Abstract
Background
Inactive behaviour is common in older adults during hospitalisation and associated with poor health outcomes. If patients at high risk of spending little time standing/walking could be identified early after admission, they could be given interventions aimed at increasing their time spent standing/walking. This study aims to identify older adults at high risk of low physical activity (PA) levels during hospitalisation.
Methods
Prospective cohort study of 165 older adults (≥ 70 years) admitted to the department of Internal Medicine of Maastricht University Medical Centre for acute medical illness. Two prediction models were developed to predict the probability of low PA levels during hospitalisation. Time spent standing/walking per day was measured with an accelerometer until discharge (≤ 12 days). The average time standing/walking per day between inclusion and discharge was dichotomized into low/high PA levels by dividing the cohort at the median (50.0%) in model 1, and lowest tertile (33.3%) in model 2. Potential predictors—Short Physical Performance Battery (SPPB), Activity Measure for Post-Acute Care (AM-PAC), age, sex, walking aid use, and disabilities in activities of daily living—were selected based on literature and analysed using logistic regression analysis. Models were internally validated using bootstrapping. Model performance was quantified using measures of discrimination (area under the receiver operating characteristic curve (AUC)) and calibration (Hosmer and Lemeshow (H–L) goodness-of-fit test and calibration plots).
Results
Model 1 predicts a probability of spending ≤ 64.4 min standing/walking and holds the predictors SPPB, AM-PAC and sex. Model 2 predicts a probability of spending ≤ 47.2 min standing/walking and holds the predictors SPPB, AM-PAC, age and walking aid use. AUCs of models 1 and 2 were .80 (95% confidence interval (CI) = .73—.87) and .86 (95%CI = .79—.92), respectively, indicating good discriminative ability. Both models demonstrate near perfect calibration of the predicted probabilities and good overall performance, with model 2 performing slightly better.
Conclusions
The developed and internally validated prediction models may enable clinicians to identify older adults at high risk of low PA levels during hospitalisation. External validation and determining the clinical impact are needed before applying the models in clinical practise.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Background
Older adults admitted to hospital with an acute medical illness show high prevalence of multimorbidity and age-related impairments, such as malnutrition, cognitive impairment, incontinence, and sensory impairment [1]. Combined with their decreased physiological and functional reserve capacity, this can result in poor outcomes [2,3,4]. Moreover, older adults spent little time standing and walking during hospitalisation. On average, between 30 and 117 min is spent standing or walking per day, the remainder of the day is spent lying in bed or sitting in a chair [5,6,7,8,9,10,11,12].
Inactive behaviour during hospitalisation is strongly associated with functional decline [2, 12,13,14], increased length of hospital stay [13], increased risk of institutionalization [2, 5, 15], and mortality [2, 6, 8, 16]. The association between physical inactivity and these negative outcomes is independent of illness severity or comorbidities [2, 6, 9, 14, 17, 18]. If patients at high risk of spending little time standing and walking could be identified early after admission, they could be given targeted interventions aimed at increasing their time spend standing and walking, such as guidance from a physiotherapist. As offering such interventions may require substantial resources, we aimed to be able to identify patients that are the least active and that are likely to benefit most from increasing their PA behaviour. Identification of these patients can therewith contribute to improved patient outcomes as well as value-based healthcare.
To our knowledge, a prognostic tool that predicts a patient’s probability of spending little time standing and walking during hospitalisation has not been developed yet. However, in recent years the number of studies investigating physical activity (PA) behaviour of older adults admitted to a hospital with an acute medical illness has grown and more insight has been gained in factors associated with inactive behaviour during hospitalisation [7,8,9,10, 14, 19,20,21,22]. Because of the association between inactive behaviour and functional decline we expect that functional assessment tools like the Short Physical Performance Battery (SPPB) and the Activity Measure for Post-Acute Care Inpatient Basic Mobility short form (AM-PAC) could help to accurately predict the probability of spending little time standing and walking during hospitalisation for older adults. Evensen et al. supported this by showing an association between SPPB-score and time spent standing and walking in older adults acutely admitted to a geriatric ward [9]. Moreover, age [7, 19], sex [19], disabilities in activities of daily living (ADL) two weeks preceding admission [7,8,9,10, 14, 19,20,21], and the use of a walking aid preceding hospitalisation [7, 20, 22] are also reported to be associated with patients’ PA behaviour during hospitalisation. Therefore, these factors may also contribute to predictive accuracy. The aim of this study is to develop and validate a prediction model that can be used early after admission to identify older adults at high risk of spending little time standing and walking during hospitalisation.
Methods
Study Design
This single centre, prospective cohort study was conducted at the department of Internal Medicine of Maastricht University Medical Centre (MUMC +) in Maastricht, the Netherlands, between October 2018 and March 2020. The Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD) statement was used as reporting guideline (Additional file 1) [23].
Study Population
Older adults, admitted to the department of Internal Medicine of the MUMC + for acute care were recruited on weekdays by their attending physician and were asked for consent to be contacted by a researcher. Patients received verbal and written information about the study from the researcher within 48 h after admittance. The researcher contacted the patients again the next day, and written informed consent was obtained before study initiation. Confidentiality of data processing and anonymity of the participant were guaranteed.
