicting results in the literature could arise from population differences, assumed endpoints, and follow-up times. Therefore, the exact role of sex in the outcome of PCI among older adults remains controversial and requires more extensive investigations.
Hypertension is a common risk factor for CAD [64] and a leading cause of mortality in older people [65]. However, only Yan et al. could demonstrate that HTN is the predictor of increased MACE risk [20]. Notably, their adjusted model consisted of fewer variables than other studies, which may have affected their results. Diabetes has been consistently associated with an increased risk of MACE/MACCE after PCI [66,67,68], especially in patients with chronic total occlusions [69]. Our meta-analysis showed that diabetes predicts a higher risk of MACE/MACCE. Moreover, triglyceride glucose-body mass index, a predictor of type II DM [70], could also predict MACE/MACCE risk in older adults after PCI [15]. However, De Luca et al. concluded that the impact of diabetes on survival in advanced age (> 74 years old) becomes unclear when adjusted for baseline confounding factors, suggesting that diabetes is mainly responsible for significant comorbidity and more bleeding complications that result in higher mortality [71].
Our study indicated that the history of MI in older people is a predictive factor of major adverse events after PCI. Previous MI is responsible for decreased LVEF and heart failure; additionally, patients are more likely to develop complex CAD, which consequently results in higher mortality and MACE following PCI [72,73,74].
In the present study, older patients with STEMI presentation had higher MACE/MACCE risk than other presentations after PCI. Likewise, Wang et al., who investigated the interaction of STEMI, sex, and age and the risk of MACE, found that older women with STEMI had the highest risk of MACE [75]. On the other hand, Chang et al. found that STEMI could independently predict a higher revascularization incidence after the index event. In comparison, non-STEMI had a higher incidence of MACE [76]. Moreover, they indicated that older adults (> 65 years old) with non-STEMI had significantly longer hospital and ICU stays besides the need for mechanical circulatory support.
Paraclinical and procedural predictors
The literature agrees that lower LVEF is associated with worse outcomes in older patients [77]. Similarly, decreased eGFR is associated with a higher risk of MACE in young and older adults undergoing PCI [78]. Consensus also exists for successful PCI as a protective factor against major adverse events [79,80,81].
Older patients receiving DES (> 75 years old) had remarkably lower risk of MACE and mortality compared to bare metal stents [82, 83]. Although our meta-analysis did not establish DES as a statistically significant protective factor against MACE/MACCE in older patients, the confidence interval was borderline (HR = 0.68, 95% CI: 0.44–1.04), suggesting that the association between DES and reduced risk of MACE/MACCE may be clinically meaningful. The advantages of DES over plain old balloon angioplasty [84] and bare metal stent [85] make DES clinically essential.
LMCA disease needs special attention due to its large amount of at-risk myocardium, and patients having MI with LCMA involvement are at significantly higher risks of cardiovascular morbidity and mortality compared to other obstructive CAD [86]. Although coronary artery bypass graft has long been the preferred treatment for LMCA with less long-term mortality and MACE/MACCE [87,88,89,90], PCI is considered in older people with higher surgical risk and frailty [91]. Our meta-analysis showed that LMCA disease in older patients predicted an increased risk of MACE/MACCE after PCI.
Limitations
The current investigation has a few limitations. First, we considered studies that reported multiple predictors of MACE/MACCE in their multivariate analysis rather than a single exposure. Second, meta-analyses were performed using HRs resulting from a multivariate model. Some studies did not use multivariate analysis or report effect sizes for statistically non-significant variables. Some studies misreported the OR instead of the HR obtained from the time-to-event model. The abovementioned shortcomings may result in publication bias in the current systematic review. Regardless, the funnel plots showed little asymmetries. Third, the included studies exhibited significant heterogeneity mainly due to differences in endpoint definitions (MACE/MACCE) and population (age cut-offs, primary or elective PCI, and CAD type). Nevertheless, it was the first systematic review with a holistic investigation of MACE/MACCE predictors in older adults after PCI.
Conclusion
We found that factors such as older age, diabetes, history of MI, STEMI presentation, lower LVEF, and LMCA disease increased the risk of MACE/MACCE after PCI in older adults. On the other hand, a higher eGFR and successful PCI were associated with a lower risk of adverse events. By identifying these predictors, healthcare providers can better assess their patients’ risk profiles and tailor interventions to mitigate adverse outcomes. Our risk of bias assessment revealed the need for more accurate study designs and statistical analysis, along with a uniform definition of MACE/MACCE.
Data availability
The datasets used during the current study are available from the corresponding author upon reasonable request.
