Abstract
Background
Most epidemiological studies have not systematically identified or categorized risk factors for urinary incontinence (UI) in older men, despite a higher prevalence than in younger men. Considering the burden of UI, an understanding of risk factors can inform cost-effective prevention/treatment programs. This scoping review aimed to identify and categorise risk factors for UI in older men, identify gaps in the evidence, and opportunities for future research.
Methods
The Joanna Briggs Institute (JBI) method for scoping reviews guided the conduct and reporting of this review alongside the Preferred Reporting Items for Systematic Reviews and Meta-analyses extension for scoping reviews checklist. JBI’s Population, Concept, and Context approach framed the inclusion criteria (all evidence sources on UI risk factors that included older men [65 +]). We employed JBI’s three-step search strategy, which included a limited initial search in Ovid MEDLINE, a detailed comprehensive database search, and a search of reference lists of included studies, Google Scholar and grey literature. There were no restrictions on language, study type, or publication date. Two independent reviewers screened, selected, and extracted eligible studies. Data were analyzed using descriptive statistics and qualitative content analysis.
Results
Forty-seven articles that met the inclusion criteria identified 98 risk factors across six categories. Behavioural risk factors, reported by only two studies, were the least investigated of all the categories, whereas medical factors/diseases were the most investigated. No genetic factors were documented. The top five risk factors were increasing age/advanced age (n = 12), Benign Prostatic Hyperplasia (n = 11), Diabetes Mellitus (n = 11), Detrusor overactivity (n = 10), limitation in physical function/ADL disability (n = 10), increased Body Mass Index (BMI)/overweight/obesity (n = 8), Dementia (n = 8), and Parkinson’s disease (n = 7).
Conclusion
There is a dearth of evidence to describe the role behavioural risk factors have in UI in older men. These factors may play a role in health promotion and disease prevention in this area.
Registration
A protocol detailing the methods was developed and published, and is registered in the Open Science Framework [Feb 07 2023; https://osf.io/xsrge/].
Similar content being viewed by others
Background
The International Continence Society defines urinary incontinence (UI) as the complaint of any involuntary leakage of urine [1]. It affects both men and women of all ages, initially affecting more women than men, but this difference in prevalence decreases in association with increasing age. Moreover, one in three older men have problems maintaining continence [2]. Epidemiological studies suggest that UI prevalence among community-dwelling men ranges between 4.81% and 32.17%, and among older men (defined here as men 65 +) between 21% and 32% [3].
In Canada, UI costs to individuals, employers, and the health care system were calculated at $8.5 billion annually in 2014 [4]. The economic burden of UI in the United States has been estimated at more than $7000 (2009 USD) per individual per year, and it totals at least $39 million for male Medicare beneficiaries over 65 [5]. In 2001, US Census Bureau data estimated that approximately 3.4 million American men over the age of 60, either in the community or in nursing homes, suffered from UI, which was also associated with an increased risk of early death [6].
UI is under-reported and under-treated [6], particularly in older men, and there have been calls for more targeted research focusing on this specific group [7, 8]. A mindset that feminizes urinary incontinence has led to health inequalities and disparities in continence services for men [9], coupled with the fact that men are less likely to seek healthcare in general [10]. Although the impact of UI on health-related quality of life in older men and women is similar, most funded research has focused on women [11].
The limited research on male UI has mainly focused on its prevalence [3, 12, 13] and associated risk factors generally [3, 13, 14]. The majority of epidemiological studies of UI have neither systematically identified nor categorized risk factors for UI in older men.
In light of the medical, psychosocial, and financial burdens of UI, understanding risk factors can inform cost-effective prevention and treatment programs like self-management, a promising and proven intervention for managing chronic conditions [15]. Identifying the factors that can be modified will allow for the development of evidence-based interventions to help older men manage their own UI, a strategy previously found to be effective for women [16, 17]. Self-management intervention packages for men currently focus on uncomplicated lower urinary tract symptoms (LUTS) associated with prostate disease. Due to the heterogeneity of these recommendations [18,19,20], the lack of clarity regarding what might constitute an optimal self-management package, and the need to address the older population specifically [7], a scoping review of risk factors for UI in older men is necessary for a comprehensive mapping of the evidence [21].
Urinary incontinence and risk factors
UI may be classified as potentially reversible or established [22]. Potentially reversible UI has a treatable cause and is more common among hospitalized older patients, and residents in long-term care [22] while established UI is chronic, and it may not be possible to identify a reversible cause. The five major types of established UI are urgency, stress (exertional), overflow, functional (disability associated), and mixed urinary incontinence [23].
Risk factors are characteristics, conditions, behaviours, or exposures that can increase the possibility of disease or injury [24]. Generally, risk factors can be grouped into categories: Behavioural risk factors relate to individuals’ actions, and can be eliminated or modified through lifestyle or behavioural changes [24]. Physiological risk factors are those relating to an individual’s body. They may be influenced by an interplay of genetics, lifestyle, and other broad factors. Demographic risk factors relate to the overall population. Environmental risk factors include social, economic, cultural, political, physical, chemical, and biological factors. Genetic risk factors are based on genetic makeup [24].