Eligible patients were included when: 70 years or older, admitted to the department of Internal Medicine with an acute medical illness, sufficient understanding of the Dutch language, living at home before hospitalisation, and able to walk independently two weeks before admission as reported on the Functional Ambulation Categories (FAC > 3) [24, 25]. Exclusion criteria were: presence of contraindications to walking or wearing an accelerometer on the upper leg, mentally incapacitated subjects, inability to follow instructions due to cognitive problems or severe agitation, (re)admittance to the intensive care unit, a life expectancy of less than three months and previous participation in this study.
The following criteria were established through performing a brief screening with the attending physician prior to the informed consent procedure: age, admission for an acute medical illness, presence of contraindications to walking or wearing an accelerometer, mental incapacity, and life expectancy. Remaining criteria were checked by patient report.
Procedure
Physical activity monitoring
PA monitoring started immediately after informed consent was obtained (t0). PA was monitored with the MOX activity monitor (MOX; Maastricht Instruments B.V., the Netherlands). The device contains a tri-axial accelerometer sensor (ADXL362; Analog Devices, Norwood, MA, USA) in a small waterproof housing (35 × 35 × 10 mm, 11 g). Raw acceleration data (± 8 g) were measured by three orthogonal sensor axes (X, Y and Z) at a 25 Hz sampling rate. PA was measured in time spent standing/walking as this was deemed a more appropriate sensor based outcome variable for hospitalised older adults than intensity levels or step counts[26]. The MOX activity monitor has been validated to differentiate lying/sitting from standing/walking in hospitalised patients [27]. A trained researcher fixated the accelerometer to the anterior thigh with a hypoallergenic plaster, 10 cm proximal of the patella. PA was continuously measured and each accelerometer was replaced with a fully charged one after seven days when needed. Nurses examined the skin for irritation every day. PA monitoring ended after twelve days or at the day of discharge, whichever came first. After removal of the accelerometer, raw accelerometer data was uploaded to a computer and participation in the study ended (t1).
A complete measurement day was defined as a 24-h interval starting and ending at midnight. If the accelerometer was temporarily removed (e.g., MRI), days with ≥ 20 h of wear time were included in the analysis. MATLAB (version 9.5 (R2018b) Natick, Massachusetts: The MathWorks Inc.: Natick, MA, USA; 2018) was used to calculate the number of minutes spent standing/walking per day. Subsequently, the average number of minutes spent standing/walking per day between t0 and t1 was calculated per patient.
For prediction model development, the average number of minutes spent standing/walking per day between t0 and t1 was dichotomized into low and high PA levels. Clinical guidelines stipulating the amount of time patients should to be standing/walking during hospitalisation do not exist yet [11, 21, 28,29,30,31]. Guidelines for healthy elderly exist, but are not suitable for hospitalised elderly [32,33,34]. As it was not possible to determine the optimal cut-off value for the dichotomization of time spent standing/walking based on existing recommendations, a data-driven approach was used with norm-referenced cut-off values instead of criterion-referenced cut-off values. Norm-referenced cut-off values were based on the prevalence of low PA levels (32%-50%) in previous studies [2, 14, 19, 35]. To enable the comparison of models with different cut-off values, two prediction models were developed with cut-off values capturing this range. For model 1, the average number of minutes spent standing/walking per day between t0 and t1 was dichotomized into low and high PA levels by dividing the cohort at the median, categorizing 50.0% of the patients as having low PA levels. For development of model 2, the cohort was divided at the lowest tertile, categorizing 33.3% of the patients as having low PA levels. The use of accelerometers allowed the assessment of low or high PA levels between t0 and t1 to remain blinded.
Potential predictors
Potential predictors were preselected based on published studies reporting factors associated with inactive behaviour of older adults admitted to a hospital with an acute medical illness [7,8,9,10, 14, 19, 20]. The following six predictors were preselected: SPPB, AM-PAC, age, sex, disabilities in ADL two weeks preceding admission, and walking aid use preceding hospitalisation.
Functional mobility was assessed by the researcher immediately after PA monitoring had started, using the SPPB and AM-PAC. The SPPB is a performance based tool to measure physical performance by assessing balance, walking speed and lower extremity strength [36, 37]. It provides a total score between 0 and 12 points, with 12 points reflecting the highest level of performance [36, 38]. The SPPB has good to excellent intra-rater and inter-rater reliability, and excellent criterion validity and responsiveness. It presents a good balance between mobility, measurement properties and applicability to an acute care geriatric unit [36].
The AM-PAC Inpatient Basic Mobility short form assesses the following daily activities: turning in bed, sitting down and standing up, moving from lying to sitting, moving from a bed to chair, walking and climbing stairs. Climbing stairs was left out of the analysis as not every patient needs to climb stairs at home. This provided a total score between 1 and 20 points, dichotomized into dependent (≤ 19 points = 0) versus independent mobility (20 points = 1) based on receiver operating characteristic (ROC) curve analysis. The AM-PAC is short and easy to use. It shows large inter-rater reliability and test–retest reliability [39, 40] and has been validated for the entire hospital population [41].
Disabilities in ADL two weeks preceding admission were reported on the Katz Index of Independence in Activities of Daily Living (Katz ADL) at t0. It rates the patient’s performance of six activities (bathing, dressing, toileting, transferring, continence, feeding) on a dichotomous scale (dependent/independent) [42, 43]. The number of disabilities was dichotomized (0/ ≥ 1 disabilities) based on ROC curve analysis. Although few reliability and validity studies exist, the Katz ADL is used extensively to assess functional capabilities of older adults in clinical settings [43, 44]. Furthermore, age, sex (0 = male/1 = female), and walking aid use preceding hospitalisation (none, walker, cane/crutch) were assessed by patient report at t0.