References
Bauersachs R, et al. Burden of Coronary Artery Disease and Peripheral Artery Disease: A literature review. Cardiovasc Ther. 2019;2019:p8295054.
Madhavan MV, et al. Coronary artery disease in patients ≥ 80 years of age. J Am Coll Cardiol. 2018;71(18):2015–40.
Malakar AK, et al. A review on coronary artery disease, its risk factors, and therapeutics. J Cell Physiol. 2019;234(10):16812–23.
Ralapanawa U, Sivakanesan R. Epidemiology and the Magnitude of Coronary Artery Disease and Acute Coronary Syndrome: a narrative review. J Epidemiol Glob Health. 2021;11(2):169–77.
Sanchis-Gomar F, et al. Epidemiology of coronary heart disease and acute coronary syndrome. Ann Transl Med. 2016;4(13):256.
Su YM, et al. Outcomes after percutaneous coronary intervention and comparison among scoring systems in predicting procedural success in elderly patients (≥ 75 years) with chronic total occlusion. Coron Artery Dis. 2019;30(7):481–7.
Shafiee A, van Bodegom D. The necessity for research on the elderly in Iran. J Tehran Univ Heart Cent. 2012;7(1):40–40.
Head T, Daunert S, Goldschmidt-Clermont PJ. The aging risk and atherosclerosis: a Fresh look at arterial homeostasis. Front Genet. 2017;8:216.
Sliman H, et al. Clinical features and outcomes of revascularization in very old patients with left main coronary artery disease. Coron Artery Dis. 2019;30(8):584–9.
Kumar S, et al. Contemporary revascularization dilemmas in older adults. J Am Heart Assoc. 2020;9(3):e014477.
Page MJ, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
UNHCR. Older persons. 2020 18 May 2020 29 November 2023]; Available from: https://emergency.unhcr.org/protection/persons-risk/older-persons
Hayden JA, et al. Assessing bias in studies of prognostic factors. Ann Intern Med. 2013;158(4):280–6.
Team RC. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.; Available from: https://www.R-project.org/
Cheng Y, et al. Association between triglyceride glucose-body mass index and cardiovascular outcomes in patients undergoing percutaneous coronary intervention: a retrospective study. Cardiovasc Diabetol. 2023;22(1):75.
Li Q et al. Bivalirudin versus Heparin on net adverse clinical events, major adverse Cardiac and cerebral events, and bleeding in Elderly Chinese patients treated with percutaneous coronary intervention. Tohoku J Exp Med, 2023.
Marschall A, et al. Outcomes prediction in Complex High-Risk indicated percutaneous coronary interventions in the older patients. Am J Cardiol. 2023;205:465–72.
Park JI, et al. Efficacy of percutaneous coronary intervention with synergy stents in patients aged ≥ 75 years: 1-Year clinical outcomes from the Synergy Elderly Registry. Am J Cardiol. 2023;204:43–52.
Shimono H, et al. Association of preoperative clinical frailty and clinical outcomes in elderly patients with stable coronary artery disease after percutaneous coronary intervention. Heart Vessels. 2023;38(10):1205–17.
Yan K, et al. Five-year prognostic value of DAPT score in older patients undergoing percutaneous coronary intervention: a large-sample study in the Real World. J Atheroscler Thromb. 2023;30(8):1057–69.
Fallahzadeh A, et al. Outcome of percutaneous coronary intervention in old patients presenting with Acute Coronary Syndrome. Arch Iran Med. 2022;25(8):523–32.
Horikoshi T, et al. A propensity score matched analysis of Statin effects on major adverse cardiac events after percutaneous coronary intervention in patients over 75 Years Old. Intern Med. 2022;61(18):2711–9.
Lang J, et al. Staged revascularization vs. culprit-only percutaneous coronary intervention for multivessel disease in elderly patients with ST-segment elevation myocardial infarction. Front Cardiovasc Med. 2022;9:943323.
Marino M, et al. Complete percutaneous revascularization in patients aged ≥ 85 years with Acute Coronary Syndrome and Multivessel Coronary Artery Disease. Am J Cardiol. 2022;180:10–6.
Otowa K, et al. One-year outcome after percutaneous coronary intervention in nonagenarians: insights from the J-PCI OUTCOME registry. Am Heart J. 2022;246:105–16.
Wang J, et al. Impact of BMI on long-term outcomes in patients with ST-Segment Elevation myocardial infarction after primary percutaneous coronary intervention. Int J Clin Pract. 2022;2022:p6210204.
Wang JL, et al. Sex-related differences in clinical outcomes and predictive factors in the very elderly patients with ACS undergoing PCI. Front Cardiovasc Med. 2022;9:950165.