Although age groups were not specified, the Sixth International Consultation on Incontinence and the European Association of Urology document some established risk factors predisposing men to UI. They include increasing age, the presence of LUTS, urinary tract infections, functional and cognitive impairment, diabetes, alcohol intake, neurological disorders, and prostatectomy [8, 25]. The aetiology of UI, particularly in older adults, is multifactorial; risk factors coexist and interact to perpetuate the condition [24]. For example, Resnick described the case of an 80-year-old incontinent man whose evaluation confirmed the coexistence of multiple factors including Parkinson’s disease with limited mobility, congestive heart failure, and anticholinergic (haloperidol) use that caused faecal impaction and urinary retention and caused discomfort and confusion [22].
As part of a larger study, this scoping review aimed to synthesise evidence on risk factors as the starting point in the creation of a self-management intervention targeting older men [21].
Objectives
This scoping review aimed to identify and categorise risk factors for UI in older men and identify gaps in the evidence. The overarching question addressed was “what are the risk factors for urinary incontinence in older men?”.
Methods
This review was conducted in accordance with the Joanna Briggs Institute (JBI) method for scoping reviews [26], and reported in line with the Preferred Reporting Items for Systematic Reviews and Meta-analyses extension for scoping reviews (PRISMA-ScR) checklist [27]. A protocol detailing the methods was developed and published [21], and is registered in the Open Science Framework [Feb 07 2023; https://osf.io/xsrge/].
Eligibility criteria
JBI’s Population, Concept, Context (PCC) framework, which highlights the relevant characteristics of the review’s participants (older men 65 +), the concept (UI risk factors), and refines the scope of the review by specifying a context (settings for older men), was used to develop the eligibility criteria. The PCC framework is detailed in the review protocol [21]. In brief, all data on UI risk factors stratified by age and sex, data on UI risk factors of 65 + males and females stratified by sex, male UI risk factors stratified by age, and data on UI risk factors of 65 + men only were included. The concept of risk factors for urinary incontinence was examined in all settings (community, acute care, post-acute care and continuing care). The sources eligible for inclusion were all study designs, including grey literature, without restrictions on publication date. For languages other than English, which comprised 10% of search results, we compared translations from two validated online language translators; DeepL translator and Google translator (https://www.deepl.com/en/translator and https://translate.google.com/).
Search strategy
Following the JBI method, the three-step search strategy comprised an initial search in Ovid MEDLINE on May 24, 2022, a detailed search in all included databases on May 28, 2022, and lastly, a search of reference lists of included studies in February and March 2023. The medical librarian (JYK) developed and executed comprehensive searches over 4 h in Ovid MEDLINE, Ovid Embase, CINAHL, Scopus, Web of Science Core Collection, Cochrane Library (via Wiley), and ProQuest Dissertations & Theses Global. Keywords and controlled vocabulary were carefully selected to capture all relevant literature pertaining to risk factors for UI in older men. Appendix I (Additional file 1) describes full-text search strategies. Relevant studies published since the inception of the databases to the date of the detailed search were included. In addition to subscription databases, the research team reviewed the first 200 results from Google Scholar for inclusion. This is a reasonable number of results to screen since Web of Science and Google Scholar overlap heavily [28]. In the third step, bibliographies of the included studies were reviewed, as well as grey literature. When searching for grey literature in electronic format at different points in the review process, we used Google and websites of national and international organizations addressing the subject matter. All identified citations from the subscription databases were imported into Covidence (Veritas Health Innovation Ltd, Melbourne); a web-based collaboration software platform that streamlines the production of systematic and other literature reviews [29]. Following automatic removal of duplicates, two reviewers (OO and BO) independently screened all titles and abstracts identified with our literature search, after pilot-testing with a random sample of 5% of studies, which showed an almost perfect inter-reviewer agreement [30] (Cohen kappa coefficient; κ value) of 0.898. The Covidence database indicated moderate inter-reviewer reliability (κ value = 0.709) based on full text review. Potential reasons for exclusion were defined a priori, categorised, recorded, and reported in the scoping review. The full text of included citations was assessed in detail against the inclusion criteria by two independent reviewers (OO and BO). Conflicts detected by Covidence during the selection process were resolved through discussion and consensus.
Data extraction
A customisable Covidence structure was used to develop the data extraction form (Appendix II/Additional file 1). Two reviewers (OO and AW) checked the draft extraction form through a calibration exercise to ensure the form captured all relevant data. The draft data extraction tool was modified and revised as necessary. Studies from Google Scholar and other sources were analysed and manually incorporated into the consensus data downloaded from Covidence. Table 1 summarizes the scoping review process and timelines.
Risk of bias
Following the JBI guidance, no quality appraisal was conducted, since the objective was to map the body of evidence without restriction in order to gain a deeper understanding and identify gaps, without testing hypotheses or trying to influence policy or practice [26].
Data analysis and presentation
Using a predetermined framework, we extracted and analysed data deductively. The data were analysed qualitatively and quantitatively, using qualitative content analysis and descriptive statistics respectively. Results were stratified by the economic status of the country where the study was conducted, ethnicity/race, health context, inclusion criteria, types of UI and categories of risk factors. Tables, charts, and figures are employed to present quantitative data while qualitative data are organised into categories and presented as narrative summaries.
