Abstract
Background
There is a contradiction in the use of microbiota-therapies, including probiotics, prebiotics, and synbiotics, to improve the condition of patients with nonalcoholic fatty liver disease (NAFLD). The aim of this review was to evaluate the effect of microbiota-therapy on liver injury, inflammation, and lipid levels in individuals with NAFLD.
Methods
Using Pubmed, Embase, Cochrane Library, and Web of Science databases were searched for articles on the use of prebiotic, probiotic, or synbiotic for the treatment of patients with NAFLD up to March 2024.
Results
Thirty-four studies involving 12,682 individuals were included. Meta-analysis indicated that probiotic, prebiotic, and synbiotic supplementation significantly improved liver injury (hepatic fibrosis, SMD = -0.31; 95% CI: -0.53, -0.09; aspartate aminotransferase, SMD = -0.35; 95% CI: -0.55, -0.15; alanine aminotransferase, SMD = -0.48; 95% CI: -0.71, -0.25; alkaline phosphatase, SMD = -0.81; 95% CI: -1.55, -0.08), lipid profiles (triglycerides, SMD = -0.22; 95% CI: -0.43, -0.02), and inflammatory factors (high-density lipoprotein, SMD = -0.47; 95% CI: -0.88, -0.06; tumour necrosis factor alpha, SMD = -0.86 95% CI: -1.56, -0.56).
Conclusion
Overall, supplementation with probiotic, prebiotic, or synbiotic had a positive effect on reducing liver enzymes, lipid profiles, and inflammatory cytokines in patients with NAFLD.
Similar content being viewed by others
Background
Nonalcoholic fatty liver disease (NAFLD) is the hepatic manifestation of metabolic syndrome, a spectrum of liver diseases ranging from simple steatosis to nonalcoholic steatohepatitis, cirrhosis, and even transformation to liver cancer [1, 2]. NAFLD is now the most prevalent liver disease worldwide, with a global prevalence between 25% and 45% [3]. In 2019, NAFLD became the number one chronic liver disease and the leading cause of abnormal liver biochemistry in health screenings [4]. The main pathogenesis of NAFLD is due to hepatic lipid accumulation and disturbed glucose metabolism [5]. Steatosis, or generalized fat buildup in vesicles that replace the cytoplasm of hepatocytes, is a hallmark of NAFLD. There is currently no established protocol for the potential mechanism and effective management of the onset and progression of NAFLD, which mostly involves dietary and lifestyle improvements. Such measures can slow the course of NAFLD and effectively control it by lowering liver lipids, enhancing the activation of liver enzymes, and decreasing plasma triglycerides [6]. According to increasing amounts of data, the gut-liver axis is linked to the development and progression of NAFLD, and is thought to be a potential approach for treating NAFLD [7,8,9,10].
In recent years, numerous studies have shown that patients with NAFLD exhibit variation in the intestinal microbiota and an increase in the occurrence of small intestinal bacterial overgrowth, and these changes seem to be related to the severity of NAFLD. Therefore, aiming to intervene in the gut flora of patients with NAFLD, the use of prebiotics, probiotics, or synbiotics has been the subject of current research [11, 12]. Prebiotics, probiotics, or synbiotics improve the health status of patients with NAFLD through effects on the intestinal flora, such as delaying the onset of the disease by balancing intestinal microbes, permeability, and inflammation when provided at an adequate dosage and for a sufficient duration [13, 14]. Moreover, this treatment protocol not only regulates intestinal microbial homeostasis, permeability, and inflammation, but also enhances the production of short-chain fatty acids such as butyrate through microbial pathways, influences energy metabolism in the gut and systemically, and achieves therapeutic effects to ameliorate disease through the gut-liver axis [15, 16].