Medical and demographic data
At t1, the following data was extracted from the electronic health record: clinical diagnosis of the current hospitalisation based on the ICD-11[45], number of comorbidities (Charlson Comorbidity Index), experienced falls in the last six months (0/ ≥ 1 falls), physiotherapy consulted during hospitalisation (yes/no), length of stay in hospital (days) and discharge location (home, geriatric rehabilitation centre, nursing home, other).
Sample Size
This study initially aimed to develop only one model, categorizing 50.0% of the patients as having low PA levels. Therefore, the sample size calculation was based on model 1. Post-hoc discussions regarding optimal cut-off values of low PA levels resulted in the development of a second model, enabling the comparison of different cut-off values. Six potential predictors were preselected. Because ‘walking aid use preceding hospitalisation’ has a categorical outcome containing three categories it had to be counted double, resulting in a sample size calculation based on seven potential predictors. It is recommended that at least 10 events should be collected per potential predictor [46]. An event is defined as the outcome status ‘low PA levels during hospitalisation’, with an estimated event rate of 50.0%. To develop a model with seven potential predictors, at least 70 events were required, resulting in a sample size of at least 140 patients (70/50*100). Based on the assumption of a 15% dropout rate, 165 patients were needed in this study.
Data analysis
Data quality and missing data
Data were checked for completeness and inconsistencies. Any inconsistencies or incomplete data were corrected or completed. Missing values were imputed using stochastic regression imputation with fully conditional specification [47, 48]. To determine whether imputation led to radically different results, a sensitivity analysis was performed by comparing the outcomes of the imputed data set with the use of complete cases only.
Study population characteristics
Characteristics of patients were compared between the low and high PA level groups. To compare proportions, the chi-square test was used. For continuous variables, the independent samples t-test or Mann–Whitney U test was used for normally and not-normally distributed data, respectively. A P-value < 0.05 was used to indicate statistical significance.
Model development
The models were developed using ‘low PA levels during hospitalisation’ as the outcome variable. Multicollinearity of potential predictor variables was checked using collinearity diagnostics (Pearson correlation coefficients, variance inflation factor (VIF) and tolerance). Additionally, continuous variables were checked for having a linear association with the log odds of the outcome. For model 1, all seven predictors were introduced in a multivariable logistic regression model. For model 2, only five predictors could be introduced as the sample size was based on model 1 and the prevalence of low PA levels was lower in model 2. Therefore, univariable regression analysis was performed as additional step to select five potential predictors. To reduce the number of predictors in the multivariable logistic regression models, backward stepwise elimination based on the Wald test was used. A liberal P-value of 0.20 was used to prevent too early deletion of potentially relevant predictors [49].
Internal validation
The models were internally validated using bootstrapping. B-bootstrap samples of the same size as the original sample (B = 1000 was used) were drawn with replacement from the original data, reflecting the drawing of samples from the underlying population. A shrinkage factor was estimated to adjust the model coefficients in order to make future predictions less extreme. After shrinkage, the model intercepts were re-estimated to prevent systematic under- or overestimation of risks.
Performance of the model
Overall performance of both models was assessed using Nagelkerke's R2 and the Brier score. The ability of the models to discriminate between patients with low and high PA levels during hospitalisation was quantified as the area under the receiver operating characteristic curve (AUC). Additionally, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated for a selection of probability cut-off values. To classify patients as being at high risk of low PA levels during hospitalisation, a probability threshold can be used. Patients are considered at high risk if their predicted probabilities are at or above this threshold. In order to have a low rate of patients misclassified as being at low risk (i.e. false-negative predictions), a probability threshold yielding a high NPV, but acceptable PPV, was chosen per model.
The agreement between predicted probabilities and observed frequencies of the outcome (accuracy) was assessed by visually inspecting the calibration plot. Furthermore, a Hosmer and Lemeshow (H–L) goodness-of-fit statistic was computed, with non-significant H–L statistics indicating good model fit. All statistical analyses were performed using SPSS version 23.0.0.2 (SPSS, Chicago, III., USA) and R version 4.0.4 (www.r-project.org).
Results
Study population characteristics
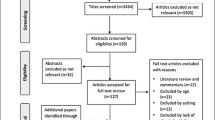
Between October 2018 and March 2020, 430 older adults admitted with an acute medical illness were screened for eligibility. In total, 215 patients were identified as eligible and 165 patients were included in this study. Of the included patients, 19 (12%) dropped out and data of 146 patients was used in the analysis (Fig. 1 TRIPOD flow chart).
Of these 146 patients, the mean age (± standard deviation (SD)) was 81.3 (6.8) years and 86 (58.9%) patients were male. The group of patients were median (± Interquartile Range (IQR)) 64.4 (34.8 – 100.1) minutes standing/walking per day and 33.3% of the patients were ≤ 47.2 min standing/walking per day. The characteristics of study participants are reported in Table 1.
Model development and internal validation
In the dependent variable ‘number of minutes spent standing/walking per day’, data was missing for 72 out of 949 measurement days (7.5%), spread over 27 patients (18%). Main reasons for missing values were the accelerometer falling off, getting lost or malfunctioning. Data of all other variables was complete. After imputation, data of all 146 patients was complete for development of the prediction model.