Results/evidence synthesis
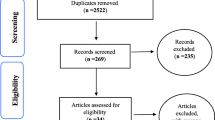
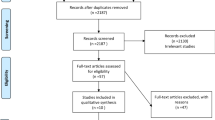
Forty-seven articles met the inclusion criteria for this review. Among the 491 eligible articles, 331 (67.4%) were excluded due to the lack of stratification of UI risk factors by age, sex, or age and sex, making them ineligible. Figure 1 shows the detailed selection process and exclusion reasons.
Characteristics of the included studies
In Table 2, thirty-seven (79%) of the included articles were primary research articles. Of the 47 evidence sources, 21 (45%) were cross-sectional studies, 12 (26%) were cohort studies, 9 (19%) were review articles and the rest included an experimental study (2%), case series (n = 2, 4%), a report summary (2%) and a prevalence study (2%). Eighteen (38%) evidence sources were published in the past 10 years.
Primary evidence sources were sparsely distributed across 12 of 195 countries. North America contributed almost half of the studies (n = 2, 5.3% from Canada and n = 15, 39.5% from the United States). Ten articles (26.3%) came from four Asian countries (China, Japan, Taiwan, and Singapore), and 10 articles (26.3%) came from six European countries (Austria, Finland, Italy, Romania, Spain, and the United Kingdom). None were from African and South American countries (Fig. 2).
Figure 3 shows that the majority (n = 29, 62%) of evidence sources were sex-stratified combined risk factor datasets for men and women 65 years and older, whereas one third focused solely on older men. Only a small percentage of included studies (n = 1, 2%) reported age-stratified data only in male samples.
Twenty-one (45%) evidence sources were community-based studies, while eight (17%) and six (13%) originated from samples from multiple settings and tertiary care facilities. Evidence from primary care settings was the least (n = 1, 2%) in Fig. 4.
Risk factors for UI in older men
Table 3 shows the details of 98 risk factors identified across six categories. A total of four behavioural risk factors, reported by only two studies, were the least investigated of all examined by these evidence sources, whereas 34 medical factors/diseases (with 111 frequency counts) were identified from 39 articles (83% of evidence sources). A total of 34 risk factors belonging to the other factors category were reported from 29 studies (62%) and were mostly medically-related entities that were not disease diagnoses. Nine physiological risk factors/age-related physiological changes were found in five studies (11%) with a frequency of 13. Four demographic factors with a frequency count of 15 were found in 14 studies (30%) and 13 environmental factors with a frequency of 18 were reported in eight studies (17%). Genetic factors were not documented. Figure 5 shows frequency counts across categories.
The top five risk factors were increasing age/advanced age (n = 12), Benign Prostatic Hyperplasia (n = 11), Diabetes Mellitus (n = 11), Detrusor overactivity (n = 10), limitation in physical function/ADL disability (n = 10), increased Body Mass Index (BMI)/overweight/obesity (n = 8), Dementia (n = 8), and Parkinson’s disease (n = 7).
For qualitative content analysis, findings are organised into categories according to the PCC framework.
UI risk factors and contexts
Five of the 20 articles focusing exclusively on the community setting suggested that age was a significant demographic risk factor [36, 46, 58, 60, 65]. Five community-based studies documented urgency UI (UUI) as the most common established UI type [31, 32, 65, 67, 68], while stress UI (SUI) was less prevalent [42, 46, 47, 69]. Physiological factors associated with UUI included increased fat mass (participants’ mean total fat mass = 24 kg), greater waist circumference (mean waist circumference = 100.6 cm) and decreased grip strength (5% or greater decrease in maximum grip strength) [31]. In this longitudinal study, higher fat mass percent and greater waist circumference were marginally associated with prevalent UI at least monthly, but strongly associated with prevalent UI at least weekly [31]. An association between UUI and medical factors/diseases (diabetes, heart disease, anxiety, depression, constipation, and brain injury) was found in a recent community-based study [32]. Gerst et al. found that prostate problems (unspecified), a higher number of comorbid conditions (mean number of chronic conditions = 2.5) and other factors such as limitations in Activities of Daily Living (ADL) were significant independent predictors of UUI [65]. UUI was also linked to frailty, faecal incontinence and depressive symptoms [67]. Increased BMI/overweight/obesity was a common medical factor in community-based studies [31, 32, 45,46,47, 68], whereas hypertension was less common [32, 68]. Tsui et al. identified increased BMI and high blood pressure as vascular risk factors for UUI [68]. At 43%, UUI was the most frequently documented type of established UI in this review [31,32,33, 40, 42, 46,47,48, 51, 54, 59, 65, 67,68,69, 72, 73, 75]. Detrusor overactivity (DO) ranked first in the other medically-related risk factors category/third overall [35, 39, 40, 51, 54, 55, 57, 71, 72, 74] and was associated with UUI [40, 51, 72] and nocturnal enuresis [39].
Identified factors associated with SUI included Diabetes mellitus [42, 46], heart disease (unspecified), poor vision and faecal incontinence [69].
Increased BMI [46, 47], increasing age [46], and other factors including poor physical function (indicated by lower Activities of Daily Living Scale (ADLS) scores) and poor sleep quality (higher Pittsburgh Sleep Quality Index (PSQI) scores) were also associated with SUI [69].
