Abstract
Background
Pneumonia is a very common infection in the cognitively impaired adult population, often leading to long-term deterioration, in physical and cognitive performance. Evidence is lacking on whether chronic comorbidities and drug use are risk factors for pneumonia in persons with Alzheimer’s disease (AD). The objective of this study was to investigate the risk factors of pneumonia in community dwellers with and without AD.
Methods
We performed a retrospective register-based study utilizing the Medication Use and Alzheimer’s disease (MEDALZ) cohort, which is based on Finnish nationwide healthcare registers and includes all community dwellers who received a verified clinical diagnosis of AD between 2005 to 2011. This study comprised 69,350 persons with AD and 69,350 persons without AD matched by age, gender, and region of residence. Association between comorbidities, drug use, and hospitalization due to pneumonia were assessed using Cox Regression.
Results
During the follow-up, 25.0% (n = 17,105) of the AD cohort and 15.8% (n = 10,966) of the non-AD cohort were hospitalized due to pneumonia. Persons with AD had a higher risk of pneumonia also after adjusting for comorbidities (HR 1.76, 95% CI 1.71–1.80). Previous pneumonia was the strongest risk factor for pneumonia in both cohorts. All comorbidities and drug use excluding biological product use were associated with a higher risk of pneumonia, but stronger associations were observed in the non-AD cohort. The risk of hospitalization following psychotropic drug use was proportional to the number of psychotropics utilized.
Conclusions
Pneumonia is a serious, potentially life-threatening illness, and risk factors for pneumonia include several potentially avoidable drugs. In addition, good care of existing comorbidities might prevent pneumonia and related hospitalization.
Similar content being viewed by others
Background
Pneumonia-related hospitalizations have been increasing during the last decades, especially in older adults [1,2,3]. This is explained, at least partly, by comorbidities and recurrent cases of pneumonia [1, 2]. Several comorbid conditions are known to predispose to pneumonia [4, 5] as well as the use of, for example, immunosuppressants [4, 6], antipsychotics [7], and benzodiazepines [8]. The relationship between proton pump inhibitors and pneumonia has been debated [9, 10].
The incidence of community-acquired pneumonia (CAP) requiring hospitalization ranges from 267 per 100 000 in the adult population to 1012 per 100,000 for persons over 65 years old [11]. In European studies, the estimated annual cost of pneumonia-related hospitalization in all age groups ranges from €2707 ($3068) to €4000 ($4533) per person [12,13,14]. According to previous studies, delirium at admission is more common in older adults who are admitted for pneumonia than for matched controls who are admitted for other causes [15].
People with cognitive disorders have a higher hospital admission rate for bacterial pneumonia (34 per 1000 person-years) than those without cognitive disorders (10 per 1000 person-years) [16]. Among persons with cognitive disorders, infections more often result in delirium [17] and may cause further cognitive and functional decline [18, 19]. Persons with cognitive disorders have been found to have more comorbidities than their peers, the most common comorbidities being hypertension, coronary artery disease, stroke, diabetes, and asthma [20,21,22]. Moreover, cognitive disorder is a risk factor for aspiration pneumonia [23] Previous studies have shown that persons with cognitive disorders have higher mortality within 30 days after pneumonia than those without cognitive disorders [24].
Evidence is lacking on whether comorbidities and utilization of psychotropics, proton pump inhibitors, oral glucocorticoids, or biological products, are risk factors for pneumonia in persons with and without Alzheimer’s disease. To our best knowledge, there are no previous studies on risk factors of pneumonia in community dwellers with Alzheimer’s disease (AD). Thus, we studied the pneumonia risk factors in community dwellers with and without Alzheimer’s disease.
Methods
Study population
The MEDALZ (Medication use and Alzheimer’s disease) is a study based on national healthcare registers. It includes 70,718 all community dwellers who have received a clinical diagnosis of AD from 2005 to 2011 in Finland [25]. To identify the persons with AD, we utilized a nationwide Special Reimbursement Register, maintained by the Social Insurance Institution of Finland (SII). This register contains information on entitlement to special reimbursements of drugs for chronic illnesses. SII centrally reviews and confirms the AD diagnosis, which was based on the NINCDS-ADRDA [26] and DMS-IV [27] criteria and includes a computed tomography or magnetic resonance imaging scan and confirmation of the diagnosis by a neurologist or a geriatrician [28]. In this study, each person with AD was matched with a comparison person without AD according to age (± one year), gender, and region of residence at the date of AD diagnosis. The matching was done with incidence density sampling without replacement. This date was assigned as an index date for the persons with AD and the comparison persons.
