Abstract
Background
This study aimed to develop and validate a clinical prediction rule to screen older patients at risk of being toxigenic Clostridioides difficile carriers at the time of hospital admission.
Methods
This retrospective case-control study was performed at a university-affiliated hospital. Active surveillance using a real-time polymerase chain reaction (PCR) assay for the toxin genes of C. difficile was conducted among older patients (≥ 65 years) upon admission to the Division of Infectious Diseases of our institution. This rule was drawn from a derivative cohort between October 2019 and April 2021 using a multivariable logistic regression model. Clinical predictability was evaluated in the validation cohort between May 2021 and October 2021.
Results
Of 628 PCR screenings for toxigenic C. difficile carriage, 101 (16.1%) yielded positive findings. To establish clinical prediction rules in the derivation cohort, the formula was derived using significant predictors for toxigenic C. difficile carriage at admission, such as septic shock, connective tissue diseases, anemia, recent use of antibiotics, and recent use of proton-pump inhibitors. In the validation cohort, the sensitivity, specificity, and positive and negative predictive values of the prediction rule, based on a cut-off value of ≥ 0.45, were 78.3%, 70.8%, 29.5%, and 95.4%, respectively.
Conclusion
This clinical prediction rule for identifying toxigenic C. difficile carriage at admission may facilitate the selective screening of high-risk groups. To implement it in a clinical setting, more patients from other medical institutions need to be prospectively examined.
Similar content being viewed by others
Introduction
In 2013, the United States Centers for Disease Control and Prevention designated Clostridioides difficile (C. difficile) as a dangerous pathogen that requires diligent monitoring and prevention activities [1]. Although C. difficile infections (CDIs) have traditionally been considered to affect patients in healthcare facilities, the disease epidemiology seems to have shifted, with patients now presenting with community-onset CDI [2].
The clinical severity of CDIs ranges from an asymptomatic carrier state to life-threatening conditions [3]. Asymptomatic carriers may serve as significant reservoirs for transmission to susceptible patients and the environment via direct or indirect contact. They are also six times more likely to develop subsequent symptomatic CDI than non-carriers [4, 5].
Older age (≥ 65 years) is a crucial contributor to CDI development and severity because of age-related immunosenescence, an increase in the use of antibiotics, and frequent exposure to medical environments [6]. Furthermore, previous epidemiological studies revealed that one out of three CDIs and two out of three healthcare-associated CDIs develop in patients aged 65 years or older. Advanced age is also significantly associated with CDI recurrence [7]. However, data on the prevalence and risk factors for toxigenic C. difficile carriage on hospital admission in older populations are limited.
Nucleic acid amplification testing (NAAT) is the only diagnostic test for the detection of toxigenic C. difficile used in many studies and may result in CDI overdiagnosis [8]. NAAT screening tools for CDI are, however, widely implemented because asymptomatic toxigenic C. difficile carriage is a major risk factor for CDI [9].
Early recognition of toxigenic C. difficile infection on hospital admission is essential for timely infection control measures to contain the transmission of nosocomial CDI [10,11,12]. Asymptomatic toxigenic C. difficile carriers are also at high risk for progression to symptomatic CDI, for which antimicrobial stewardship measures should be implemented. Therefore, this study aimed to develop and validate a clinical prediction rule to identify older toxigenic C. difficile carriers on hospital admission.
Methods
Hospital setting
This study was conducted at a 1,048-bed university-affiliated hospital in Seoul, Republic of Korea. Since October 2019, the hospital has run an active surveillance program of toxigenic C. difficile carriers, targeted at older patients (≥ 65 years) within 48 h of their admission to the Division of Infectious Diseases, Department of Internal Medicine. As part of the pilot project, a program was implemented to strengthen the antimicrobial stewardship for the targeted population and promote the early detection of symptomatic patients with CDI. However, strict contact isolation, including private room use or cohorting, could not be implemented because of the lack of medical resources.