Four included studies focused exclusively on nursing homes (NH) [34, 35, 61, 64], and documented environmental risk factors such as poor lighting, cold weather, lack of commodes, use of cot-sides/bedrails, reliance on draw sheets and pads, and forgotten call bells [35]. UI was also correlated with medical factors/diseases including Dementia [34, 35], UTI, BPH/bladder stones, Stroke, Parkinson’s disease (PD), faecal impaction [35], and depressive symptoms [64]. Race, as a demographic factor [61], was reported along with other factors like medications [35], poor physical function and poor cognitive status [64]. African-Americans, especially African-American men, had higher UI odds [61]. The development of nocturnal enuresis was associated with age-related physiological changes related to detrusor instability/overactivity, reduction in functional bladder capacity, increased post-void residual volume, renal function decline, increased night-time urine production, decreased awareness of bladder filling, and decreased bladder emptying efficiency [35]. Diabetes was frequently correlated with UI in geriatric care facilities [42].
Studies in tertiary healthcare facilities identified behavioural, demographic [49], disease-related [49, 52], and other factors [50]. These are detailed below in relation to their corresponding patient characteristics.
UI risk factors and population characteristics
Among older men with frailty, Landi et al. found that urinary tract infection, physical restraints, and environmental barriers were potentially reversible risk factors. Non-reversible UI risk factors included advanced age, physical limitations, cognitive impairment, and diabetes mellitus [58]. Diabetes was also a primary risk factor for urinary and faecal incontinence among the oldest old men in a Canadian longitudinal study [76].
Among men with BPH, Prada et al. identified tobacco smoking and alcohol use, urban dwelling and occupation (work requiring a high degree of physical effort and jobs that require sitting for longer periods), and medical/disease-related factors, such as Heart failure, Diabetes mellitus and having at least three other comorbidities [49].
Among men following post-robotic radical prostatectomy, myosteatosis (low average total psoas density), low obturator internus muscle thickness and short membranous urethral length were recently reported by Yamashita et al., with myosteatosis being considered a novel predictor of post-prostatectomy incontinence [50]. In a cohort of older men with SUI, the majority of whom were offered prostatectomy for prostate cancer, male SUI was associated with multi-morbidity, functional dependence, and frailty [77].
Among men undergoing artificial urethral sphincter (AUS) placement post-prostatectomy, low serum testosterone was reported as a risk factor for stress UI [44]. In community-dwelling older male cancer patients, diagnoses of prostate and bladder cancers had the strongest associations with UI, compared to colorectal and lung cancers [43]. Furthermore, Kopp et al. identified prostatectomy, post-radiation therapy, observation/watchful waiting in prostate cancer (Ca) and androgen deprivation therapy for prostate Ca as risk factors for post-prostatectomy incontinence among elderly prostate Ca survivors [66]. Moore also described prostatectomy-related neural injury, ischemia during surgery, scar tissue immobilizing the sphincter, short membranous urethral length, surgical technique and preoperative radiotherapy as causative factors for post-prostatectomy incontinence along with increasing age [74].
Among relatively healthy older men, Cheng et al. showed a causal relationship between UI and increasing age, as well as diabetes mellitus [46].
Discussion
In this scoping review, evidence in all contexts was systematically synthesised in relation to older men. Despite the lack of systematically conducted reviews identifying and categorising UI risk factors in older men, we found systematic reviews on UI in nursing home residents as well as on male UI risk factors in general, although uncategorised, with which to compare our findings [3, 78]. Our findings revealed that behavioural risk factors were the least investigated. Lifestyle factors including sedentary behaviour are rarely the focus of UI research [79]. In an effort to fill the evidence gap, Farrés-Godayol and colleagues found that nursing home residents with UI spent significantly more sedentary time compared to continent residents [79]. According to the Seventh International Consultation on Incontinence, rigorous studies on lifestyle interventions are needed [80]. This emphasises the need for more research that explores the breadth of lifestyle/behavioural factors to inform lifestyle interventions.
Age was the top risk factor, consistent with findings from a systematic review of UI and associated risk factors in nursing home residents, which found that age and sex were the most frequently studied risk factors out of 45 from 16 studies [78]. The pooled prevalence of UI increased with age and functional dependency [3]. Many medical conditions have been implicated in the multifactorial aetiology of UI in older adults [40]. Similar to our findings, an association was found between BPH and UI in men aged 60 + [81]. A systematic review also reported associations between UI in community-dwelling men and stroke, diabetes, poor general health, radiation, and prostate cancer surgery, although age groups were not specified [3]. The European Patients’ Academy on Therapeutic Innovation noted the importance of the coexistence of multiple factors, an understanding of which is crucial to evaluating patients and developing relevant interventions for older adults with urinary incontinence effectively [24].
Studies show that DO is the most common cystometric abnormality in patients with PD and is one of the most common forms of urinary dysfunction in people with idiopathic PD (IPD) [82, 83]. The finding of ADL disability is consistent with reports on Hispanic longitudinal data from community-dwelling older adults, which included functional impairment and ageing as risk factors for incident incontinence [84]. A bidirectional relationship between functional decline/disability and UI was described by Coll-Planas et al., in which continence reduction leads to functional decline, and functional decline leads to further continence decline [85]. In other studies, obesity was associated with an increased odds of UI [86]. Depression increased the odds of UI among elderly people in the Brazilian SABE study, and UI prevalence was higher when there was high physical dependence [87]. According to a German UI survey, five or more comorbid conditions increased incontinence risk to 100% [88].
Institutionalisation increased UI prevalence following facility admission [72]. For NH, urinary incontinence is a quality of care indicator and a high prevalence of UI is often regarded as a sign of poor quality care [61, 89].