Pneumonia
We obtained data on pneumonia diagnoses from Care Register for Health Care, which contains information on inpatient days, including the date and reason for the hospital stay. These diagnoses were based on the International Statistical Classification of Diseases and Related Health Problems 10th revision (ICD-10). Pneumonia diagnoses utilized in this study were the following: J10-12 (viral pneumonia), J13-15 (bacterial pneumonia), J16 (pneumonia due to other infectious organisms, not elsewhere classified), J17 (pneumonia in diseases classified elsewhere), and J18 (pneumonia, organism unspecified). The same codes were used to retrieve information on pneumonia from the Cause of Death register. Previous hospital-treated pneumonia was defined as any of the previous diagnoses six months before AD diagnosis or index date.
Participant characteristics
Socioeconomic position was defined as the highest occupational social class between 1972 and the index date and the information were obtained from Statistics Finland, as described previously [29]. We retrieved data on comorbidities from Special Reimbursement Register and Care Register for Health Care from 1972 until the index date or AD diagnosis. Relevant comorbidities were defined according to existing literature: chronic obstructive pulmonary disease (COPD), asthma, diabetes, cardiovascular disease, stroke, and chronic renal or liver disease [4, 5]. Special Reimbursement Register included data on the diagnosis of asthma/COPD, diabetes, rheumatoid arthritis or other connective tissue diseases, and cardiovascular disease. More detailed information on definitions of these characteristics is given in Supplementary Table 1.
In addition, data on the use of benzodiazepines and related drugs, antidepressants, antipsychotics, antiepileptics, opioids, biological products, oral glucocorticoids, and proton pump inhibitors were obtained from Prescription Register, which includes information on all purchased and reimbursed prescription drugs from Finnish pharmacies. Drugs were classified according to the WHO Anatomical Therapeutic Chemical (ATC) classification system [30] Drug use was investigated one year before AD diagnosis or index date. Psychotropics include antipsychotics, benzodiazepines, or related drugs and antidepressants. The total number of psychotropics in use for one person could reach a maximum number of three and the number could include drugs from all psychotropic classes. Similarly, antiepileptic and opioid use during the year preceding AD diagnosis or index date was investigated and the utilized ATC codes are listed in Supplementary Table 1. Care Register for Health Care included data on previous hospitalizations due to pneumonia, stroke, and liver or kidney disease. More detailed information on definitions of these characteristics is given in Supplementary Table 1.
Persons who were hospitalized for pneumonia within a six-month time window before the index date, including those with ongoing hospitalization with pneumonia diagnosis on the index date, were excluded. In addition, persons who received active cancer treatment within six months before the index date were excluded from the data. The study population consisted of 69,350 persons with AD and 69,350 persons without AD. The same exclusion criteria were used for both cohorts. Data sources and periods for identifying exclusion criteria are presented in Supplementary Table 1 and Fig. 1. The follow-up began on the index date and ended on the date of pneumonia diagnosis, death (due to other causes than pneumonia), the beginning of acute cancer treatment, or the end of the data linkage (31.12.2015), whichever occurred first. In addition, the comparison persons were censored on the date of AD diagnosis if that occurred before the other censoring criteria.
Statistical analyses
χ2 test, independent samples T-test, and Mann–Whitney test were utilized to report the p-values for categorical and continuous variables. Cox regression was utilized to define the incidence of pneumonia and reported as hazard ratios (HRs) with 95% confidence intervals (CIs). The proportionality assumption was confirmed with Kaplan–Meier curves. Multivariable models were adjusted for: AD, age, gender, socioeconomic position, asthma/COPD, rheumatoid arthritis, cardiovascular disease, diabetes, stroke, liver or kidney disease, benzodiazepine or related drug use, antidepressant use, antipsychotic use, antiepileptic use, opioid use, use of biological products, oral glucocorticoid use, and proton pump inhibitor use. The association of AD and other risk factors with pneumonia were evaluated in the entire study population including both persons with and without AD: To assess whether the association of risk factor was similar in persons with and without AD, models with AD*risk factor interaction term were fitted. As there was statistical evidence for different association between the cohorts, the analyses were also stratified by AD. Statistical analysis was performed using IBM SPSS Statistics for Mac (version 25, IBM Corporation, Armonk, New York, USA). In addition, competing risks regression models with death for other causes than pneumonia as competing risk, according to the method of Fine and Gray were fitted with Stata MP17.0 [31].