Study design
This retrospective cohort study was performed using separate derivative and validation datasets to generate and validate a clinical prediction rule for identifying patients who are toxigenic C. difficile carriers at the time of their hospital admission. In the derivation cohort, 1:1 case-control was conducted to identify the risk factors associated with toxigenic C. difficile carriage upon hospital admission between October 2019 and April 2021. The formula derived from the multivariable logistic regression analysis was used to establish the clinical prediction rules. A case was defined as an older (≥ 65 years) toxigenic C. difficile carrier when confirmed by real-time polymerase chain reaction (PCR) screening using a stool specimen or rectal swab at the time of admission to the Division of Infectious Diseases, Department of Internal Medicine. A control subject was defined as an older patient (≥ 65 years) who did not have toxigenic C. difficile at hospital admission. Subsequently, an internal validation was performed on the derived clinical prediction rule in the validation cohort between May 2021 and October 2021.
Data collection
The following potential predictive variables for toxigenic C. difficile carriage or CDI were collected from a computerized hospital database for each patient: age, sex, comorbid conditions, history of procedures or operations over the past month, receipt of proton-pump inhibitors or immunosuppressants, exposure to a medical environment, antibiotic use for more than 3 days over the past month, diagnosis on admission, intensive care unit stay over the past month, and multidrug-resistant microorganisms isolated from clinical specimens during hospitalization. Diarrhea was defined as the passage of three or more loose or liquid stools per day. An asymptomatic carrier was defined as a person infected with C. difficile, detected by PCR, without diarrhea.
The study was approved by our hospital’s institutional review board [2022AN0356]. Since the clinical data were obtained through a routine hospital surveillance program for infection control and antimicrobial stewardship, the requirement for informed consent was waived.
Microbiological methods
Stool samples or rectal swabs were obtained from each patient within 48 h of hospital admission. Toxigenic C. difficile carriers were identified with a real-time PCR assay, which simultaneously detects toxin A (TcdA enterotoxin, encoded by tcdA) and toxin B (TcdB cytotoxin, encoded by tcdB) (AdvanSure CD Real-Time PCR Kit; LG Life Science, Seoul, Korea). Enzyme-linked immunosorbent assay (ELISA) (C. DIFFICILE TOX A/B II, TECHLAB, USA) was used to evaluate the stool samples for toxin A and B production.
Statistical analyses
Existing active surveillance data for toxigenic C. difficile carriage were divided into derivation and validation datasets to build a clinical predictive model and validate the clinical performance of the model. The derivation cohort included all carriers of toxigenic C. difficile identified at our center between October 2019 and April 2021. A control group was randomly selected from the pool of eligible patients with no toxigenic C. difficile, based on a 1:1 pairing. The validation cohort included all subjects who underwent PCR testing for toxigenic C. difficile carriage between May 2021 and October 2021. The risk factors for toxigenic C. difficile carriage were compared between the case and the control group using Chi-squared tests or Fisher’s exact test for ordinal and dichotomous variables, respectively. Two-sample Student’s t-tests or Mann–Whitney U-test were used to compare continuous independent variables with normal or non-normal distributions, respectively.
Multivariable logistic regression analysis was conducted using stepwise variable selection based on the Wald statistic criterion. Variables with P < 0.05 were included in the final logistic regression model. The Hosmer–Lemeshow goodness-of-fit test was performed to evaluate the final selected model.
A receiver operating characteristic (ROC) curve analysis using the clinical prediction model was conducted to generate a risk index to identify the patients having a higher probability of carrying toxigenic C. difficile. The discriminative ability of the models to predict toxigenic C. difficile carriage upon hospital admission was assessed through area under the ROC curve (AUC) analysis. The optimal ROC cut-off value was derived from Youden’s index. Furthermore, the performance of the final multivariable logistic regression model was confirmed by evaluating its predictive accuracy using the leave-one-out cross-validation (LOOCV) method and a test dataset. IBM SPSS Statistics, version 20.0 (IBM, Armonk, NY, USA) and SAS 9.4 (SAS Institute Inc., Cary, NC, USA) were used to perform the multivariable logistic regression analysis and to simulate the validation of estimates, respectively.