For development of model 1, all predictors were entered in the multivariable regression model (SPPB, AM-PAC, age, sex, disabilities in ADL, and walking aid use). For development of model 2, univariable regression analysis was first performed on all potential predictors, after which SPPB, AM-PAC, age, and walking aid use preceding hospitalisation were entered in the multivariable regression model. Table 2 shows the original and internally validated models that can be used to compute the probability of low PA levels during hospitalisation. Internal validation yielded a shrinkage factor of 0.95 and 0.90 in model 1 and 2, respectively. The equations in Table 2 can be used to compute the individual probability of low PA levels during hospitalisation.
Performance of the models
All model performance measures are shown in detail in Additional file 2. The AUCs of model 1 and 2 were 0.80 (95% confidence interval (CI) = 0.73—0.87) and 0.86 (95%CI = 0.79—0.92), respectively, indicating good discriminatory ability. The optimism-corrected AUCs were 0.79 and 0.84 for model 1 and 2, respectively. Figure 2 shows the ROC curves of both models. Both models show good calibration, as indicated by calibration plots showing good agreement between actual and predicted probabilities (Additional file 3). Additionally, both H–L goodness-of-fit tests were non-significant (p = 0.755 and p = 0.209). Overall, model 2 showed a slightly better performance.
A selection of probability cut-off values and their corresponding sensitivity, specificity, PPV and NPV are reported in Additional file 4 and Additional file 5. Probability thresholds of 0.41 and 0.30 were chosen for model 1 and 2, respectively. The corresponding classification tables are shown in Fig. 3.
Classification tables showing the actual and predicted number of patients with low or high PA levels during hospitalisation and their corresponding sensitivity, specificity, PPV and NPV, using (A) model 1 (probability threshold .41) and (B) model 2 (probability threshold .30). PA = Physical Activity, TP = True Positive, FP = False Positive, FN = False Negative, TN = True Negative, PPV = Positive Predictive Value, NPV = Negative Predictive Value
The sensitivity analyses showed similar results for the imputed and the original non-imputed data sets and we conclude that imputation did not result in large differences.
Discussion
In this study we developed and internally validated two prediction models that can be used to predict the probability of low PA levels during hospitalisation for older adults admitted to a hospital with an acute medical illness. The first model predicts a patient’s probability of spending less than an average of 64.4 min standing/walking per day and holds three predictors: SPPB, AM-PAC and sex. The second model predicts a patient’s probability of spending less than an average of 47.2 min standing/walking per day and holds four predictors: SPPB, AM-PAC, age and walking aid use preceding hospitalisation. Both models showed good discriminative ability and accurate prediction of spending little time standing/walking during hospitalisation, with the second model performing slightly better.
To our knowledge, this is the first study that aims to identify older adults at high risk of spending little time standing/walking during hospitalisation. One of the challenges in developing a suitable prediction model was the lack of criterion-referenced cut-off values regarding the classification of low or high PA levels [11, 21, 28,29,30,31]. Although many studies have shown that PA contributes to the prevention of negative outcomes, the optimal-dose response relationship remains unknown. Baldwin et al. provided the first international consensus for recommendations on PA and sedentary behaviour for older adults hospitalised with an acute medical illness. They recommend that older adults should: be as physically active as their abilities and condition allow; minimise time spent sedentary for extended periods; and, move more and sit less throughout the day. Additionally, muscle strengthening and balance exercises are also advised [29]. Although this provides some guidance, criterion-referenced cut-off values regarding the classification of low and high PA levels are still lacking [11, 21, 28,29,30,31]. Therefore, the current prediction models were developed using a data-driven approach with norm-referenced cut-off values capturing the range of low PA levels identified in previous studies [2, 14, 19, 35]. We do not know whether the resulting cut-off values of 64.4 or 47.2 min standing/walking are sufficient to prevent the negative effects of inactivity. This may also be influenced by many individual factors such as preadmission status, illness severity or daily caloric intake, necessitating more personalised recommendations. However, as the PA behaviour of older adults admitted for acute illness was heterogeneous in previous literature, we aimed to be able to identify patients that are the least active. As offering interventions (e.g., monitoring patients’ PA behaviour using wearables) may require substantial financial resources, the prediction models allow to identify patients that are likely to benefit most from such interventions. Although criterion-referenced cut-off values are lacking, the chosen cut-off values are relevant and contribute to providing value-based healthcare.
PA was measured in time spent in different activities (lying/sitting, standing/walking), as this was deemed a more meaningful sensor based outcome variable for hospitalised older adults than intensity levels (activity counts) or step counts [26]. First, the intensity of PA as perceived by patients may deviate from the intensity measured by the accelerometer. When patients are feeling ill they may perceive walking at low walking speeds as a high intensity activity, while the accelerometer objectively classifies this as a low intensity activity. Second, recovery or deterioration during the admission period may result in fluctuations in perceived intensity within patients. Third, many older adults admitted to hospital with an acute medical illness require a walking aid. Moreover, slow and impaired gaits are common [50]. Several studies have shown that these factors decrease the validity of activity trackers to measure step counts [51,52,53,54]. Lastly, movements of the arms or legs performed in bed or on a chair may result in an overestimation of step count. Therefore, time spent in different activities is deemed the most appropriate sensor-based outcome measure [26].