Ethics
The MEDALZ study plan was approved by the register maintainers. All data were pseudonymized before submission to the research team and study participants were not contacted, thus, according to Finnish law, ethics committee approval or informed consent was not required.
Results
Characteristics
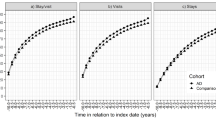
The study population is described in detail in Table 1. Altogether 8.6% (n = 5983) of the AD cohort and 7.4% (n = 5126) of the non-AD cohort had had previous hospital-treated pneumonia before the follow-up. Cardiovascular disease, diabetes, and stroke, as well as the use of psychotropics and antiepileptics during one year before AD diagnosis, were more common in the AD cohort. The median follow-up was 4.5 years (IQR 2.5–9.2, range 1 day – 11 years) in the AD cohort and 5.2 years (IQR 3.2–7.2, range 1 day-11 years) years in the non-AD cohort. During the follow-up, 25.0% (n = 17,105) of the AD cohort and 15.8% (n = 10,966) of the non-AD cohort were hospitalized due to incident pneumonia and the median of hospital days due to pneumonia was 11 days for both groups. The incidence of pneumonia was higher, 5.5/100 person-years in persons with AD and 3.0/100 person-years in persons without AD. Persons with AD were more likely to be censored due to death than those without AD (42.7% and 26.5%, respectively). A detailed description of censoring reasons in both cohorts is provided in Supplementary Table 2.
In both AD and non-AD cohorts, chronic comorbidities (excluding liver and kidney disease in the AD cohort) were more common in persons with incident pneumonia (Table 2). In addition, the use of benzodiazepines and related drugs and antipsychotics, as well as antiepileptics, opioids, oral glucocorticoids, and proton pump inhibitors, was more common in this group.
Risk factors for hospitalization due to pneumonia
Out of all the investigated risk factors, previous hospital-treated pneumonia had the strongest association with incident pneumonia in the whole population with incident pneumonia (aHR 2.25, 95% CI: 2.18–2.33) (Table 3). Male gender (aHR 2.09, 95% CI 2.04–2.14), AD (aHR 1.76, 95% CI 1.71–1.80), all comorbidities (highest aHR in asthma/COPD 1.59 95% CI 1.54–1.65) and drug use (highest aHR for oral glucocorticoids (1.68 95% CI 1.32–2.15) were associated with incident pneumonia. The association of all risk factors except oral glucocorticoids and biological products was different in AD and the non-AD cohorts (P for interaction < 0.05).
Persons with AD had a higher risk of pneumonia than those without AD also after adjusting for comorbidities. Higher risk of pneumonia in persons with AD was still observed after adjusting for competing risk of death (aHR, 95% CI 1.54, 1.51–1.58). The association of other risk factors was weaker in these competing risk analyses, but except for biological products and liver and kidney disease, they were still associated with a higher risk of pneumonia (Table 3).
When risk factor analyses were performed separately in the AD and non-AD cohorts, previous pneumonia was a strong risk factor for pneumonia in both cohorts (persons with AD aHR 2.00, 95% CI: 1.91–2.09, persons without AD aHR 2.61, 95% CI 2.48–2.75) (Table 4). Except for antidepressants, biological products, benzodiazepines or related drugs, and liver or kidney disease that had no association with pneumonia in the AD cohort, all comorbidities, and drug use were associated with the increased risk of pneumonia in both cohorts. The associations were stronger in the non-AD cohort.
The use of any psychotropic before the follow-up was associated with a higher risk of pneumonia in both cohorts, and the risk increase was proportional to the number of different psychotropics. The strongest risk factors for pneumonia in the AD cohort were male gender (aHR 2.27, 95% CI: 2.20–2.34) and oral glucocorticoid use (aHR 1.63, 95% CI: 1.19–2.23). In the non-AD cohort, the strongest associations were observed for use of psychotropics from all three psychotropic classes during the year preceding the index date (unadjusted aHR 2.43, 95% CI 2.07–2.84) and for use of two psychotropic classes during the year preceding the index date (unadjusted aHR 2.00, 95% CI 1.87–2.13).