Lattuca B, et al. Bleeding in the Elderly: risk factors and impact on clinical outcomes after an Acute Coronary Syndrome, a sub-study of the Randomized ANTARCTIC Trial. Am J Cardiovasc Drugs. 2021;21(6):681–91.
Lim M, et al. Clinical outcomes in older patients undergoing percutaneous coronary intervention for Non-ST-Elevation Acute Coronary syndromes. Heart Lung Circ. 2021;30(2):275–81.
Kalyoncuoğlu M et al. Predicting one-year deaths and major adverse vascular events with the Controlling Nutritional Status score in Elderly patients with Non-ST-Elevated myocardial infarction undergoing percutaneous coronary intervention. J Clin Med, 2021. 10(11).
Kanwar A, et al. Poor quality of life in patients with and without frailty: co-prevalence and prognostic implications in patients undergoing percutaneous coronary interventions and cardiac catheterization. Eur Heart J Qual Care Clin Outcomes. 2021;7(6):591–600.
Maruyama S, et al. Impact of nutritional index on long-term outcomes of elderly patients with coronary artery disease: sub-analysis of the SHINANO 5 year registry. Heart Vessels. 2021;36(1):7–13.
Morici N, et al. Residual SYNTAX score and one-year outcome in Elderly patients with Acute Coronary Syndrome. CJC Open. 2020;2(4):236–43.
Zhang W, et al. Prognostic value of serum calprotectin level in elderly diabetic patients with acute coronary syndrome undergoing percutaneous coronary intervention: a cohort study. Med (Baltim). 2020;99(33):e20805.
Berezhnoi K, Kokov L, Vanyukov A. Effects of complete revascularization on long-term treatment outcomes in patients with multivessel coronary artery disease over 80 years of age admitted for acute coronary syndrome. Cardiovasc Diagn Ther. 2019;9(4):301–9.
Huang J, et al. Systemic Immune-Inflammatory Index predicts clinical outcomes for Elderly patients with Acute Myocardial Infarction receiving percutaneous coronary intervention. Med Sci Monit. 2019;25:9690–701.
Aghajani H, et al. Predictors of long-term major adverse cardiac events following percutaneous coronary intervention in the Elderly. Arch Iran Med. 2018;21(8):344–8.
de La Torre Hernandez JM, et al. Multivessel disease in patients over 75years old with ST elevated myocardial infarction. Current management strategies and related clinical outcomes in the ESTROFA MI + 75 nation-wide registry. Cardiovasc Revasc Med. 2018;19(5 Pt B):580–8.
De Rosa R, et al. High on-treatment platelet reactivity and outcome in elderly with non ST-segment elevation acute coronary syndrome - insight from the GEPRESS study. Int J Cardiol. 2018;259:20–5.
Gerber RT, et al. Age is not a bar to PCI: insights from the long-term outcomes from off-site PCI in a real-world setting. J Interv Cardiol. 2017;30(4):347–55.
Wei Z, et al. Comparison of percutaneous coronary intervention Versus Coronary artery bypass graft in aged patients with unprotected left main artery lesions. Int Heart J. 2016;57(6):682–8.
Yu XF, et al. Staged versus one-time multivessel intervention in elderly patients with non-ST-elevation acute coronary syndrome. J Geriatr Cardiol. 2016;13(9):760–7.
Uthamalingam S, et al. Long term outcomes in octogenarians undergoing percutaneous coronary intervention: comparison of bare metal versus drug eluting stent. Int J Cardiol. 2015;179:385–9.
Liu W, et al. Impact of diabetes on long term follow-up of elderly patients with chronic total occlusion post percutaneous coronary intervention. J Geriatr Cardiol. 2013;10(1):16–20.
Chen J, et al. Incomplete revascularization in the drug eluting stent era permits meaningful long-term (12–78 months) outcomes in patients ≥ 75 years with acute coronary syndrome. J Geriatr Cardiol. 2012;9(4):336–43.
López-Palop R, et al. Safety and efficacy of coronary drug-eluting stents in octogenarians. Rev Esp Cardiol. 2009;62(11):1250–9.
Ma HY, et al. Long-term outcome of patients of over 85 years old with acute coronary syndrome undergoing percutaneous coronary stenting: a comparison of bare metal stent and drug eluting stent. Chin Med J (Engl). 2008;121(10):887–91.
Gach O, et al. Predictors of early and late outcome of percutaneous coronary intervention in octogenarians. Acta Cardiol. 2003;58(4):289–94.
Rumiz E, et al. Long-term outcomes and predictors of morbi-mortality according to age in stemi patients with multivessel disease: impact of an incomplete revascularization. Catheter Cardiovasc Interv. 2018;92(7):E512–7.