Results
Prevalence of toxigenic C. difficile carriers on hospital admission
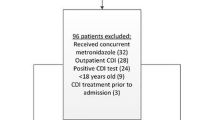
During the study period, 1,045 out of the 1,586 patients admitted to the Division of Infectious Diseases of our hospital were of older age (≥ 65 years). PCR screening for toxigenic C. difficile carriage was conducted for 628 (60.1%) out of these 1,045 patients (Fig. 1). PCR screening was not performed in the remaining 417 patients because stool samples or rectal swab specimens could not be collected within 48 h of admission or the patient refused to provide rectal swabs or undergo a stool examination.
Of the 628 screened patients, 101 (16.1%) were toxigenic C. difficile carriers. The monthly prevalence of toxigenic C. difficile carriage at admission ranged from 4.0 to 15.9 per 10,000 patient-days, with an average of 8.8 per 10,000 patient-days during the study period (Fig. 2). The demographic and clinical characteristics of the screened patients are shown in Table 1. Of the 101 patients with positive PCR test results, 55 (54.5%) were diagnosed with symptomatic CDI (45 healthcare-associated and 10 community-acquired cases) and underwent antibiotic therapy for CDI. In particular, nine patients were diagnosed with symptomatic CDI at admission, and 24, including these patients, started CDI treatment within 48 h of admission.
Construction of the clinical prediction model
Carriers of toxigenic C. difficile among our screened patients were more likely to reside in a facility than at home and be exposed to medical procedures 30 days before admission than those who were non-carriers (Table 1). Carriers had more neurological, chronic pulmonary, and connective tissue as well as hematologic diseases than non-carriers (Table 1). Only three patients with toxigenic C. difficile had a history of CDI 90 days before admission. Multidrug-resistant microorganisms, such as carbapenem-resistant Acinetobacter baumannii or methicillin-resistant Staphylococcus aureus were more commonly isolated from clinical samples during hospitalization in carriers than in non-carriers (Table 1).
In the multivariable logistic regression model, septic shock, connective tissue diseases, anemia, recent use of antibiotics, and recent use of proton-pump inhibitors were significant risk factors associated with toxigenic C. difficile carriage at the time of admission in older patients admitted to the Division of Infectious Diseases, Department of Internal Medicine (Table 2).
We calculated the predictive probability of patients being toxigenic C. difficile carriers using the following formula:
A: If septic shock, positive = 1 / negative = 0; B: If connective tissue diseases, positive = 1 / negative = 0; C: If anemia, positive = 1 / negative = 0; D: If recent use of antibiotics, positive = 1 / negative = 0; and E: If recent use of proton-pump inhibitors, positive = 1 / negative = 0.
When a cut-off value of ≥ 0.45 was applied to the clinical prediction model, the AUC value was 0.80, with a 95% confidence interval (CI) of 0.74–0.86 in the derivation cohort. The sensitivity, specificity, positive predictive value, and negative predictive value of this prediction rule were 75.3% (95% CI, 65.7–83.3), 74.3% (95% CI, 64.6–82.4), 74.5% (95% CI, 67.3–80.6), and 75.0% (95% CI: 67.7–81.1), respectively (Table 3). As shown in Table 3, the test and training data sets had similar accuracy, sensitivity, and specificity. The Hosmer–Lemeshow goodness-of-fit test result for the final model was P = 0.58, yielding no evidence for a lack of fit.
Validation of the clinical prediction model
The validation cohort included 170 patients who underwent PCR screening upon admission for toxigenic C. difficile carriage between April 2021 and October 2021. PCR screening was not performed in 33 patients because stool samples or rectal swab specimens could not be collected within 48 h of admission. This prediction risk model showed an AUC of 0.84 (95% CI, 0.78–0.90) in the validation dataset. The sensitivity, specificity, positive predictive value, and negative predictive value of this prediction rule were 78.3% (95% CI, 56.3–92.5), 70.75% (95% CI, 62.7–78.0), 29.5% (95% CI, 23.1–36.8), and 95.4% (95% CI, 90.5–97.8), respectively (Table 3). Of the 170 screened patients, 23 were positive for C. difficile toxin and 147 were negative. Our prediction rule confirmed that 27 were false positives and six were false negatives.