The developed models show that functional assessments combined with easily acquired clinical parameters have potential to identify patients at high risk of low PA levels during hospitalisation. As we felt it important that patients at high risk would not miss out on an intervention, we opted for models with low rates of false-negative predictions. In choosing a probability threshold for each model we therefore aimed for a high NPV and accepted a higher rate of false-positive predictions.
Using model 1 with a probability threshold of 0.41 resulted in misclassification of 8.9% of all patients as being at low risk of spending less than 64.4 min standing/walking (false-negative predictions). Moreover, 20.6% were misclassified as being at high risk (false-positive predictions) and would be given an intervention while actually having high PA levels. The predictive abilities of model 2 are slightly better, resulting in less misclassifications. Using model 2 with a probability threshold of 0.30 will result in a 4.8% false-negative prediction rate and a 15.1% false-positive prediction rate of spending less than 47.2 min standing/walking.
However, a certain level of misclassifications seems inevitable when predicting the PA behaviour of hospitalised patients early after admission. Previous studies have shown that the PA behaviour of hospitalised patients is influenced by many different factors, such as complications or symptoms developing throughout the hospital stay, patient motivation, using medical devices that limit walking (e.g., IV-poles, urinary catheters, lack of portable oxygen), referral to physiotherapy, or a lack of availability of healthcare staff to provide assistance during walking [55,56,57,58,59,60,61]. As some of these factors are unknown yet early after admission, they cannot be included in the models as predictors and may therefore contribute to misclassifications.
In retrospect, we consider the rate of misclassifications of both models acceptable for use in clinical practice. Although performance of model 2 was slightly better, the choice for either one of the models depends on whether roughly one hour or three quarters of an hour standing/walking are preferred as cut-off value for low PA levels during hospitalisation. Moreover, it also depends on the availability of resources, as model 1 will classify more patients as being at high risk of low PA levels.
Strengths and limitations
The present study has several strengths, including a prospective data collection, recruitment of consecutive patients, and inclusion of patients with mild cognitive impairments. Moreover, all predictors were selected from literature based on previous evidence [7,8,9,10, 14, 19, 20] while the methodology of this study followed the TRIPOD guideline for prediction modelling [23]. We corrected for missing data, performed a sensitivity analysis and an internal validation procedure of the developed models.
We also recognise several limitations. First, with the current study design we cannot determine if patients changed their time spent standing/walking due to potential confounding factors that were unknown early after admission, such as receiving physiotherapy guidance or complications which may have developed during hospital stay. Second, we initially aimed to develop one prediction model. Due to post-hoc discussions regarding an optimal cut-off value we decided to develop a second model as well, enabling the comparison of different cut-off values. The power calculation was originally meant for model 1, allowing for seven potential predictors and an event rate of 50.0%. Because the event rate of model 2 was 33.3%, only five potential predictors could be introduced in the analysis. To prevent overfitting of the model by introducing to many predictors, univariable analysis had to be performed as an additional step. Third, we dichotomized the categorical AM-PAC and Katz ADL outcomes to improve the clinical applicability, which may have led to loss of information. Lastly, the algorithm of the MOX activity monitor is unable to differentiate time spent standing from time spent walking. This limited the development of a prediction model that can be used to identify older adults at high risk of spending little time walking during hospitalisation.
Clinical implications and recommendations for future research
The developed prediction models can be used in clinical practice by performing a simple screening early after admission, consisting of two functional assessments combined with self-reported information. The prediction models can be adapted into an easy-to-use calculator that can be used during screening. Using the prediction models to identify patients at high risk of low PA levels early after admission is an important first step in preventing the negative effects associated with spending little time standing/walking during hospitalisation. Patients at high risk can subsequently be given interventions aimed at increasing their time spend standing/walking. However, as few studies have investigated the efficacy of interventions aimed at increasing the PA behaviour of older adults during hospitalisation, further research is advised, comparing different types of interventions and with detailed reporting of frequency, intensity and duration [4, 29,30,31]. Moreover, the accelerometer algorithm used in the current study was not able to differentiate standing from walking. Therefore, we recommend future studies to develop a prediction model using an optimised accelerometer algorithm that allows to differentiate between standing and walking in hospitalised patients. Furthermore, the current study included seven potential predictors that were associated with low PA levels of older adults admitted to hospital with an acute medical illness. However, the PA behaviour of hospitalised older adults may be influenced by many other factors as well, such as ‘reason for hospitalisation’ or ‘history of falls’ [7, 20,21,22, 55,56,57,58,59,60,61]. In order to improve the prediction of older adults at risk of low PA levels, we recommend that this study should be followed by a larger study that allows to include more potential predictor variables. Lastly, before implementing the prediction models into clinical practice, future research should also focus on assessing the next steps within prediction modelling: determining the external validity and clinical impact of the models. Because this has not been performed yet in the current study, our results should be interpreted with caution. In order to choose a cut-off value for low PA levels during hospitalisation based on empirical evidence, future research should also focus on developing guidelines regarding the recommended PA levels of older adults admitted to a hospital with an acute medical illness.