Strengths and limitations
This scoping review focused on the collation, identification and categorisation of risk factors for UI in older men by mapping and synthesising the breadth of evidence and identifying knowledge gaps in a systematic and comprehensive manner. An exhaustive search across all sources was conducted that produced robust evidence. Study type or publication date was not restricted, and there was no language restriction.
The lack of age stratification in most data on men in general and the paucity of data specifically on older men limited the amount of eligible evidence sources.
Conclusions
This scoping review found limited evidence on factors, other than those related to medical diagnoses, that might contribute to UI in older men. Available data are limited by the inability to extract data specific to older men, rather than men or older adults in general. The primary evidence sources originated from only 6% of countries in the world, making generalisations difficult.
Recommendations for future studies
There is a need for more primary research focusing on behavioural risk factors for UI in older men due to the lack of evidence on this topic. These factors may play a role in health promotion and disease prevention in this area. It is imperative that more UI research be conducted in areas where there are no existing data on the topic. More research should be encouraged in primary care settings since primary care is the first point of care for the vast majority of patients.
Availability of data and materials
A reasonable request may be made to the corresponding author to obtain the dataset used in this study.
References
D’Ancona C, Haylen B, Oelke M, Abranches-Monteiro L, Arnold E, Goldman H, et al. The International Continence Society (ICS) report on the terminology for adult male lower urinary tract and pelvic floor symptoms and dysfunction. Neurourol Urodyn. 2019;38(2):433–77.
Yates A. Addressing the gender gap in urinary continence care. Br J Community Nurs. 2021;26(5):228–34.
Shamliyan TA, Wyman JF, Ping R, Wilt TJ, Kane RL. Male urinary incontinence: prevalence, risk factors, and preventive interventions. Rev Urol. 2009;11(3):145–65.
The Canadian Continence Foundation. The impact of incontinence in Canada. A briefing document for policy makers. 2014. Available from: https://www.canadiancontinence.ca/pdfs/en-impact-of-incontinence-in-canada-2014.pdf.
Stothers L, Thom D, Calhoun E. Urologic diseases in america project: Urinary incontinence in males—Demographics and economic burden. J Urol. 2005;173(4):1302–8. https://doi.org/10.1097/01.ju.0000155503.12545.4e.
Johnson TM, Bernard SL, Kincade JE, Defriese GH. Urinary incontinence and risk of death among community-living elderly people: results from the National Survey on Self-Care and Aging. J Aging Health. 2000;12(1):25–46.
Wagg A. Male overactive bladder: underappreciated, under-researched. More please? Eur Urol. 2021;79(4):505–6.
Abrams,P, Cardozo, L, Wagg, A, Wein, A. (Eds) Incontinence 6th Edition. Bristol UK: ICI-ICS. International Continence Society. 2017. ISBN: 978-0956960733.
Nursing Times. Best practice: identifying and managing male incontinence problems. Available from: https://tinyurl.com/nk5ykvyp. Cited 2023 Mar 15.
Lancet T. Raising the profile of men’s health. Lancet. 2019;394(10211):1779. https://doi.org/10.1016/S0140-6736(19)32759-X.
Moore KN, Gray M. Urinary incontinence in men: current status and future directions. Nurs Res. 2004;53(6 Suppl):S36-41.
Herschorn S, Gajewski J, Schulz J, Corcos J. A population-based study of urinary symptoms and incontinence: the Canadian Urinary Bladder Survey. BJU Int. 2007;22(5):52–8.
Abrams P, Cardozo L, Khoury S, Wein A, editors. Incontinence. 5th ed. 2013. Available from: https://www.ics.org/Publications/ICI_5/INCONTINENCE.pdf.
Fernández-Cuadros ME, Nieto-Blasco J, Geanini-Yagüez A, Ciprián-Nieto D, Padilla-Fernández B, Lorenzo-Gómez MF. Male urinary incontinence: associated risk factors and electromyography biofeedback results in quality of life. Am J Mens Health. 2016;10(6):NP127-35.
Grady PA, Gough LL. Self-management: a comprehensive approach to management of chronic conditions. Am J Public Health. 2014;104(8):e25-31.
Tannenbaum C, van den Heuvel E, Fritel X, Southall K, Jutai J, Rajabali S, et al. Continence Across Continents To Upend Stigma and Dependency (CACTUS-D): study protocol for a cluster randomized controlled trial. Trials. 2015;16(1):565.
Holroyd-Leduc JM, Straus S, Thorpe K, Davis DA, Schmaltz H, Tannenbaum C. Translation of evidence into a self-management tool for use by women with urinary incontinence†. Age Ageing. 2011;40(2):227–33.
Albarqouni L, Sanders S, Clark J, Tikkinen KAO, Glasziou P. Self-management for men with lower urinary tract symptoms: a systematic review and meta-analysis. Ann Fam Med. 2021;19(2):157–67.
Brown CT, Yap T, Cromwell DA, Rixon L, Steed L, Mulligan K, et al. Self management for men with lower urinary tract symptoms: randomised controlled trial. BMJ. 2007;334(7583):25.
Brown CT, van der Meulen J, Mundy AR, O’Flynn E, Emberton M. Defining the components of a self-management programme for men with uncomplicated lower urinary tract symptoms: a consensus approach. Eur Urol. 2004;46(2):254–62 discussion 263.