In the competing risk models, in persons with AD cardiovascular diseases, liver and kidney disease, oral glucocorticoids, and biological products were not associated with pneumonia. In persons without AD, the associations of liver and kidney disease and biological products were no longer associated with increased risk of pneumonia in the competing risk models.
Discussion
In this study, the AD cohort had a higher incidence of pneumonia than the non-AD cohort. Previously experienced hospital-treated pneumonia had the strongest association with incident pneumonia in both cohorts, and several comorbidities and drug use were also associated with incident pneumonia, especially in the non-AD cohort. The use of more than one kind of psychotropic drug during the preceding year of AD diagnosis or index date was a strong risk factor for pneumonia, especially in the non-AD cohort. Nearly all of the associations remained after accounting for the competing interest of death.
A previous study on the incidence of bacterial pneumonia showed a higher risk of hospitalization for persons with cognitive impairment [16]. Our findings are similar; however, we included a broader range of pneumonia diagnoses including viral pneumonia caused by for example influenza virus and other common respiratory viruses. This implies that the risk is higher in persons with AD not only for bacterial pneumonia but also for viral pneumonia.
Although the same risk factors were associated with a higher risk of pneumonia in both cohorts, nearly all risk factors had stronger associations in the AD cohort. This is consistent with our earlier studies on risk factors of hip fracture [32] and mortality [33] in the same MEDALZ study and implies that AD itself is such a strong risk factor for pneumonia that it overrules the relative impact of other risk factors, although these risk factors are still associated with a higher risk of pneumonia in them. The risk of hospitalization from incident pneumonia was strongly associated with the male gender, especially in the AD cohort. According to a systematic review of risk factors of pneumonia, there is no definitive conclusion on the role of gender as a pneumonia risk factor [34]. However, smoking, and associated COPD are significant risk factors for pneumonia [34] and men are more frequent smokers than women [35], which could explain this finding.
We found a strong association between all comorbidities and incident pneumonia, especially in the non-AD cohort. The observed strong association between previous hospital-treated pneumonia and incident pneumonia in both cohorts is likely explained by persistent risk factors for pneumonia, for example, lifestyle factors including smoking and oral hygiene or immunosuppressive conditions, e.g., inflammatory bowel disease, that were not included in our study.
Interestingly, in our study, the use of biological products was not associated with a higher risk of incident pneumonia, but the use of oral glucocorticoids and existing rheumatoid arthritis were associated with a higher pneumonia risk. Oral glucocorticoid use and immunosuppressive therapy have previously been associated with increased pneumonia risk in the adult population [34]. However, users of biological products might be an exceptional group among older adults, especially individuals with AD. It could be possible that the threshold for prescribing biological products for these groups is very high and individuals more prone to pneumonia could be excluded from the users in this process. Furthermore, older adults who utilize biological products could be more frequently vaccinated with influenza and pneumococcal vaccine [36].
Psychotropic use was associated with a higher risk of pneumonia and the risk increase was proportional to the number of different psychotropics. This finding highlights the importance of avoiding psychotropic polypharmacy, as instructed by treatment guidelines [37]. In addition, clinicians should consider deprescribing psychotropics without current indication or if these are prescribed for neuropsychiatric symptoms or sleep disorders [38, 39]. When this is impossible, switching to a safer alternative or dose reduction should be considered. Out of the individual psychotropics, the strongest associations were observed with antipsychotics, especially in the non-AD cohort. A previous systematic review reported an increased risk for pneumonia following the use of first- and second-generation antipsychotics [40] and according to another review of studies conducted in older adults, similar associations were observed in older adults, also those with dementia or AD [41]. Our findings on the association between antipsychotic use at the beginning of the follow-up are in line with these earlier observations. We have also previously shown that antipsychotic users with AD had a higher risk of pneumonia [42]. However, there are important differences between this and the previous study as that study was restricted to persons who initiated antipsychotic use after AD diagnosis, and exposure was modeled as time-dependent, whereas the current study assessed antipsychotic use during one year before AD diagnosis.