Discussion
In this study, we generated a clinical prediction rule for identifying patients with toxigenic C. difficile at hospital admission through active surveillance of older patients (≥ 65 years old) hospitalized with infectious diseases with a high probability of using antibiotics. Surprisingly, our analyses show that the prevalence of toxigenic C. difficile carriage on admission was as high as 16.1%. Considering the high cost of PCR testing, this scoring system may be valuable for selective screening to reduce the screening volumes at our hospital.
Previous studies have reported that C. difficile carriage prevalence rates in healthcare settings among the older population range from 1.6% for patients in the community to 21% for those in short- or long-term care facilities [13]. In our study, the 16.1% prevalence of toxigenic C. difficile carriage on admission was acceptable, considering that only 51.5% of the older patients were admitted without exposure to other healthcare facilities. These prevalence rates may vary according to the type of medical institution and surveillance methods used. Previous studies have reported a C. difficile carriage prevalence among older adults on admission of approximately 10% in culture-based and 16.4% in PCR-based screenings [14, 15]. Commercial PCR assays can provide a rapid and sensitive alternative to sample culture screenings for C. difficile, despite their high cost and rising concerns about false positivity.
We found that 50.5% (46/92) of asymptomatic toxigenic C. difficile carriers developed symptoms of CDI during hospitalization. A previous study demonstrated that toxigenic C. difficile carriers identified in an active screening had a 23-fold greater risk of developing CDI than non-carriers [16]. Other studies have also suggested that 2 − 37% of asymptomatic C. difficile carriers become symptomatic [17, 18].
In our study, septic shock, connective tissue diseases, anemia, recent use of antibiotics, and recent use of proton-pump inhibitors were significant risk factors associated with toxigenic C. difficile carriage at the time of admission in older patients with infectious diseases. Similar to our findings, previous studies have identified old age, underlying diseases, prior hospitalization, low Norton scores, pressure sores, and recent use of antibiotics, proton-pump inhibitors, and corticosteroids as independent risk factors for C. difficile colonization or infection [19,20,21,22]. In the present study, anemia and septic shock were significant predictors of toxigenic C. difficile carriage among older individuals. These conditions compromise the immune function and may be associated with underlying chronic diseases. A recent study suggested that anemia with hemoglobin levels < 10 g/dL is a risk factor associated with poor CDI outcomes [23]. Furthermore, iron appears to contribute to C. difficile colonization and CDI pathogenesis in mouse models [24]. Although there is no convincing evidence linking septic shock with toxigenic C. difficile carriage, CDI may develop during antibiotic treatment for worsening sepsis caused by a separate bacterial infection.
Our study provides a clinical model to predict toxigenic C. difficile carriage in older patients. However, previous studies have developed clinical rules to predict primary CDI onset, as well as CDI recurrence, complications, and mortality, with a sensitivity of 60 − 98% and a specificity of 44 − 95% [25,26,27,28,29]. The variables included in the clinical prediction rules for predicting the primary CDI onset were heterogeneous, such as old age, recent-onset diarrhea, development of infection during a prior admission, residing in a long-term care facility, admission to an intensive care unit, length of stay of 7 days or longer, endoscopy within 30 days, recent use of broad-spectrum antibiotics such as cephalosporins or fluoroquinolones, use of laxatives, gastric acid suppressors, or antimotility drugs, low body mass index (< 25), hypoalbuminemia, CDI pressure, and hemodialysis [25,26,27,28].
Asymptomatic C. difficile carriage is well known as a major risk factor for developing symptomatic CDI. Furthermore, although asymptomatic carriers pose a substantial reservoir for the transmission of CDI, control measures focus almost entirely on symptomatic patients [30]. While the current guidelines do not recommend active surveillance or the isolation of asymptomatic C. difficile carriers as measures to prevent C. difficile transmission, several studies have shown that active screening for C. difficile carriage using PCR assays is effective for infection control and prevention of C. difficile infection [10,11,12]. Ongoing research on new strategies, such as screening for asymptomatic C. difficile carriage, is needed to optimize the containment of these infections.