Live microbial nutrient supplements called “probiotics” help the equilibrium of intestinal bacteria in the host organism [17]. A previous study showed that the administration of probiotic organisms could exert a lipid-lowering effect to maintain cardiovascular well-being [18,19,20]. Prebiotics are organic compounds that the host does not digest or absorb; instead, they selectively encourage the growth and multiplication of healthy bacteria such as Bifidobacteria, which enhances the host’s wellness [21]. Previous systematic reviews have explored the potential of prebiotics in the treatment of NAFLD by Stachowska et al. [21], who demonstrated that prebiotics could improve anthropometric parameters and liver enzyme levels. Synbiotics, in a preparation called symbiosis, combine probiotics and prebiotics, sometimes with the addition of vitamins, trace minerals, etc. Probiotics and prebiotics can cooperate to prevent disease and retain the microecological balance of the organism by bringing into play both the physiological and bacterial activities of the former [22]. A systematic review and meta-analysis by Khan et al. [23] revealed that probiotics and synbiotics could not only reduce liver enzymes, but also decrease inflammatory cytokines in patients with NAFLD. However, regarding the potential efficacy of employing microbiota treatment in the clinical care of patients with NAFLD, the available evidence is contradictory.
The potential for improving NAFLD with probiotics, prebiotics, and synbiotics has already been examined in meta-analyses and systematic reviews [17, 23,24,25,26]. Nevertheless, other meta-analysis studies included only a small number of published articles, some of which focused solely on the effectiveness of probiotics or prebiotics, and concentrated on the outcomes of insulin resistance and lipid profiles (and not liver-specific outcomes) [11, 27,28,29]. We investigated the improvements in liver-specific indicators, lipid profiles, and inflammation induced by probiotics, prebiotics, and synbiotics through a meta-analysis to obtain more conclusive results.
Methods
Search strategy
This systematic review and meta-analysis of randomized controlled trials (RCTs) were carried out following the PRISMA guidelines, which recommend reporting items for systematic reviews and meta-analyses. The literature search was performed in Embase, PubMed, Cochrane Library, and Web of Science from inception to March 2024. The following search strings were used in the search process: (“probiotic” OR “synbiotic” OR “prebiotic”) in combination with (“non-alcoholic fatty liver disease”) and (“randomized controlled trials”), and a lookup of the relevant free words in databases of foreign language literature such as PubMed. To identify intersections, we used the subjects and free words with the Boolean operation AND/OR to search for target articles in abstract keywords or titles, and the language was limited to English. Moreover, the reference or citation lists of the retrieved articles were checked to search for further relevant studies. The full search approach is detailed in Appendix 1 Supplemental Table 1.
Inclusion criteria and exclusion criteria
Inclusion criteria
Original studies were included if they met the following criteria: (1) each subject included in the literature complied with the Chinese Medical Association’s Guidelines for the Treatment of Non-Alcoholic Fatty Liver Disease (2010 version); (2) the inclusion of data with spelled out means, standard deviations, or standard deviations that can be computed mathematically; and (3) randomized controlled trial with a control group or placebo, and an intervention group of probiotic, prebiotic, or synbiotic for NAFLD. No restrictions on the utilization of blinding or allocation concealment were placed on the randomized controlled trial. The research question for the systematic review was established using criteria (Table 1).
Exclusion criteria
Original studies were excluded if they met the following criteria: (1) were duplicate literature; (2) were reviews, case reports, conference proceedings, or article for which data were unavailable, for which the article statements were not available; (3) had viral hepatitis, alcoholic hepatitis, drug-related hepatitis, autoimmune hepatitis, or chronic liver diseases caused by genetic metabolic diseases; (4) had apparent errors in the statistical methods or contradictory experimental results; or (5) were nonrandomized controlled trials or animal experiments (Table 1).
Study screening and data extraction
Duplicate literature was removed using Endnote software and manual reading, and the read titles and abstracts of the remaining publications were then assessed to determine whether the literature met the inclusion criteria. Data extraction was independently conducted using a standardized data collection by two investigators (Y.Y., W.M.), and a third evaluator (C.Y.) was asked to jointly discuss whether to include the literature that had different opinions. The data extracted included the basic information of the included studies, including the first author, year of publication, case number, age, intervention measures, treatment course, and outcome indicators.