Theoretically, benzodiazepines may cause aspiration possibly leading to pneumonia. We found that benzodiazepines and related drugs increased the risk of pneumonia in the non-AD cohort. Our previous study on persons with AD showed an increased risk of pneumonia in benzodiazepine users [43]. However, it must be noted that in this study, drug exposure was assessed from a different time window. A study on nursing home residents, on the other hand, showed a lower risk for pneumonia in persons using benzodiazepines [44]. However, their use is not recommended in older adults for various reasons, including the risk of falls and negative effects on cognition.
We found that antidepressants increased the risk of pneumonia in the non-AD cohort. Previous studies on the use of antidepressants and the risk of pneumonia have shown conflicting results [41]. Antidepressant use was a risk factor for pneumonia in persons with Parkinson’s Disease [45] but another study on nursing home residents did not link antidepressant use to pneumonia [44]. However, contrary to that study, all our study participants were community-dwelling at the beginning of the follow-up. We found antidepressant use to be a risk factor for pneumonia only in the non-AD cohort, which is likely explained by the fact that AD itself is a strong risk factor for pneumonia.
Persons who had used both antiepileptics and opioids during the year preceding the beginning of the follow-up had a higher risk of pneumonia than those who had used only one of these drugs. Both drugs have been linked to pneumonia in previous studies and one of the potential mechanisms for that could be sedation and aspiration [46, 47].
Previous systematic reviews and meta-analyses have shown an increased risk for pneumonia with proton pump inhibitor use [48], but the most recent systematic review raised the question of heterogeneity between reviewed studies as well as both publication and protopathic bias [49]. According to that review, studies that considered protopathic bias did not show an increased risk for pneumonia. We observed a slight increase in the risk for pneumonia following proton pump inhibitor use but it is noteworthy that this study did not include incident proton pump inhibitor users, and this higher risk could be explained by NSAID and/or oral glucocorticoid use.
Strengths and limitations
A key strength of our study is the utilization of nationwide register-based data which provides valid information on AD diagnoses [50], prescription drug purchases [51], and diagnosed comorbidities [52]. Registers in this study covered all residents regardless of their region or socioeconomic status, minimizing selection bias. Importantly, however, our data did not contain information on several lifestyle factors, for example, smoking, alcohol consumption, nutritional status, and dental hygiene, and therefore we were not able to study their association with pneumonia, or how much the increased risk of pneumonia in persons with AD is explained by these factors. Another limitation of our study is that we could not access information on the severity of AD and thus we were unable to analyze the incidence rate of pneumonia at different stages of the disease.
Conclusions
Community-dwellers with AD had a higher incidence of pneumonia than community-dwellers without AD. These findings raise the question of whether comorbidities are optimally treated and why the use of psychotropics and especially psychotropic polypharmacy is so prevalent, especially in persons with AD. In clinical practice, we should assess the state of comorbidities and optimize their care in older adults and especially among vulnerable older adults with AD. Furthermore, we need to review drug use, and carefully consider deprescribing of psychotropics without a current indication.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author, but restrictions apply to the availability of these data, so they are not publicly available. Data are available from the authors upon reasonable request and with the permission of the register maintainers.
Abbreviations
- AD:
-
Alzheimer’s disease
- aHR:
-
Adjusted hazard ratio
- ATC:
-
Anatomical therapeutic chemical
- CAP:
-
Community-acquired pneumonia
- CI:
-
Confidence interval
- COPD:
-
Chronic obstructive pulmonary disease
- DSM‐IV:
-
Diagnostic and statistical manual of mental disorders
- HR:
-
Hazard ratio
- MEDALZ:
-
Medication use and Alzheimer’s disease
- NINCDS‐ADRDA:
-
National institute of neurological and communicative disorders and stroke and the Alzheimer’s disease and related disorders association
- SII:
-
Social insurance institution
References
Sogaard M, Nielsen RB, Schonheyder HC, Norgaard M, Thomsen RW. Nationwide trends in pneumonia hospitalization rates and mortality, Denmark 1997–2011. Respir Med. 2014;108(8):1214–22.
Millett ERC, Quint JK, Smeeth L, Daniel RM, Thomas SL. Incidence of community-acquired lower respiratory tract infections and pneumonia among older adults in the United Kingdom: a population-based study. PLoS One. 2013;8(9):e75131. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24040394.