This study has a few limitations. First, this was a retrospective, single-center study that used random sampling of control cases. The study participants were limited to patients at our hospital. Therefore, a longitudinal prospective validation of the performance of this model in an external population is needed. Afterwards, an intervention study is needed to evaluate the effect of infection control or antimicrobial stewardship programs, including a screening tool for C. difficile carriage. Second, screening compliance was not universal. Because the unscreened patients might be less frail or sick than the screened patients, a selection bias might have occurred and, as a result, toxigenic C. difficile carriage might have been overestimated in our datasets. Third, owing to the small number of study subjects in our analysis, it was not possible to identify the risk factors that differentiate between asymptomatic C. difficile carriers and symptomatic patients with CDI on admission. Finally, PCR assays rather than cultures were used to identify toxigenic C. difficile carriers; the former may, however, be oversensitive in detecting C. difficile toxins.
Conclusion
Consistent with those of recent reports, our findings report that older patients with toxigenic C. difficile at hospital admission are common. Thus, our clinical prediction rule, as an initial screening tool, followed by PCR screening for prediction rule-positive patients, could reduce the PCR screening volumes. However, clinical prediction rules specific to local hospitals should be periodically verified, and these strategies should be fully integrated into existing infection control programs that include thorough contact precautions, cohorts, and environmental disinfection.
Data Availability
The data that support the findings of this study are available on request from the corresponding author.
Abbreviations
- AUC:
-
Area under the curve
- CD:
-
Clostridioides difficile
- CDI:
-
Clostridioides difficile infection
- CRAB:
-
Carbapenem-resistant Acinetobacter baumannii
- CRE:
-
Carbapenem-resistant Enterobacteriaceae
- CRPA:
-
Carbapenem-resistant Pseudomonas aeruginosa
- CVC:
-
Central venous catheter
- ER:
-
Emergency room
- MDROs:
-
Multi-drug resistant organisms
- MRSA:
-
Methicillin-resistant Staphylococcus aureus
- PCR:
-
Polymerase chain reaction
- PPI:
-
Proton-pump inhibitor
- VRE:
-
Vancomycin-resistant enterococci
References
Centers for Disease Control & Prevention. Antibiotic resistance threats in the United States, https://stacks.cdc.gov/view/cdc/20705, [accessed 22 November 2022]; 2013. United States Department of Health and Human Services.
Gerding DN, Lessa FC. The epidemiology of Clostridium difficile infection inside and outside health care institutions. Infect Dis Clin North Am. 2015;29:37–50. https://doi.org/10.1016/j.idc.2014.11.004.
Czepiel J, Dróżdż M, Pituch H, Kuijper EJ, Perucki W, Mielimonka A, et al. Clostridium difficile infection: review: review. Eur J Clin Microbiol Infect Dis. 2019;38:1211–21. https://doi.org/10.1007/s10096-019-03539-6.
Riggs MM, Sethi AK, Zabarsky TF, Eckstein EC, Jump RL, Donskey CJ. Asymptomatic carriers are a potential source for transmission of epidemic and nonepidemic Clostridium difficile strains among long-term care facility residents. Clin Infect Dis. 2007;45:992–8. https://doi.org/10.1086/521854.
Zacharioudakis IM, Zervou FN, Pliakos EE, Ziakas PD, Mylonakis E. Colonization with toxinogenic C. difficile upon hospital admission, and risk of infection: a systematic review and meta-analysis. Am J Gastroenterol. 2015;110:381–90. https://doi.org/10.1038/ajg.2015.22. quiz 391.
Asempa TE, Nicolau DP. Clostridium difficile infection in the older: an update on management. Clin Interv Aging. 2017;12:1799–809. https://doi.org/10.2147/CIA.S149089.