Conclusions
We developed and internally validated two prediction models that can be used to predict the probability of low PA levels during hospitalisation for older adults admitted to a hospital with an acute medical illness. Both models showed a good overall performance, with a good discriminative ability and accurate prediction of low PA levels. This study showed that both models hold promise as prediction tools that enable clinicians to accurately identify older adults at high risk of low PA levels during hospitalisation.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- PA :
-
Physical Activity
- SPPB:
-
Short Physical Performance Battery
- AM-PAC:
-
Activity Measure for Post-Acute Care Inpatient Basic Mobility short form
- ADL:
-
Activities of Daily Living
- MUMC + :
-
Maastricht University Medical Centre
- TRIPOD:
-
Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis
- FAC :
-
Functional Ambulation Categories
- MOX :
-
MOX Activity Monitor
- ROC:
-
Receiver Operating Characteristic
- ICD:
-
International Classification of Diseases for mortality and morbidity
- VIF:
-
Variance Inflation Factor
- AUC:
-
Area Under the receiver operating characteristic Curve
- PPV:
-
Positive Predictive Value
- NPV:
-
Negative Predictive Value
- H–L :
-
Hosmer and Lemeshow
- SD:
-
Standard Deviation
- IQR:
-
Interquartile Range
- CCI:
-
Charlson Comorbidity Index
- LOS:
-
Length Of hospital Stay
- CI:
-
Confidence Interval
References
Buurman BMHJ, de Haan RJ, Abu-Hanna A, Lagaay AM, Verhaar HJ, et al. Geriatric conditions in acutely hospitalized older patients: prevalence and one-year survival and functional decline. PLoS ONE. 2011;6(11):e26951.
Brown CJ, Friedkin RJ, Inouye SK. Prevalence and outcomes of low mobility in hospitalized older patients. J Am Geriatr Soc. 2004;52(8):1263–70.
Boyd CM, Landefeld CS, Counsell SR, Palmer RM, Fortinsky RH, Kresevic D, et al. Recovery of activities of daily living in older adults after hospitalization for acute medical illness. J Am Geriatr Soc. 2008;56(12):2171–9.
Scheerman K, Raaijmakers K, Otten RHJ, Meskers CGM, Maier AB. Effect of physical interventions on physical performance and physical activity in older patients during hospitalization: a systematic review. BMC Geriatr. 2018;18(1):288.
Brown CJ, Redden DT, Flood KL, Allman RM. The underrecognized epidemic of low mobility during hospitalization of older adults. J Am Geriatr Soc. 2009;57(9):1660–5.
Ostir GV, Berges IM, Kuo YF, Goodwin JS, Fisher SR, Guralnik JM. Mobility activity and its value as a prognostic indicator of survival in hospitalized older adults. J Am Geriatr Soc. 2013;61(4):551–7.
Fisher SR, Goodwin JS, Protas EJ, Kuo YF, Graham JE, Ottenbacher KJ, et al. Ambulatory activity of older adults hospitalized with acute medical illness. J Am Geriatr Soc. 2011;59(1):91–5.
Pedersen MM, Bodilsen AC, Petersen J, Beyer N, Andersen O, Lawson-Smith L, et al. Twenty-four-hour mobility during acute hospitalization in older medical patients. J Gerontol A Biol Sci Med Sci. 2013;68(3):331–7.
Evensen S, Sletvold O, Lydersen S, Taraldsen K. Physical activity among hospitalized older adults - an observational study. BMC Geriatr. 2017;17(1):110.
Villumsen M, Jorgensen MG, Andreasen J, Rathleff MS, Molgaard CM. Very Low Levels of Physical Activity in Older Patients During Hospitalization at an Acute Geriatric Ward: A Prospective Cohort Study. J Aging Phys Act. 2015;23(4):542–9.
Hartley P, DeWitt AL, Forsyth F, Romero-Ortuno R, Deaton C. Predictors of physical activity in older adults early in an emergency hospital admission: a prospective cohort study. BMC Geriatr. 2020;20(1):177.
Pavon JM, Sloane RJ, Pieper CF, Colón-Emeric CS, Cohen HJ, Gallagher D, et al. Accelerometer-Measured Hospital Physical Activity and Hospital-Acquired Disability in Older Adults. J Am Geriatr Soc. 2020;68(2):261–5.
Covinsky KE, Pierluissi E, Johnston CB. Hospitalization-associated disability: “She was probably able to ambulate, but I’m not sure.” JAMA. 2011;306(16):1782–93.
Zisberg A, Shadmi E, Sinoff G, Gur-Yaish N, Srulovici E, Admi H. Low mobility during hospitalization and functional decline in older adults. J Am Geriatr Soc. 2011;59(2):266–73.
Mahoney JE, Sager MA, Jalaluddin M. Use of an ambulation assistive device predicts functional decline associated with hospitalization. J Gerontol A Biol Sci Med Sci. 1999;54(2):M83–8.
Covinsky KE, Palmer RM, Fortinsky RH, Counsell SR, Stewart AL, Kresevic D, et al. Loss of independence in activities of daily living in older adults hospitalized with medical illnesses: increased vulnerability with age. J Am Geriatr Soc. 2003;51(4):451–8.
McCullagh R, Dillon C, Dahly D, Horgan NF, Timmons S. Walking in hospital is associated with a shorter length of stay in older medical inpatients. Physiol Meas. 2016;37(10):1872–84.
Fisher SR, Graham JE, Ottenbacher KJ, Deer R, Ostir GV. Inpatient Walking Activity to Predict Readmission in Older Adults. Arch Phys Med Rehabil. 2016;97(9 Suppl):S226–31.