Trotter CL, Stuart JM, George R, Miller E. Increasing hospital admissions for pneumonia. England Emerg Infect Dis. 2008;14(5):727–33.
Koivula I, Sten M, Makela PH. Risk factors for pneumonia in the elderly. Am J Med. 1994;96(4):313–20.
Torres A, Peetermans WE, Viegi G, Blasi F. Risk factors for community-acquired pneumonia in adults in Europe: a literature review. Thorax. 2013;68(11):1057–65.
Loeb M, McGeer A, McArthur M, Walter S, Simor AE. Risk factors for pneumonia and other lower respiratory tract infections in elderly residents of long-term care facilities. Arch Intern Med. 1999;159(17):2058–64.
Tolppanen AM, Koponen M, Tanskanen A, Lavikainen P, Sund R, Tiihonen J, et al. Antipsychotic use and risk of hospitalization or death due to pneumonia in persons with and those without Alzheimer disease. Chest. 2016;150(6):1233–41.
Taipale H, Tolppanen AM, Koponen M, Tanskanen A, Lavikainen P, Sund R, et al. Risk of pneumonia associated with incident benzodiazepine use among community-dwelling adults with Alzheimer disease. CMAJ. 2017;189(14):E519–29.
Lambert AA, Lam JO, Paik JJ, Ugarte-Gil C, Drummond MB, Crowell TA. Risk of community-acquired pneumonia with outpatient proton-pump inhibitor therapy: a systematic review and meta-analysis. PLoS ONE. 2015;10(6):e0128004.
Wang CH, Li CH, Hsieh R, Fan CY, Hsu TC, Chang WC, et al. Proton pump inhibitors therapy and the risk of pneumonia: a systematic review and meta-analysis of randomized controlled trials and observational studies. Expert Opin Drug Saf. 2019;18(3):163–72.
Marston BJ, Plouffe JF, File TMJ, Hackman BA, Salstrom SJ, Lipman HB, et al. Incidence of community-acquired pneumonia requiring hospitalization. Results of a population-based active surveillance Study in Ohio the community-based pneumonia incidence study group. Arch Intern Med. 1997;157(15):1709–18.
Baldo V, Cocchio S, Baldovin T, Buja A, Furlan P, Bertoncello C, et al. A population-based study on the impact of hospitalization for pneumonia in different age groups. BMC Infect Dis. 2014;5(14):485.
Antunes C, Pereira M, Rodrigues L, Organista D, Cysneiros A, Paula F, et al. Hospitalization direct cost of adults with community-acquired pneumonia in Portugal from 2000 to 2009. Pulmonology. 2000;26(5):264–7.
Spoorenberg SMC, Bos WJW, Heijligenberg R, Voorn PGP, Grutters JC, Rijkers GT, et al. Microbial aetiology, outcomes, and costs of hospitalisation for community-acquired pneumonia; an observational analysis. BMC Infect Dis. 2014;17(14):335.
Riquelme R, Torres A, El-Ebiary M, Mensa J, Estruch R, Ruiz M, et al. Community-acquired Pneumonia in the Elderly. Am J Respir Crit Care Med. 1997;156(6):1908–14.
Phelan EA, Borson S, Grothaus L, Balch S, Larson EB. Association of incident dementia with hospitalizations. JAMA. 2012;307(2):165–72.
George J, Bleasdale S, Singleton SJ. Causes and prognosis of delirium in elderly patients admitted to a district general hospital. Age Ageing. 1997;26(6):423–7.
Fick DM, Agostini JV, Inouye SK. Delirium superimposed on dementia: a systematic review. J Am Geriatr Soc. 2002;50(10):1723–32.
Fong TG, Jones RN, Marcantonio ER, Tommet D, Gross AL, Habtemariam D, et al. Adverse outcomes after hospitalization and delirium in persons with Alzheimer disease. Ann Intern Med. 2012;156(12):56 (W296).
Bunn F, Burn AM, Goodman C, Rait G, Norton S, Robinson L, et al. Comorbidity and dementia: a scoping review of the literature. BMC Med. 2014;31(12):192.
Formiga F, Fort I, Robles MJ, Riu S, Sabartes O, Barranco E, et al. Comorbidity and clinical features in elderly patients with dementia: differences according to dementia severity. J Nutr Health Aging. 2009;13(5):423–7.