Olagundoye O, Kung JY, Gibson W, Wagg A. Urinary incontinence in older men: protocol for a scoping review of risk factors. BMJ Open. 2023;13(2):e068956.
Resnick NM. Voiding dysfunction and urinary incontinence. In: Cassel CK, Riesenberg DE, Sorensen LB, Walsh JR, editors. Geriatric medicine. New York: Springer New York; 1990. p. 501–18. Available from: https://link.springer.com/10.1007/978-1-4757-2093-8_38. Cited 2023 Apr 4.
Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Neurourol Urodyn. 2002;21(2):167–78.
EUPATI Open Classroom. Risk factors in health and disease. Available from: https://learning.eupati.eu/mod/book/view.php?id=215. Cited 2022 Aug 17.
Thüroff JW, Abrams P, Andersson KE, Artibani W, Chapple CR, Drake MJ, et al. EAU guidelines on urinary incontinence. Eur Urol. 2011;59(3):387–400.
Peters M, Godfrey C, McInerney P, Munn Z, Trico A, Khalil H. Chapter 11: Scoping Reviews. In: Aromataris E, Munn Z, editors. JBI Manual for Evidence Synthesis. JBI; 2020. Available from: https://wiki.jbi.global/display/MANUAL/Chapter+11%3A+Scoping+reviews. Cited 2022 Sep 27.
Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467–73.
Haddaway NR, Collins AM, Coughlin D, Kirk S. The role of google scholar in evidence reviews and its applicability to grey literature searching. Wray KB, editor. PLoS One. 2015;10(9):e0138237.
Covidence systematic review software. Melbourne: Veritas Health Innovation. Available from: www.covidence.org.
McHugh ML. Interrater reliability: the kappa statistic. Biochem Medica. 2012;22(3):276–82.
Bauer S, Grimes B, Suskind A, Cawthon P, Cummings S, et al. Urinary incontinence and nocturia in older men: associations with body mass, composition and strength in the health ABC study. J Urol. 2019;202(5):1017–22.
Zhang Y, Wang X, Song Y, Peng R, Tang T, Li M, et al. Epidemiology of frequent/urgent urination in older adults in China: a multicenter, cross-sectional study. Front Public Health. 2021;9:669070.
Nuotio M, Jylhä M, Luukkaala T, Tammela TLJ. Urgency, urge incontinence ana voiding symptoms in men and women aged 70 years and over. BJU Int. 2002;89(4):350–5.
Palmer MH. Urinary continence status of newly admitted elderly nursing home residents: a longitudinal study over a one year period. Johns Hopkins University; 1990. Available from: https://login.ezproxy.library.ualberta.ca/login?url=https://search.ebscohost.com/login.aspx?direct=true&db=rzh&AN=109869530&site=ehost-live&scope=site.
Nazarko L. Treatment of nocturnal enuresis in nursing homes. Prof Nurse. 1995;10(10):657–60.
Maggi S, Minicuci N, Langlois J, Pavan M, Enzi G, Crepaldi G. Prevalence rate of urinary incontinence in community-dwelling elderly individuals: the Veneto study. J Gerontol - Ser Biol Sci Med Sci. 2001;56(1):M14–8.
Ostbye T, Hunskaar S, Sykes E. Predictors and incidence of urinary incontinence in elderly Canadians with and without dementia - a five-year follow up: the Canadian Study of Health and Aging. Can J Aging. 2002;21(1):95–102.
Zunzunegui Pastor MV, Rodriguez-Lasob A, Garcia de Yebenes MJ, Aguilar Conesa MD, Lazaro y de Mercado P, Otero Puime A. Prevalence of urinary incontinence and linked factors in men and women over 65. Aten Primaria. 2003;32(6):337–42.
Lin ADY, Lin ATL, Chen KK, Chang LSS. Nocturnal enuresis in older adults. J Chin Med Assoc. 2004;67(3):136–40.
Madersbacher H, Madersbacher S. Men’s bladder health: urinary incontinence in the elderly (Part I). J Mens Health Gend. 2005;2(1):31–7.
Matsukawa Y, Hattori R, Komatsu T, Funahashi Y, Mizutani K, Gotoh M. Declined urethral sphincter function related to aging comtributes to urinary incontinence after radical prostatectomy. Neurourol Urodyn. 2009;28(7):692.
Wehrberger C, Madersbacher S, Jungwirth S, Fischer P, Tragl KH. Lower urinary tract symptoms and urinary incontinence in a population-based 85-years old cohort. Eur Urol Suppl. 2012;11(1):e581.
White AJ, Reeve BB, Chen RC, Stover AM, Irwin DE. Urinary incontinence and health-related quality of life among older Americans with and without cancer: a cross-sectional study. BMC Cancer. 2013;13:377 (White, Irwin) Departments of Epidemiology, University of North Carolina, Chapel Hill, NC, United States (Reeve, Chen, Stover, Irwin) Lineberger Comprehensive Cancer Center, University of North Carolina, Chapel Hill, NC, United States (Reeve) Health Policy.
McKibben MJ, Fuentes J, Shakir N, Fuchs JS, Viers BR, Pagliara TJ, et al. Low serum testosterone is present in nearly half of men undergoing artificial urinary sphincter placement. Urology. 2018;118:208–12.