The primary outcome indicators included liver-related outcomes, namely, serum Alanine aminotransferase (ALT), aspartate aminotransferase (AST) levels, changes in hepatic fibrosis via elastography, and changes in hepatic steatosis via ultrasound. The secondary outcomes were body mass index (BMI), g-glutamyltransferase (GGT), alkaline phosphatase (ALP), total cholesterol (TC), triglyceride (TG), high-density lipoprotein (HDL), low-density lipoprotein (LDL), high-sensitivity C-reactive protein (hs-CRP), tumour necrosis factor α (TNF-α), lipopolysaccharide (LPS), and interleukin 6 (IL-6) levels.
Study quality assessment
The quality of the included literature was evaluated based on the Cochrane risk of bias assessment tool, which was divided into the following areas: (1) selection bias (random sequence generation) (2) selection bias (allocation concealment) (3) implementation bias (blinding of investigators and subjects) (4) measurement bias (blinded evaluation of study outcomes) (5) follow-up bias (completeness of outcome) (6) reporting bias (selective reporting of study results), and (7) other bias (other sources of bias). RevMan 5.4 software was used to classify the above biases as “low risk”, “high risk”, or “unclear risk”. We comprehensively evaluated the “risk” levels and the reasonableness and stringency of each article. Funnel plots were also used to evaluate publication bias.
Statistical analysis
The data from the included studies were synthesized and analysed by Stata MP 16, and the quality of the included literatures was estimated by RevMan 5.4. The included studies must contain at least 1 of the above outcomes of interest for this review and include the baseline and endpoint values or net changes between them with the mean and standard deviation available. Considering the different scales for some outcomes used in the original studies, we calculated the standardized mean difference (SMD) and its 95% confidence interval (95% CI) to observe continuous outcomes, while effect size will be represented by odds ratios (ORs) and 95% CI for continuous data if the assessment tools were the same across the original studies.
The heterogeneity among studies was examined through the Q test and the I2 value. Random-effects or fixed-effects models were used based on the results of the heterogeneity test; P < 0.05 or I2 > 50% was considered to indicate significant heterogeneity, and a random-effect model was used to conduct the meta-analysis (I2 > 25%); otherwise, a fixed-effect model was used (I2 ≤ 25%). Meta-regression was conducted to examine the characteristics of the studies that were hypothesized to influence the observed treatment effects. The association between the overall estimate of effect sizes and potential moderator variables, including intervention type, country, intervention duration, and sample size, was assessed.
Further subgroup analyses were performed to explore the impacts of certain characteristics: intervention duration (≤ 12 weeks and > 12 weeks), country (Asian and Europe & US (European and American countries)), intervention type (probiotic, prebiotic, and synbiotic), and sample size (> 40 and ≤ 40). The Begg’s and Egger’s test tests were used to assess the publication bias of the studies included in the final analysis. The alpha level for statistical significance was set at 0.05.
Results
Selection and characteristics of studies
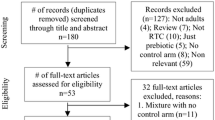
Figure 1 illustrates the procedure for extracting the data. Using the planned search technique, the last electronic database search was completed on March 17, 2024, and 2,079 articles were retrieved. Ultimately, the qualitative review included 34 double-blind, randomized, placebo-controlled trials [30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63].
The characteristics of the included trials are summarized in Table 2. Except for fibrosis (I2 = 22.00%) and steatosis (I2 = 0.00%), all included studies employed a random-effects model. All included studies were conducted between 2012 and 2024. Of the 34 included studies, 20 were conducted in Asia, and the remaining 14 were conducted in Europe, America and Africa. The therapeutic interventions were prebiotic in 5 studies, probiotic included 19 studies, and synbiotic in 10 studies. The intervention duration of the studies ranged from 8 to 56 weeks. The age of the participants was less than 18 years in 2 studies, and the age of the remaining 32 studies was more than 18 years. Patient and control sample sizes ranged from 14 to 140. The risk of bias was assessed as shown in Fig. 2 and Appendix 1 Supplementary Fig. 1.