Clague F, Mercer SW, McLean G, Reynish E, Guthrie B. Comorbidity and polypharmacy in people with dementia: insights from a large, population-based cross-sectional analysis of primary care data. Age Ageing. 2017;46(1):33–9.
Manabe T, Teramoto S, Tamiya N, Okochi J, Hizawa N. Risk factors for aspiration pneumonia in older adults. PLoS ONE. 2015;10(10):e0140060.
Graversen SB, Pedersen HS, Sandbaek A, Foss CH, Palmer VJ, Ribe AR. Dementia and the risk of short-term readmission and mortality after a pneumonia admission. PLoS ONE. 2021;16(1):e0246153.
Tolppanen AM, Taipale H, Koponen M, Lavikainen P, Tanskanen A, Tiihonen J, et al. Cohort profile: the Finnish Medication and Alzheimer’s disease (MEDALZ) study. BMJ Open. 2016;6(7):12100.
McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA work group under the auspices of department of health and human services task force on Alzheimer’s disease. Neurology. 1984;34(7):939–44.
First MB. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: American Psychiatric Assoc; 1994.
Finnish Medical Society Duodecim. Current care: Memory Disorders. Vol. 2017. 2021. Available from: http://www.kaypahoito.fi
Statistics Finland. Classification of Occupations. https://www2.stat.fi/fi/luokitukset/ammatti/. 2010.
WHO Collaborating center for Drug Statistics Methodology. The Anatomical Therapeutic Chemical Classification System. Vol. 2017. Available from: https://www.whocc.no/atc_ddd_index/
Fine JP, Gray RJ. A Proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509.
Tolppanen AM, Taipale H, Tanskanen A, Tiihonen J, Hartikainen S. Comparison of predictors of hip fracture and mortality after hip fracture in community-dwellers with and without Alzheimer’s disease - exposure-matched cohort study. BMC Geriatr. 2016;16(1):204.
Rajamaki B, Hartikainen S, Tolppanen AM. The effect of comorbidities on survival in persons with Alzheimer’s disease: a matched cohort study. BMC Geriatr. 2021;21(1):173.
Almirall J, Serra-Prat M, Bolíbar I, Balasso V. Risk factors for community-acquired pneumonia in adults: a systematic review of observational studies. Respiration. 2017;94(3):299–311.
Lugo A, la Vecchia C, Boccia S, Murisic B, Gallus S. Patterns of smoking prevalence among the elderly in Europe. Int J Environ Res Public Health. 2013;10(9):4418–31.
Melmed GY. Vaccination strategies for patients with inflammatory bowel disease on immunomodulators and biologics. Inflamm Bowel Dis. 2009;15(9):1410–6.
APA Work Group on Alzheimer’s Disease and other Dementias, Rabins PV, Blacker D, Rovner BW, Rummans T, Schneider LS, et al. American Psychiatric Association practice guideline for the treatment of patients with Alzheimer’s disease and other dementias. Second edition. Am J Psychiatry. 2007;164(12):5–56.
Reus VI, Fochtmann LJ, Eyler AE, Hilty DM, Horvitz-Lennon M, Jibson MD, et al. The American Psychiatric Association Practice Guideline on the Use of Antipsychotics to Treat Agitation or Psychosis in Patients With Dementia. Am J Psychiatry. 2016;173(5):543–6.
Seppala LJ, Petrovic M, Ryg J, Bahat G, Topinkova E, Szczerbińska K, et al. STOPPFall (Screening Tool of Older Persons Prescriptions in older adults with high fall risk): a Delphi study by the EuGMS task and finish group on fall-risk-increasing drugs. Age Ageing. 2021;50(4):1189–99.
Dzahini O, Singh N, Taylor D, Haddad PM. Antipsychotic drug use and pneumonia: Systematic review and meta-analysis. J Psychopharmacol. 2018;32(11):1167–81.
Rajamaki B, Hartikainen S, Tolppanen AM. Psychotropic drug-associated pneumonia in older adults. Drugs Aging. 2020;37(4):241–61.
Tolppanen AM, Koponen M, Tanskanen A, Lavikainen P, Sund R, Tiihonen J, et al. Antipsychotic use and risk of hospitalization or death due to pneumonia in persons with and those without alzheimer disease. Chest. 2016;150(6):1233–41.