Hu MY, Katchar K, Kyne L, Maroo S, Tummala S, Dreisbach V, et al. Prospective derivation and validation of a clinical prediction rule for recurrent Clostridium difficile infection. Gastroenterology. 2009;136:1206–14. https://doi.org/10.1053/j.gastro.2008.12.038.
van Prehn J, Reigadas E, Vogelzang EH, Bouza E, Hristea A, Guery B et al. European Society of Clinical Microbiology and Infectious Diseases: 2021 update on the treatment guidance document for Clostridioides difficile infection in adults. Clin Microbiol Infect. European Society of Clinical Microbiology and Infectious Diseases. 2021;27 (Suppl 2):S1–S21. https://doi.org/10.1016/j.cmi.2021.09.038 Supp. l. 2:S1-S21.
Koo HL, Van JN, Zhao M, Ye XY, Revell PA, Jiang ZD, et al. Real-time polymerase chain reaction detection of asymptomatic Clostridium difficile colonization and rising C. difficile-associated disease rates. Infect Control Hosp Epidemiol. 2014;35:667–73. https://doi.org/10.1086/676433.
Longtin Y, Paquet-Bolduc B, Gilca R, Garenc C, Fortin E, Longtin J, et al. Effect of detecting and isolating Clostridium difficile carriers at hospital admission on the incidence of C difficile infections: a quasi-experimental controlled study. JAMA Intern Med. 2016;176:796–804. https://doi.org/10.1001/jamainternmed.2016.0177.
Maghdoori S, Moghadas SM. Assessing the effect of patient screening and isolation on curtailing Clostridium difficile infection in hospital settings. BMC Infect Dis. 2017;17:384. https://doi.org/10.1186/s12879-017-2494-6.
Lanzas C, Dubberke ER. Effectiveness of screening hospital admissions to detect asymptomatic carriers of Clostridium difficile: a modeling evaluation. Infect Control Hosp Epidemiol. 2014;35:1043–50. https://doi.org/10.1086/677162.
Rea MC, O’Sullivan O, Shanahan F, O’Toole PW, Stanton C, Ross RP, et al. Clostridium difficile carriage in older subjects and associated changes in the intestinal microbiota. J Clin Microbiol. 2012;50:867–75. https://doi.org/10.1128/JCM.05176-11.
Brazier JS, Fitzgerald TC, Hosein I, Cefai C, Looker N, Walker M, et al. Screening for carriage and nosocomial acquisition of Clostridium difficile by culture: a study of 284 admissions of older patients to six general hospitals in Wales. J Hosp Infect. 1999;43:317–9. https://doi.org/10.1016/s0195-6701(99)90431-0.
Nissle K, Kopf D, Rösler A. Asymptomatic and yet C. difficile-toxin positive? Prevalence and risk factors of carriers of toxigenic Clostridium difficile among geriatric in-patients. BMC Geriatr. 2016;16:185. https://doi.org/10.1186/s12877-016-0358-3.
Baron SW, Ostrowsky BE, Nori P, Drory DY, Levi MH, Szymczak WA, et al. Screening of Clostridioides difficile carriers in an urban academic medical center: understanding implications of disease. Infect Control Hosp Epidemiol. 2020;41:149–53. https://doi.org/10.1017/ice.2019.309.
Samore MH, DeGirolami PC, Tlucko A, Lichtenberg DA, Melvin ZA, Karchmer AW. Clostridium difficile colonization and diarrhea at a tertiary care hospital. Clin Infect Dis. 1994;18:181–7. https://doi.org/10.1093/clinids/18.2.181.
McFarland LV, Mulligan ME, Kwok RY, Stamm WE. Nosocomial acquisition of Clostridium difficile infection. N Engl J Med. 1989;320:204–10. https://doi.org/10.1056/NEJM198901263200402.
Meltzer E, Smollan G, Huppert A, Fluss R, Tal I, Gilboa M, et al. Universal screening for Clostridioides difficile in a tertiary hospital: risk factors for carriage and clinical disease. Clin Microbiol Infect. 2019;25:1127–32. https://doi.org/10.1016/j.cmi.2019.02.002.
Owens RC Jr, Donskey CJ, Gaynes RP, Loo VG, Muto CA. Antimicrobial-associated risk factors for Clostridium difficile infection. Clin Infect Dis. 2008;46(Suppl 1):19–31. https://doi.org/10.1086/521859.