Among the 34 studies, 91.18% reported adequate random sequence generation but were considered high risk in one study and unclear in the remaining two. The risk of bias in allocation concealment was 61.76%, and the risk in one trial was high and unclear in thirteen studies. The outcome assessment was double- or triple-blinded in 55.88% of the trials and was unclear in fifteen trials. A total of 70.59% of the trials had a low risk of bias due to the blinding of participants and key researchers, and ten trials had an unclear risk of bias. Additionally, a low risk of bias was shown in most of the trials based on incomplete outcome data and selective outcome reporting but was unclear in the two studies. The biases were mainly derived from blinding and unrealized allocation concealment in the outcome assessment, followed by nonspecific implementer and participant double-blinding.
Effects of primary outcomes
Hepatic fibrosis
The effect of probiotics, prebiotics, and synbiotics on improvingt in hepatic fibrosis, as measured by elastography, was assessed in 6 studies (339 participants). The combined SMD for hepatic fibrosis significantly decreased (SMD = -0.31; 95% CI: -0.53, -0.09) (Fig. 3A). There was low between-study heterogeneity among the studies (I2 = 22.00%). Subgroup analysis revealed that probiotics could effectively improve hepatic fibrosis (SMD = -0.58; 95% CI: -0.94, -0.22). Furthermore, there was a promising effect on treating hepatic fibrosis when the duration of intervention was more than 12 weeks (SMD = -0.35; 95% CI: 0.61, -0.10). Country and sample size may be factors influencing heterogeneity (Table 3). There was no evidence of publication bias (Egger’s tests = 0.33; Begg’s tests = 0.76) (Appendix 2 Figure S1). As shown in Appendix 1 Supplemental Table 2, the quality of evidence for hepatic fibrosis was rated as high.
Forest plot of the effect of microbiota therapies on primary outcomes. (A) The role of microbiota-therapy on hepatic fibrosis on different interventions. (B) The role of microbiota-therapy on hepatic steatosis on different interventions. (C) The role of microbiota-therapy on AST on different interventions. (D) The role of microbiota-therapy on ALT on different interventions
Hepatic steatosis
Five studies with 205 participants evaluated the impact of probiotics, prebiotics, and synbiotics on reducing hepatic steatosis determined by liver ultrasound. Neither probiotics, nor prebiotics, nor synbiotics improved moderate/severe hepatic steatosis (OR: 0.95; 95% CI: 0.62, 1.41) (Fig. 3B). There was low between-study heterogeneity among the studies (I2 = 0.00%). No significant small-study effects were found using Begg’s tests and Egger’s (P = 0.55 and P = 0.69), respectively). As shown in Appendix 1 Supplemental Table 2, the quality of evidence for hepatic fibrosis was rated as moderate (based on inconsistency).
AST
According to the meta-analysis of 26 studies (1515 participants), probiotics, prebiotics, and synbiotics significantly reduced AST levels, and the pooled estimate of SMD = -0.35 (95% CI: -0.55, -0.15; I2 = 73.10% was reported. Country, intervention duration, sample size, and intervention type were detected as sources of heterogeneity (Table 3). Subgroup analysis revealed that the reducing effects of probiotic supplementation were greater than those of other intervention types (SMD = -0.39; 95% CI: -0.63, -0.15) (Fig. 3C), and the effectiveness of probiotic, prebiotic, and synbiotic supplementation on decreasing AST levels was greater in studies with sample sizes > 40. Moreover, the beneficial effect on alleviating AST levels occurred irrespective of the intervention time and country. No significant small-study effect was shown using Egger’s and Begg’s tests (P = 0.70 and P = 0.49, respectively) (Appendix 2 Figure S3). GRADE results showed that the evidence for AST was of had high quality (Appendix 1 Supplemental Table 2).