Zisberg A, Syn-Hershko A. Factors related to the mobility of hospitalized older adults: A prospective cohort study. Geriatric nursing (New York, NY). 2016;37(2):96–100.
Fisher SR, Graham JE, Brown CJ, Galloway RV, Ottenbacher KJ, Allman RM, et al. Factors that differentiate level of ambulation in hospitalised older adults. Age Ageing. 2012;41(1):107–11.
Tasheva P, Kraege V, Vollenweider P, Roulet G, Méan M, Marques-Vidal P. Accelerometry assessed physical activity of older adults hospitalized with acute medical illness - an observational study. BMC Geriatr. 2020;20(1):382.
Tasheva P, Vollenweider P, Kraege V, Roulet G, Lamy O, Marques-Vidal P, et al. Association Between Physical Activity Levels in the Hospital Setting and Hospital-Acquired Functional Decline in Elderly Patients. JAMA Netw Open. 2020;3(1):e1920185.
Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. BMJ. 2015;350:g7594.
de Morton NA, Berlowitz DJ, Keating JL. A systematic review of mobility instruments and their measurement properties for older acute medical patients. Health Qual Life Outcomes. 2008;6:44.
Holden MK, Gill KM, Magliozzi MR, Nathan J, Piehl-Baker L. Clinical gait assessment in the neurologically impaired. Reliability and meaningfulness Physical therapy. 1984;64(1):35–40.
van Dijk-Huisman HC, Weemaes ATR, Boymans T, Lenssen AF, de Bie RA. Smartphone App with an Accelerometer Enhances Patients’ Physical Activity Following Elective Orthopedic Surgery: A Pilot Study. Sensors (Basel, Switzerland). 2020;20(15):4317.
Bastings SJL. Klinische validatie van de MOX en algoritme voor activiteitenmonitoring bij orthopedische en oncologische patiënten tijdens postoperatieve ligduur. Heerlen: Zuyd Hogeschool; 2017.
Baldwin C, van Kessel G, Phillips A, Johnston K. Accelerometry Shows Inpatients With Acute Medical or Surgical Conditions Spend Little Time Upright and Are Highly Sedentary: Systematic Review. Phys Ther. 2017;97(11):1044–65.
Baldwin CE, Phillips AC, Edney SM, Lewis LK. Recommendations for older adults’ physical activity and sedentary behaviour during hospitalisation for an acute medical illness: an international Delphi study. Int J Behav Nutr Phys Act. 2020;17(1):69.
Ley L, Khaw D, Duke M, Botti M. The dose of physical activity to minimise functional decline in older general medical patients receiving 24-hr acute care: A systematic scoping review. J Clin Nurs. 2019;28(17–18):3049–64.
Resnick B, Boltz M. Optimizing Function and Physical Activity in Hospitalized Older Adults to Prevent Functional Decline and Falls. Clin Geriatr Med. 2019;35(2):237–51.
Gezondheidsraad. Beweegrichtlijnen 2017. Den Haag: Gezondheidsraad; 2017.
Bull FC, Al-Ansari SS, Biddle S, Borodulin K, Buman MP, Cardon G, et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med. 2020;54(24):1451–62.
WHO Guidelines Approved by the Guidelines Review Committee. WHO Guidelines on Physical Activity and Sedentary Behaviour. Geneva: World Health Organization © World Health Organization; 2020.
Agmon M, Zisberg A, Gil E, Rand D, Gur-Yaish N, Azriel M. Association Between 900 Steps a Day and Functional Decline in Older Hospitalized Patients. JAMA Intern Med. 2017;177(2):272–4.
Soares Menezes KVR, Auger C, de Souza Menezes WR, Guerra RO. Instruments to evaluate mobility capacity of older adults during hospitalization: A systematic review. Arch Gerontol Geriatr. 2017;72:67–79.
Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85-94.
McCullagh R, O’Connell E, O’Meara S, Perry I, Fitzgerald A, O’Connor K, et al. A study protocol of a randomised controlled trial to measure the effects of an augmented prescribed exercise programme (APEP) for frail older medical patients in the acute setting. BMC Geriatr. 2016;16:79.
Jette DU, Stilphen M, Ranganathan VK, Passek S, Frost FS, Jette AM. Interrater Reliability of AM-PAC “6-Clicks” Basic Mobility and Daily Activity Short Forms. Phys Ther. 2015;95(5):758–66.
Hoyer EH, Young DL, Klein LM, Kreif J, Shumock K, Hiser S, et al. Toward a Common Language for Measuring Patient Mobility in the Hospital: Reliability and Construct Validity of Interprofessional Mobility Measures. Phys Ther. 2018;98(2):133–42.
Jette DU, Stilphen M, Ranganathan VK, Passek SD, Frost FS, Jette AM. Validity of the AM-PAC “6-Clicks” inpatient daily activity and basic mobility short forms. Phys Ther. 2014;94(3):379–91.
Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of adl: a standardized measure of biological and psychosocial function. Jama. 1963;185:914–9.
Liebzeit D, King B, Bratzke L. Measurement of function in older adults transitioning from hospital to home: an integrative review. Geriatr Nurs. 2018;39(3):336–43.
Hartigan I. A comparative review of the Katz ADL and the Barthel Index in assessing the activities of daily living of older people. Int J Older People Nurs. 2007;2(3):204–12.