Shanmugam VB, et al. An overview of PCI in the very elderly. J Geriatr Cardiol. 2015;12(2):174–84.
Biscaglia S, et al. Complete or culprit-only PCI in older patients with myocardial infarction. N Engl J Med. 2023;389(10):889–98.
Hanna JM, et al. Complex percutaneous coronary intervention outcomes in older adults. J Am Heart Association. 2023;12(19):e029057.
Brenes-Salazar JA, Forman DE. Advances in Percutaneous Coronary interventions for Elderly patients. Prog Cardiovasc Dis. 2014;57(2):176–86.
Nanna Michael G, et al. Assessment and Management of older adults undergoing PCI, part 1. Volume JACC. Advances; 2023. p. 100389. 4.
Floyd KC, et al. Age-based differences of percutaneous coronary intervention in the drug-eluting stent era. J Interv Cardiol. 2006;19(5):381–7.
Kim DW, et al. Incremental age-related one-year MACCE after acute myocardial infarction in the drug-eluting stent era (from KAMIR-NIH registry). J Geriatr Cardiol. 2018;15(9):574–84.
Kumar S et al. Sex-differences in outcomes after percutaneous coronary intervention of chronic total occlusions: insights from a large single-center registry. Eur Heart J, 2021.
Kosmidou I, et al. Long-term outcomes in women and men following percutaneous coronary intervention. J Am Coll Cardiol. 2020;75(14):1631–40.
Alkhouli M, et al. Age-stratified sex-related differences in the incidence, management, and outcomes of Acute myocardial infarction. Mayo Clin Proc. 2021;96(2):332–41.
Tonet E, et al. The impact of sex and physical performance on long-term mortality in older patients with myocardial infarction. BMC Med. 2022;20(1):15.
Tonet E, et al. Nutritional status and all-cause mortality in older adults with acute coronary syndrome. Clin Nutr. 2020;39(5):1572–9.
Pancholy SB, et al. Sex differences in short-term and long-term all-cause mortality among patients with ST-segment elevation myocardial infarction treated by primary percutaneous intervention: a meta-analysis. JAMA Intern Med. 2014;174(11):1822–30.
Xu N, et al. Sex-based differences in bleeding and long-term adverse events after percutaneous coronary intervention in older patients with coronary artery disease. J Interv Cardiol. 2018;31(3):345–52.
Weber T, et al. Hypertension and coronary artery disease: epidemiology, physiology, effects of treatment, and recommendations: a joint scientific statement from the Austrian Society of Cardiology and the Austrian Society of Hypertension. Wien Klin Wochenschr. 2016;128(13–14):467–79.
Wu CY, et al. High blood pressure and all-cause and Cardiovascular Disease mortalities in Community-Dwelling older adults. Med (Baltim). 2015;94(47):e2160.
Dehdar Karsidani S, et al. Intelligent prediction of major adverse cardiovascular events (MACCE) following percutaneous coronary intervention using ANFIS-PSO model. BMC Cardiovasc Disord. 2022;22(1):389.
Zhao Y, Guo M, Shi G. Prediabetes predicts adverse cardiovascular outcomes after percutaneous coronary intervention: a meta-analysis. Biosci Rep, 2019. 40.
Alzaky M, et al. Glycemic variability as a predictor of major adverse cardiac events after percutaneous coronary intervention. Volume 9. JOURNAL OF INDIAN COLLEGE OF CARDIOLOGY; 2019. pp. 148–53.
Latif A, et al. Impact of diabetes Mellitus on outcomes of Percutaneous Coronary intervention in chronic total occlusions: a systematic review and Meta-analysis. Cardiovasc Revasc Med. 2022;37:68–75.
Song B, et al. Triglyceride glucose-body Mass Index and Risk of Incident Type 2 diabetes Mellitus in Japanese People with Normal Glycemic Level: a Population-based longitudinal cohort study. Front Endocrinol (Lausanne). 2022;13:907973.
De Luca G et al. Impact of diabetes on clinical outcome among elderly patients with acute coronary syndrome treated with percutaneous coronary intervention: insights from the ELDERLY ACS 2 trial. J Cardiovasc Med, 2020.
Nishihira K, et al. Outcomes of Elderly patients with Acute myocardial infarction and heart failure who undergo percutaneous coronary intervention. Circ Rep. 2022;4(10):474–81.
Numasawa Y, et al. Comparison of outcomes after percutaneous coronary intervention in Elderly patients, including 10 628 nonagenarians: insights from a Japanese Nationwide Registry (J-PCI Registry). J Am Heart Association. 2019;8(5):e011183.