Yang B, Lee DR, Lo JC, Gordon NP. A cross-sectional survey of urinary incontinence among adults age 65–79 years. J Am Geriatr Soc. 2019;67(Supplement 1):S302.
Cheng J, Simons K, Crozier J, Kew N, Danny L, Mcneil J, et al. Urinary incontinence in healthy Australian adults over 70: prevalence and causal associations. BJU Int. 2020;125(Supplement 1):9.
Cheng J, Koen S, Crozier J, Liew D, McNeil J, O’Connell H. Impact of body mass index (BMI) on urinary incontinence in community dwelling Australian adults aged 70 years and above. Neurourol Urodyn. 2021;40(SUPPL 2):S127–8.
Tse WY, Ch Siu D, Yeung CK, Chung KW, Leung SY, Hui EMT. Prevalence of urinary incontinence in Chinese elderly male in primary caresetting and their quality of life in Hong Kong. Hong Kong Pract. 2021;43(2):35–45.
Prada GI, Nacu RM, Bajenaru OL, Nuta CR, Kozma A, Alexa ID, et al. Benign prostate hypertrophy and urinary incontinence in older people. Eur Geriatr Med. 2021;12(SUPPL 1):S371.
Yamashita S, Kawabata H, Deguchi R, Ueda Y, Higuchi M, Muraoka S, et al. Myosteatosis as a novel predictor of urinary incontinence after robot-assisted radical prostatectomy. Int J Urol. 2022;29(1):34–40.
Ouslander JG. Urinary incontinence in the elderly. West J Med. 1981;135(6):482–91.
Murray PK. Cervical spondylotic myelopathy: a cause of gait disturbance and urinary incontinence in older persons. J Am Geriatr Soc. 1984;32(4):324–30.
Resnick NM, Yalla SV. Detrusor hyperactivity with impaired contractile function. An unrecognized but common cause of incontinence in elderly patients. JAMA. 1987;257(22):3076–81.
Chan KM, Lim PH, Chee YC, Jayaratnam FJ. Urinary symptoms and urodynamic diagnosis of patients in one geriatric department. Ann Acad Med Singapore. 1992;21(6):813–7.
Anonymous. Incontinence. Causes, management and provision of services. A working party of the Royal College of Physicians. J R Coll Physicians Lond. 1995;29(4):272–4.
Wetle T, Scherr P, Branch LG, Resnick NM, Harris T, Evans D, et al. Difficulty with holding urine among older persons in a geographically defined community: prevalence and correlates. J Am Geriatr Soc. 1995;43(4):349–55.
Heath T, Watson R. The causes of urinary incontinence in men. Nurs Older People. 2002;14(6):15–9.
Landi F, Cesari M, Russo A, Onder G, Lattanzio F, Bernabei R, et al. Potentially reversible risk factors and urinary incontinence in frail older people living in community. Age Ageing. 2003;32(2):194–9.
Nuotio M, Jylha M, Luukkaala T, Tammela TLJ. Urinary incontinence in a Finnish population aged 70 and over. Prevalence of types, associated factors and self-reported treatments. Scand J Prim Health Care. 2003;21(3):182–7.
Kim H, Yoshida H, Hu X, Yukawa H, Shinkai S, Kumagai S, et al. Risk factors associated with onset of urinary incontinence in a community-dwelling elderly population: a 4-year follow-up study. Nihon Koshu Eisei Zasshi. 2004;51(8):612–22.
Boyington JEA, Howard DL, Carter-Edwards L, Gooden KM, Erdem N, Jallah Y, et al. Differences in resident characteristics and prevalence of urinary incontinence in nursing homes in the southeastern United States. Nurs Res. 2007;56(2):97–107.
Markland AD, Goode PS, Burgio KL, Redden DT, Richter HE, Sawyer P, et al. Correlates of urinary, fecal, and dual incontinence in older African-American and white men and women. J Am Geriatr Soc. 2008;56(2):285–90.
Goode PS, Burgio KL, Redden DT, Markland A, Richter HE, Sawyer P, et al. Population based study of incidence and predictors of urinary incontinence in black and white older adults. J Urol. 2008;179(4):1449–54.
Chen YM, Hwang SJ, Chen LK, Chen DY, Lan CF. Urinary incontinence among institutionalized oldest old Chinese men in Taiwan. Neurourol Urodyn. 2009;28(4):335–8.
Gerst K, Ray LA, Samper-Ternent R, Espino DV, Markides KS. Self-reported urge urinary incontinence (UUI) among older Mexican-American men: risk factors and psycho-social consequences. J Immigr Minor Health. 2011;13(6):1110–5.
Kopp RP, Marshall LM, Wang PY, Bauer DC, Barrett-Connor E, Parsons JK. The burden of urinary incontinence and urinary bother among elderly prostate cancer survivors. Eur Urol. 2013;64(4):672–9.
Wang CJ, Hung CH, Tang TC, Chen LY, Peng LN, Hsiao FY, et al. Urinary incontinence and its association with frailty among men aged 80 years or older in Taiwan: a cross-sectional study. Rejuvenation Res. 2017;20(2):111–7.
Tsui A, Kuh D, Cardozo L, Davis D. Vascular risk factors for male and female urgency urinary incontinence at age 68 years from a British birth cohort study. BJU Int. 2018;122(1):118–25.