Taipale H, Tolppanen AM, Koponen M, Tanskanen A, Lavikainen P, Sund R, et al. Risk of pneumonia associated with incident benzodiazepine use among community-dwelling adults with Alzheimer disease. CMAJ. 2017;189(14):E519–29.
Huybrechts KF, Rothman KJ, Silliman RA, Brookhart MA, Schneeweiss S. Risk of death and hospital admission for major medical events after initiation of psychotropic medications in older adults admitted to nursing homes. CMAJ. 2011;183(7):E411–9.
Kuo WY, Huang KH, Kuan YH, Chang YC, Tsai TH, Lee CY. Antidepressants usage and risk of pneumonia among elderly patients with the parkinson’s disease: a population-based case-control study. Front Med (Lausanne). 2022;9:740182.
Steffens C, Sung M, Bastian LA, Edelman EJ, Brackett A, Gunderson CG. The association between prescribed opioid receipt and community-acquired pneumonia in adults: a systematic review and meta-analysis. J Gen Intern Med. 2020;35(11):3315–22.
Taipale H, Lampela P, Koponen M, Tanskanen A, Tiihonen J, Hartikainen S, et al. Antiepileptic drug use is associated with an increased risk of pneumonia among community-dwelling persons with alzheimer’s disease-matched cohort study. J Alzheimers Dis. 2019;68(1):127–36.
Lambert AA, Lam JO, Paik JJ, Ugarte-Gil C, Drummond MB, Crowell TA. Risk of community-acquired pneumonia with outpatient proton-pump inhibitor therapy: a systematic review and meta-analysis. PLoS ONE. 2015;10(6):e0128004.
Wang CH, Li CH, Hsieh R, Fan CY, Hsu TC, Chang WC, et al. Proton pump inhibitors therapy and the risk of pneumonia: a systematic review and meta-analysis of randomized controlled trials and observational studies. Expert Opin Drug Saf. 2019;18(3):163–72.
Solomon A, Ngandu T, Soininen H, Hallikainen MM, Kivipelto M, Laatikainen T. Validity of dementia and Alzheimer’s disease diagnoses in Finnish national registers. Alzheimers Dement. 2014;10(3):303–9.
Wettermark B, Zoega H, Furu K, Korhonen M, Hallas J, Norgaard M, et al. The Nordic prescription databases as a resource for pharmacoepidemiological research–a literature review. Pharmacoepidemiol Drug Saf. 2013;22(7):691–9.
Sund R. Quality of the Finnish Hospital Discharge Register: a systematic review. Scand J Public Health. 2012;40(6):505–15.
Acknowledgements
Not applicable.
Funding
This work was supported by grants from Päivikki and Sakari Sohlberg foundation and Uulo Aarhio foundation. The sponsor did not have any role in the design, methods, subject recruitment, data collection, analysis, or preparation of the paper.
Author information
Authors and Affiliations
Contributions
Study concept and design: HJ, AMT, SH. Acquisition of data: AMT, SH. Analysis, and interpretation of data: HJ, AMT, SH. Drafting of the manuscript: HJ. Critical revision of the manuscript for important intellectual content: HJ, AMT, SH. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All methods were carried out in accordance with relevant guidelines and regulations including Declaration of Helsinki, the European General Data Protection Regulation, and national legislation. The MEDALZ study plan was approved by the register maintainers. All data were pseudonymized before submission to the research team and study participants were not contacted. Thus, according to Finnish law (including Personal Data Act 23/1999, Act on the Openness of Government Activities 621/1999 and Act on the Secondary Use of Health and Social Data 552/2019 (and previous Act on the National Healthcare registers, not official English translation as this is not available 556/1989), the study has been granted an exemption from requiring ethics approval or informed consent.
Consent for publication
Not applicable.
Competing interests
HJ has no competing interests to declare. AMT reports a research grant from Amgen, paid through the institution she is employed by, outside of the submitted work. SH reports a lecture fee from Eisai.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Supplementary Table 1. Definitions of comorbidities and drug use and data sources.
Additional file 2: Supplementary Table 2.
Reasons for censoring in both cohorts.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Järvinen, H., Tolppanen, AM. & Hartikainen, S. Risk factors of pneumonia in persons with and without Alzheimer’s disease: a matched cohort study. BMC Geriatr 23, 227 (2023). https://doi.org/10.1186/s12877-023-03940-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12877-023-03940-z