ALT
The effects of probiotics, prebiotics, and synbiotics on ALT levels were reported in 27 studies (1501 participants). Our analysis revealed a significant reduction in ALT levels (SMD = -0.48; 95% CI: -0.71, -0.25). Compared with control, probiotics (SMD = -0.41; 95% CI: -0.66, -0.12) and prebiotics (SMD = -1.51; 95% CI: -2.19, -0.83) were associated with a significantly greater reduction in ALT (Fig. 3D). Subgroup analysis revealed that probiotics, prebiotics, and synbiotics supplementation contributed to a more robust decrease in ALT levels in studies with duration of ≤ 12 weeks. Moreover, there was an improvement in lowering ALT levels irrespectively of country and sample size (Table 3). Egger weighted regression statistics (P < 0.01) indicated that there was publication bias. Additional analysis with the trim and fill method revealed four missing studies, and after imputation, the overall effect size did not change (SMD= -0.44; 95% CI: -0.54, -0.35; P < 0.01). Based on the GRADE method, there was a moderate level of evidence for ALT (based on inconsistency) (Appendix 1 Supplemental Table S3).
Effects of secondary outcomes
BMI
A meta-analysis of the relevant data from 19 studies (1058 participants) revealed 89.80% heterogenety, and the results of a random effects model revealed that probiotics (SMD = -0.98; 95% CI: -1.87, -0.08) had a discernible impact on BMI (Fig. 4A). Subgroup analysis revealed that patients from Asia (SMD = -0.52; 95% CI: -0.95, -0.08) and duration of treatment greater than 12 weeks (SMD = -0.77; 95% CI: -1.38, -0.16) were associated with a reduced BMI. In, addition, there was decreasing effect on BMI regardless of sample size (> 40: SMD = -0.58; 95% CI: -1.12, -0.02; ≤ 40: SMD = -0.43; 95% CI: -0.75, -0.10) (Appendix 1 Supplemental Table 3). There was a significant small-study effects using Egger’s tests (P = 0.02). The trim-and-fill analysis suggested that five iterations of the iterative technique did not significantly change the pooled effect size estimates did not significantly change (SMD = -0.61, 95% CI: -0.72, -0.50), which indicates that the results are generally stable and that publication bias has little impact. Appendix 1 Supplemental Table 2 presents the quality of evidence (calculated by the GRADE method) for BMI that was high.
ALP
Eight studies (338 participants) evaluated the effect of probiotics, prebiotics, and synbiotics supplementation on ALP levels among NAFLD patients. Overall, there was significant reduction compared to that in placebo group (SMD = -0.81; 95% CI: -1.55, -0.8) (Fig. 4B). Subgroup analysis revealed the effectiveness of probiotics, prebiotics, and synbiotics supplementation in lowering ALP levels in studies with a treatment duration ≤ 12 weeks (SMD = -1.39; 95% CI: -2.59, -0.19) and in individuals from Asia (SMD = -1.09; 95% CI: -2.05, -0.13) (Appendix 1 Supplemental Table 3). Begg’s (P = 0.07) and Egger’s tests (P = 0.11) suggested no publication bias. ALP has a moderate level of evidence due to inconsistency based on the GRADE method (Appendix 2 Supplemental Table 2).
GGT
Consistently, a forest plot of 15 datasets (860 participants) did not indicate a significant reduction in GGT levels after taking probiotics, prebiotics, or synbiotics compared to the placebo (SMD = -0.23; 95% CI: -0.57, 0.16; I2 = 82.60%) (Appendix 1 Supplemental Table 3). Subgroup analysis by study population, sample size, and intervention duration did not reveal the source of heterogeneity Furthermore, symbiotics, but not probiotics or prebiotics, were associated with a greater reduction in GGT levels (Fig. 4C). There was no publication bias according to the Begg’s (P = 0.36) and Egger’s tests (P = 0.79). The result of the GRADE results showed that the evidence for GGT was of moderate quality, which was reduced by inconsistency (Appendix 1 Supplemental Table 2).
HDL
Twenty trials (1307 participants) reported the effect of probiotics, prebiotics, and synbiotics on HDL (Fig. 5A). The pooled effect size indicated no significant improvement in HDL compared to the placebo (SMD = -0.10; 95% CI: -0.32, 0.13, I2 = 74.30%). Subgroup analysis indicated that according to the study population, sample size, intervention duration and intervention type did not improve HDL levels (Appendix 1 Supplemental Table 4). No significant small-study effect was shown using Egger’s and Begg’s tests (P = 0.22 and P = 0.41, respectively). GRADE results showed that the evidence for HDL was of high quality (Appendix 1 Supplemental Table 2).