Organization WH. International Classification of Diseases for Mortality and Morbidity Statistics (11th Revision) 2019 [11:[Available from: https://icd.who.int/browse11/l-m/en#/http://id.who.int/icd/entity/2136808878.
Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49(12):1373–9.
Donders AR, van der Heijden GJ, Stijnen T, Moons KG. Review: a gentle introduction to imputation of missing values. J Clin Epidemiol. 2006;59(10):1087–91.
Steyerberg EW. Clinical Prediction Models. 1st ed. New York, NY: Springer; 2009.
Harrell F. Regression Modeling Strategies. New York, NY: Springer; 2001.
Peel NM, Kuys SS, Klein K. Gait speed as a measure in geriatric assessment in clinical settings: a systematic review. J Gerontol A Biol Sci Med Sci. 2013;68(1):39–46.
Floegel TA, Florez-Pregonero A, Hekler EB, Buman MP. Validation of Consumer-Based Hip and Wrist Activity Monitors in Older Adults With Varied Ambulatory Abilities. J Gerontol A Biol Sci Med Sci. 2017;72(2):229–36.
Beevi FH, Miranda J, Pedersen CF, Wagner S. An Evaluation of Commercial Pedometers for Monitoring Slow Walking Speed Populations. Telemed J E Health. 2016;22(5):441–9.
Martin JB, Krč KM, Mitchell EA, Eng JJ, Noble JW. Pedometer accuracy in slow walking older adults. Int J Ther Rehabil. 2012;19(7):387–93.
Cyarto EV, Myers A, Tudor-Locke C. Pedometer accuracy in nursing home and community-dwelling older adults. Med Sci Sports Exerc. 2004;36(2):205–9.
So C, Pierluissi E. Attitudes and expectations regarding exercise in the hospital of hospitalized older adults: a qualitative study. J Am Geriatr Soc. 2012;60(4):713–8.
Brown CJ, Williams BR, Woodby LL, Davis LL, Allman RM. Barriers to mobility during hospitalization from the perspectives of older patients and their nurses and physicians. J Hosp Med. 2007;2(5):305–13.
Boltz M, Capezuti E, Shabbat N. Nursing staff perceptions of physical function in hospitalized older adults. Appl Nurs Res. 2011;24(4):215–22.
Doherty-King B, Bowers B. How nurses decide to ambulate hospitalized older adults: development of a conceptual model. Gerontologist. 2011;51(6):786–97.
Moore JE, Mascarenhas A, Marquez C, Almaawiy U, Chan WH, D’Souza J, et al. Mapping barriers and intervention activities to behaviour change theory for Mobilization of Vulnerable Elders in Ontario (MOVE ON), a multi-site implementation intervention in acute care hospitals. Implement Sci. 2014;9:160.
Boltz M, Capezuti E, Shabbat N, Hall K. Going home better not worse: older adults’ views on physical function during hospitalization. Int J Nurs Pract. 2010;16(4):381–8.
Koenders N, van Oorsouw R, Seeger JPH, Nijhuis-van der Sanden MWG, van de Glind I, Hoogeboom TJ. “I’m not going to walk, just for the sake of walking…”: a qualitative, phenomenological study on physical activity during hospital stay. Disabil Rehabil. 2020;42(1):78–85.
Acknowledgements
We would like to thank all physiotherapists, nurses and physicians working at the department of Internal Medicine of the MUMC+ for their assistance in this study. We would also like to thank Bo Brans-Brabant, Anouk Weemaes and Marissa Gerards for their assistance in the data collection.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
HCvDH, AFL, and RdB were involved in the conceptualization and methodology of the study. HCvDH, MW and FJHM have performed the investigation. W.B. contributed to the software and HCvDH, AFL, RdB, WB, and SMJvK performed the formal analysis. HCvDH and SMJvK were responsible for the visualization and AFL and RdB for the recourses and supervision. HCvDH performed the project administration, data curation, and writing of the original draft. All authors reviewed and edited the final version, and read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Medical Ethics Committee of the University Hospital Maastricht and Maastricht University (METC azM/UM), registration number METC18-103, date of approval 4 October 2018. Informed consent was obtained from all subjects involved in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
TRIPOD checklistPrediction Model Development. Checklist for transparent reporting of amultivariable prediction model for individual prognosis or diagnosis (TRIPOD):The TRIPOD statement.
Additional file 2.
Performance of theprediction models. Model performance measures of the two prediction models forpredicting the probability of low physical activity levels duringhospitalisation for older adults admitted to a hospital with an acute medicalillness.
Additional file 3.
Calibration plots. Calibrationplots with the observed frequency of low physical activity levels by predictedprobability of model 1 and 2.
Additional file 4.
Probability cut-offvalues model 1. Sensitivity, specificity, positive predictive value, andnegative predictive value for low physical activity levels duringhospitalisation at a selection of consecutive cut-off points of the predictedprobability of model 1.
Additional file 5.
Probability cut-offvalues model 2. Sensitivity, specificity, positive predictive value, andnegative predictive value for low physical activity levels duringhospitalisation at a selection of consecutive cut-off points of the predictedprobability of model 2.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
van Dijk-Huisman, H.C., Welters, M.H.P., Bijnens, W. et al. Development and internal validation of a prediction model to identify older adults at risk of low physical activity levels during hospitalisation: a prospective cohort study. BMC Geriatr 22, 479 (2022). https://doi.org/10.1186/s12877-022-03146-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12877-022-03146-9