Luo Y, Zou P, Wang K, Li X, Wang J. Prevalence and risk factors of urinary incontinence among elderly adults in rural china: a cross-sectional survey. J Wound Ostomy Cont Nurs. 2022;49(1):78–86.
Bauer SR, Jin C, Kamal P, Suskind AM. Association between lower urinary tract symptoms and frailty in older men presenting for urologic care. Urology. 2021;148:230–4.
Hester AG, Kretschmer A, Badlani G. Male Incontinence: the etiology or basis of treatment. Eur Urol Focus. 2017;3(4–5):377–84.
Griebling TL. Urinary incontinence and voiding dysfunction in elderly men. Curr Bladder Dysfunct Rep. 2008;3(4):241–6.
Miller SW, Miller MS. Urological disorders in men: urinary incontinence and benign prostatic hyperplasia. J Pharm Pract. 2011;24(4):374–85.
Moore K. A review of the anatomy of the male continence mechanism and the cause of urinary incontinence after prostatectomy. J Wound Ostomy Continence Nurs. 1999;26(2):86–93.
Neki N. Urinary incontinence in elderly. J Krishna Inst Med Sci Univ. 2016;5(1):5–13.
Østbye T, Seim A, Krause KM, Feightner J, Hachinski V, Sykes E, et al. A 10-year follow-up of urinary and fecal incontinence among the oldest old in the community: the Canadian Study of Health and Aging. Can J Aging. 2004;23(4):319–31.
Hampson LA, Suskind AM, Breyer BN, Lai L, Cooperberg MR, Sudore RL, et al. Understanding the health characteristics and treatment choices of older men with stress urinary incontinence. Urology. 2021;154:281–7.
Offermans MPW, Du Moulin MFMT, Hamers JPH, Dassen T, Halfens RJG. Prevalence of urinary incontinence and associated risk factors in nursing home residents: a systematic review. Neurourol Urodyn. 2009;28(4):288–94.
Farrés-Godayol P, Jerez-Roig J, Minobes-Molina E, Yildirim M, Goutan-Roura E, Coll-Planas L, et al. Urinary incontinence and sedentary behaviour in nursing home residents in Osona, Catalonia: protocol for the OsoNaH project, a multicentre observational study. BMJ Open. 2021;11(4):e041152.
Cardozo L, Rovner E, Wagg A, Wein A, Abrams P. (Eds) Incontinence 7th Edition (2023). Bristol UK: ICI-ICS. International Continence Society. ISBN: 978-0-9569607-4-0.
Park J, Son Hong GR. Association of functional ability and benign prostatic hyperplasia with urinary incontinence in older Korean men. Int Neurourol J. 2016;20(2):137–42.
Yeo L, Singh R, Gundeti M, Barua JM, Masood J. Urinary tract dysfunction in Parkinson’s disease: a review. Int Urol Nephrol. 2012;44(2):415–24.
Blackett H, Walker R, Wood B. Urinary dysfunction in Parkinson’s disease: a review. Parkinsonism Relat Disord. 2009;15(2):81–7.
Miles TP, Palmer RF, Espino DV, Mouton CP, Lichtenstein MJ, Markides KS. New-onset incontinence and markers of frailty: data from the Hispanic established populations for epidemiologic studies of the elderly. J Gerontol A Biol Sci Med Sci. 2001;56(1):M19-24.
Coll-Planas L, Denkinger MD, Nikolaus T. Relationship of urinary incontinence and late-life disability: implications for clinical work and research in geriatrics. Z Gerontol Geriatr. 2008;41(4):283–90.
Muscatello DJ, Rissel C, Szonyi G. Urinary symptoms and incontinence in an urban community: prevalence and associated factors in older men and women. Intern Med J. 2001;31(3):151–60.
Tamanini JTN, Lebrão ML, Duarte YAO, Santos JLF, Laurenti R. Analysis of the prevalence of and factors associated with urinary incontinence among elderly people in the Municipality of São Paulo, Brazil: SABE Study (Health, Wellbeing and Aging). Cad Saúde Pública. 2009;25(8):1756–62.
Welz-Barth A, Füsgen I, Melchior HJ. 1999 rerun of the 1996 German urinary incontinence survey: will doctors ever ask? World J Urol. 2000;18(6):436–8.
Schnelle JF, Cadogan MP, Yoshii J, Al-Samarrai NR, Osterweil D, Bates-Jensen BM, et al. The minimum data set urinary incontinence quality indicators: do they reflect differences in care processes related to incontinence? Med Care. 2003;41(8):909–22.
Acknowledgements
We appreciate Dr. Andrea C. Tricco for sharing resources on the JBI method for scoping reviews.
Funding
The review has received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
AW and OO conceptualized the study and contributed to the analysis and interpretation of the data. OO, AW and WG drafted and critically reviewed the manuscript for its intellectual content. OO, BO and JYK were involved in data acquisition. JYK and BO provided technical/material support, while OO handled statistical analysis. All authors are responsible for the overall content as guarantors.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not required.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
APPENDICES: Search strategies, data extraction form and PRISMA-ScR checklist.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Olagundoye, O., Odusanya, B., Kung, J.Y. et al. A scoping review of risk factors for urinary incontinence in older men. BMC Geriatr 23, 534 (2023). https://doi.org/10.1186/s12877-023-04249-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12877-023-04249-7