Forest plot of the effect of microbiota therapies on lipid profiles in patients with NAFLD. (A) The role of microbiota-therapy on HDL on different interventions. (B) The role of microbiota-therapy on LDL on different interventions. (C) The role of microbiota-therapy on TG on different interventions. (D) The role of microbiota-therapy on TC on different interventions
LDL
Nineteen trials (1242 participants) evaluated the effect of probiotics, prebiotics, and synbiotics on LDL levels among NAFLD patients. Overall, there was no significant reduction in LDL compared to that in the placebo group (SMD = -0.21; 95% CI: -0.48, 0.06) (Appendix 1 Supplemental Table 4). Subgroup analysis revealed that prebiotic (SMD = -1.22; 95% CI: -2.23, -0.22) and synbiotic (SMD = -0.47; 95% CI: -0.91, -0.02) supplementation attenuated LDL levels (Fig. 5B). In addition, the effectiveness of the intake of prebiotics, probiotics, and synbiotics had a reducing-effect on LDL levels in subjects from Asia (SMD = -0.50; 95% CI: -0.75, -0.25) and those with an intervention time less than 12 weeks (SMD = -0.34; 95% CI: -0.60, -0.08). No significant small-study effects were found using Egger’s and Begg’s tests (P = 0.20 and P = 0.54, respectively). LDL has a moderate level of evidence due to inconsistency based on the GRADE method (Appendix 1 Supplemental Table 2).
TG
Twenty-four trials (1493 participants) reported the effect of microbiota treatment on TG. The meta-analysis revealed that the administration of probiotics, prebiotics, and synbiotics decreased TG levels (SMD = -0.22; 95% CI: -0.43, -0.02; I2 = 72.70%) (Appendix 1 Supplemental Table 4). Subgroup analysis showed that the lowering effects of probiotics, prebiotics, and synbiotics occurred in subjects from Asia (SMD = -0.36; 95% CI: -0.64, -0.09) and a positive effect on TG was detected in individuals supplemented with prebiotic (SMD = -0.89, 95% CI: -1.68, -0.10) (Fig. 5C). No evidence of publication bias was detected according to Egger’s (P = 0.76)) and Begg’s tests (P = 0.38). TG had a high level of evidence based on the GRADE method (Appendix 1 Supplemental Table 2).
TC
The pooled effect size of twenty-one studies (1281 participants) revealed that there was no significant effect of the probiotics, prebiotics, or synbiotics treatment on TC (SMD = -0.26; 95% CI: -0.54, 0.03) (Appendix 1 Supplemental Table 4). Subgroup analysis revealed a greater reduction in patients receiving prebiotics (SMD = -0.69; 95% CI: -0.98, -0.40) (Fig. 5D). Furthermore, there was a positive effect on alleviating TC levels when subjects were Asian (SMD = -0.57; 95% CI: -0.93, -0.20), had a duration of treatment less than 12 weeks (SMD = -0.52; 95% CI: -0.96, -0.09), and had a sample size less than forty (SMD = -0.87; 95% CI: -1.64, -0.10). No evidence of publication bias was detected according to Egger’s (P = 0.89) and Begg’s tests (P = 0.56). There was a moderate level of evidence for TC due to inconsistency based on the GRADE method (Appendix 1 Supplemental Table 2).
hs-CRP
The quantitative analysis of hs-CRP values (12 trials, 637 participants) indicated a significant reduction in hs-CRP compared to that in the placebo group (SMD = -0.47; 95% CI: -0.88, -0.06) with high heterogeneity across studies (I2 = 84.00%) (Fig. 6A). Subgroup analysis indicated that by study population, sample size, intervention duration and intervention types did not have a promising effect on improving hs-CRP levels (Appendix 1 Supplemental Table 5). No evidence of publication bias was detected according to Egger’s (P = 0.09) and Begg’s tests (P = 0.64). Appendix 1 Supplemental Table 2 presents the quality of evidence (calculated by the GRADE method) for hs-CRP which was moderate due to inconsistency.