Trifan A, Stanciu C, Girleanu I, Stoica OC, Singeap AM, Maxim R, et al. Proton pump inhibitors therapy and risk of Clostridium difficile infection: systematic review and meta-analysis. World J Gastroenterol. 2017;23:6500–15. https://doi.org/10.3748/wjg.v23.i35.6500.
Kalakuntla AS, Nalakonda G, Nalakonda K, Pidikiti CV, Aasim SA. Probiotics and Clostridium difficile: a review of dysbiosis and the rehabilitation of gut microbiota. Cureus. 2019;11:e5063. https://doi.org/10.7759/cureus.5063.
Lee E, Song KH, Bae JY, Yoon D, Hwang JH, Choe PG, et al. Risk factors for poor outcome in community-onset Clostridium difficile infection. Antimicrob Resist Infect Control. 2018;7:75. https://doi.org/10.1186/s13756-018-0365-6.
Deshpande A, Olaitan AO, Mckelvey AM, Rutherford JT, Hurdle JG. The ferrous iron transporter FeoB1 is essential for Clostridioides difficile toxin production and pathogenesis in mice.bioRxiv. 2022.03.03.482942.
Smith LA, Chan CK, Halm M, Slattery W, Lindquist R, Savik K. Development and validation of a Clostridium difficile risk assessment tool. AACN Adv Crit Care. 2014;25:334–46. https://doi.org/10.1097/NCI.0000000000000046.
Chandra S, Thapa R, Marur S, Jani N. Validation of a clinical prediction scale for hospital-onset Clostridium difficile infection. J Clin Gastroenterol. 2014;48:419–22. https://doi.org/10.1097/MCG.0000000000000012.
Dubberke ER, Yan Y, Reske KA, Butler AM, Doherty J, Pham V, et al. Development and validation of a Clostridium difficile infection risk prediction model. Infect Control Hosp Epidemiol. 2011;32:360–6. https://doi.org/10.1086/658944.
Tanner J, Khan D, Anthony D, Paton J. Waterlow score to predict patients at risk of developing Clostridium difficile-associated disease. J Hosp Infect. 2009;71:239–44. https://doi.org/10.1016/j.jhin.2008.11.017.
Abou Chakra CN, Pepin J, Valiquette L. Prediction tools for unfavourable outcomes in Clostridium difficile infection: a systematic review. PLOS ONE. 2012;7:e30258. https://doi.org/10.1371/journal.pone.0030258.
Gilboa M, Houri-Levi E, Cohen C, Tal I, Rubin C, Feld-Simon O, et al. Environmental shedding of toxigenic Clostridioides difficile by asymptomatic carriers: a prospective observational study. Clin Microbiol Infect. 2020;26:1052–7. https://doi.org/10.1016/j.cmi.2019.12.011.
Acknowledgements
None.
Funding
This study was partly supported by grants from Korea University Anam Hospital, Seoul, Republic of Korea and the National Research Foundation of Korea (NRF) grant, funded by the Korean Government (Ministry of Science and ICT) (No. NRF-2022R1A2C1010808). The funding sources had no role in the study design, data collection, data analysis, decision to publish, or manuscript preparation.
Author information
Authors and Affiliations
Contributions
Yoon YK conceived, designed, and performed this study. Lee KB, Lee M, Suh JW, Kim JY, Kim SB, Sohn JW, and Chung Y contributed to the data collection and analysis. Yang KS contributed to the statistical analysis and revision of the manuscript. Yoon YK and Lee KB wrote the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the institutional review board of Korea University Anam Hospital [2022AN0356]. Clinical data were obtained through a routine hospital surveillance program for infection control and antimicrobial stewardship. Therefore, the requirement for informed consent was waived. All methods were carried out in accordance with the relevant guidelines and regulations. This study was performed in line with the principles of the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing Interests
The author(s) declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lee, KB., Lee, M., Suh, J.W. et al. Clinical prediction rule for identifying older patients with toxigenic clostridioides difficile at the time of hospital admission. BMC Geriatr 23, 127 (2023). https://doi.org/10.1186/s12877-023-03808-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12877-023-03808-2