Saada M, et al. Prognosis of PCI in the older Adult Population: outcomes from the Multicenter prospective e-ULTIMASTER Registry. J Soc Cardiovasc Angiography Interventions. 2022;1(5):100442.
Cuiping W, et al. Interactions of ST-elevation myocardial infarction, age, and sex and the risk of major adverse cardiovascular events among Chinese adults: a secondary analysis of a single-centre prospective cohort. BMJ Open. 2022;12(7):e058494.
Chang SS, et al. Prognosis between ST-Elevation and Non-ST-elevation myocardial infarction in older adult patients. Front Cardiovasc Med. 2021;8:749072.
Velagaleti RS, et al. Change in left ventricular ejection Fraction with coronary artery revascularization and subsequent risk for adverse Cardiovascular outcomes. Volume 15. Circulation: Cardiovascular Interventions; 2022. p. e011284. 4.
Zhu X, et al. Effect of glomerular filtration rate in patients undergoing percutaneous coronary intervention: a systematic review and meta-analysis. Med (Baltim). 2022;101(44):e31498.
Chen Q, et al. Safety and effectiveness of percutaneous coronary intervention (PCI) in elderly patients. A 5-year consecutive study of 201 cases with PCI. Arch Gerontol Geriatr. 2010;51(3):312–6.
Farshidi H, et al. Major adverse cardiovascular event (MACE) after percutaneous coronary intervention in one-year follow-up study. Electron Physician. 2018;10(2):6383.
Rao SV, et al. Temporal trends in percutaneous coronary intervention outcomes among older patients in the United States. Am Heart J. 2013;166(2):273–81. e4.
Bae S, et al. Efficacy and safety of drug-eluting stents in elderly patients: a meta-analysis of randomized trials. Cardiol J. 2021;28(2):223–34.
Varenne O, et al. Drug-eluting stents in elderly patients with coronary artery disease (SENIOR): a randomised single-blind trial. Lancet. 2018;391(10115):41–50.
Mitomo S, et al. Comparison between Plain Old Balloon Angioplasty and Drug-Eluting Stent Implantation for the treatment of Stent Fracture. J Interv Cardiol. 2015;28(4):365–73.
Lai CH, et al. Comparison of Bare-Metal Stent and Drug-Eluting Stent for the treatment of patients undergoing percutaneous coronary intervention for unprotected left main coronary artery disease - long-term result from a single Center experience. Acta Cardiol Sin. 2015;31(5):381–9.
Park S, Park SJ, Park DW. Percutaneous coronary intervention for Left Main Coronary Artery Disease: Present Status and Future perspectives. JACC Asia. 2022;2(2):119–38.
Tam Derrick Y, et al. Real-world examination of revascularization strategies for Left Main Coronary Disease in Ontario, Canada. Volume JACC. Cardiovascular Interventions; 2023. pp. 277–88. 3.
Holm NR, et al. Percutaneous coronary angioplasty versus coronary artery bypass grafting in the treatment of unprotected left main stenosis: updated 5-year outcomes from the randomised, non-inferiority NOBLE trial. Lancet. 2020;395(10219):191–9.
Persson J, et al. PCI or CABG for left main coronary artery disease: the SWEDEHEART registry. Eur Heart J. 2023;44(30):2833–42.
Park DW, et al. 10-Year outcomes of stents Versus Coronary Artery Bypass Grafting for Left Main Coronary Artery Disease. J Am Coll Cardiol. 2018;72(23 Pt A):2813–22.
Lawton JS, et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery revascularization: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice guidelines. J Am Coll Cardiol. 2022;79(2):e21–129.
Acknowledgements
We appreciate the Tehran Heart Center Research Institute for supporting us in conducting this review.
Funding
None.
Author information
Authors and Affiliations
Contributions
A.J. and A.S. conceptualized the review and registered the protocol. A.H. and S.N. searched the online databases. S.N., M.S.N., Z.K., and M.D. screened and selected the studies. A.H. and M.D. extracted the data. S.N. and M.S.N. performed the risk of bias assessment. S.N. performed the meta-analysis and designed the plots. A.J., A.H., and M.S.N. drafted the primary manuscript. A.J., A.S., S.N., A.H., M.S.N., Z.K., and M.D. revised and confirmed the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Jalali, A., Hassanzadeh, A., Najafi, M.S. et al. Predictors of major adverse cardiac and cerebrovascular events after percutaneous coronary intervention in older adults: a systematic review and meta-analysis. BMC Geriatr 24, 337 (2024). https://doi.org/10.1186/s12877-024-04896-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12877-024-04896-4