Forest plot of the effect of microbiota therapies on inflammation factors in patients with NAFLD. (A) The role of microbiota-therapy on hs-CRP on different interventions. (B) The role of microbiota-therapy on TNF-α on different interventions. (C) The effect of microbiota therapies on IL-6. (D) The effect of microbiota therapies on LPS
TNF-α
The quantitative analysis of TNF-α (10 trials, 412 participants) indicated a significant reduction compared to that in the placebo group (SMD = -0.86; 95% CI: -1.56, -0.56) with high heterogeneity across studies (I2 = 90.50%). Subgroup analysis revealed a more prominent effect of synbiotics supplementation on TNF-α levels (SMD = -0.74; 95% CI: -1.38, -0.10) (Fig. 6B). Moreover, the observed decreasing impact was greater in studies with larger sample sizes (≤ 40), and with Asian subjects (Appendix 1 Supplemental Table 5). Egger’s test revealed publication bias (P = 0.04). The trim-and-fill analysis suggested that three iterations of the iterative technique did not significantly change the pooled effect size estimates (SMD = -1.04, 95% CI: -1,79, -0.30), which indicates that the results are generally stable and that publication bias has little impact. There was a moderate level of evidence TNF-α due to inconsistency (Appendix 1 Supplemental Table 2).
IL-6
Eight trials were conducted (379 participants) to evaluate the effect of the probiotics, prebiotics, and synbiotics supplementation on IL-6 levels among NAFLD patients. There was no significant reduction in the mean difference in IL-6 (SMD = -0.55; 95% CI: -1.21, 0.12; I2 = 89.00%) (Appendix 1 Supplemental Table 5). Subgroup analysis indicated that the study population, sample size, intervention duration and intervention types did not improve IL-6 levels (Fig. 6C). There was no publication bias, as determined by the Begg’s (P = 0.20) and Egger’s tests (P = 0.15). There was a had a moderate level of evidence for IL-6 due to inconsistency based on the GRADE method (Appendix 1 Supplemental Table 2).
LPS
The meta-analysis of 3 trials (115 participants) revealed that probiotics, prebiotics, or synbiotics supplementation did not affect the reduction of LPS levels (SMD = -1.15; 95% CI: -3.18, 0.87) (Fig. 6D). We did not perform further subgroup analyses due to the small number of literatures. No significant small-study effect was shown using Egger’s and Begg’s tests (P = 0.37 and P = 0.06, respectively). Appendix 1 Supplemental Table 2 presents the quality of evidence (calculated by the GRADE method) for LPS which was moderate due to inconsistency.
Discussion
A total of 34 RCTs assessing microbial treatments in NAFLD patients were found in this meta-analysis. We observed that probiotics, prebiotics, and synbiotics supplementation improved not only liver histology (hepatic fibrosis) and liver function (AST, ALT, and ALP) but also TG levels and BMI. Moreover, it significantly decreased the levels of inflammatory markers including TNF-α and hs-CRP. Intervention duration, study population, sample size, and treatment types were potential sources of heterogeneity in the different subgroup analyses.
Improvement in liver function in patients with NAFLD is clinically measured by quantifying established clinical diagnostic indicators of liver dysfunction, such as ALT, AST, and ALP, which are thought to be reliable signs of liver damage. It has been discovered that microbiota treatments are successful in lowering liver enzymes in the NAFLD patients [12, 64]. When the intestinal microbiota is dysbiosis, the enterotoxins secreted by pathogenic bacteria, such as endotoxin (lipopolysaccharide), increase the permeability of the intestinal mucosa, leading to bacterial translocation, which can cause endotoxemia and long-term damage to liver cells [65]. Probiotics can regulate intestinal ecological disorders and improve the integrity of the intestinal mucosal barrier, thereby reducing the inflammatory response of the liver [66]. According to this analysis, supplementation with probiotics was linked to lower levels of AST, ALT and ALP, which may have a protective impact by changing the gut’s microbial makeup and metabolism in NAFLD patients [67]. Subgroup analysis of liver enzyme levels showed that intervention durations ≤ 12 weeks were more conducive to reducing ALT and ALP levels, while a reduction in AST levels occured regardless of the duration of treatment. A systematic review and meta-analysis also showed that probiotics supplements should be continued for at least 12 weeks [68]. Another 12-week study showed that probiotics supplementation was able to decrease ALT and AST compared to control group [37]. In addition, we carefully examined the duration of the included treatment and discovered that the majority of the intervention time in the literature were approximately 12 weeks [31, 37, 39, 69,70,71]. Therefore, we speculated that 12 weeks might be the required time for probiotics to fully take effect. Furthermore, our study demonstrated that probiotics had a more beneficial impact on hepatic fibrosis. Some studies have shown that the development of pathogenic intestinal bacteria results in an increase in endotoxin, which can worsen the hepatic fibrosis process in cirrhotic rats and increase blood levels of AST and ALT. Endotoxin increases the permeability of hepatocytes to potassium ions, leading to mitochondrial swelling and impaired adenosine triphosphate (ATP) production, resulting in swelling or necrosis of hepatocytes, and leading to fibrotic changes [72].
Researchers investigated the BMI of NAFLD patients in the included studies and revealed that probiotics, preciotics, or synbiotics supplementation could reduce BMI. Probiotic supplements have been shown in earlier research to benefit weight loss, lower body fat mass, and decrease waist circumference in overweight people, improving body composition and fat distribution [73]. Previous studies have shown that probiotics can produce short chain fatty acids (SCFAs). The binding of SCFAs to specific g-protein coupled receptors can stimulate the release of glucagon like peptides (GLP-1) and GLP-2 to maintain energy homeostasis and enhance fat storage, which is consistent with our results [68, 74, 75]. The results of meta-analysis revealed that microbial treatments could significantly decrease lipid levels, which was consistent with the findings of Musazadeh et al. [76]. Subgrouping the studies based on the intervention type showed that prebiotics supplementation had more beneficial effects on lipid profiles compared to probiotics or synbiotics. Prebiotics are water-soluble dietary fibers that cannot be directly digested and absorbed by the human body; they can regulate the intestinal environment and selectively promote the proliferation of various beneficial bacteria in the intestine [77]. Research has demonstrated that prebiotics can control the expression of genes related to lipogenic enzymes, lessen the production of hepatic fatty acids at the source, and consequently lower the levels of hepatic triglycerides [78]. An animal study also showed that prebiotics could reduce liver fat and lower serum cholesterol levels in non-alcoholic fatty liver disease model rats [79]. The study population is another important factor that changes the overall effect. Supplementation of Asian populations with probiotics, prebiotics, or synbiotics leads to improvements in TG, TC, and LDL levels. The reason for this observation may be the diet structure, the Asian diet is dominated by grains, with a large number of vegetables and fruits, supplemented by bean products and fish and shellfish. Hence, compared to those of individuals in Europe, America, and Africa, the intestinal environment may be more normal [80].
Previous meta-analysis have indicated that microbial therapy can effectively decrease TNF-α and hs-CRP levels [11, 24, 26, 81]. Consistently, our study demonstrated that it had a statistically significant effect on lowering TNF-α and hs-CRP. Another RCT involving 52 NAFLD patients found that taking synbiotic for 28 weeks can greatly decrease TNF-α and hs-CRP levels [82]. Clinical studies have also shown that almost all patients with NAFLD have abnormal levels of inflammatory cytokines, which trigger inflammatory response pathways in the NAFLD gut flora [83]. Microbial treatments may be a potential target for local mucosal inflammation, such as hs-CRP and TNF-α [84]. It has been established that supplementation with synbiotics can improve NAFLD by increasing SCFA synthesis and decreasing the expression of genes associated with inflammation [85]. SCFAs have anti-inflammatory effects by controlling the release of cytokines, reactive oxygen species (ROS), and immune cell chemotaxis [86]. Although we found that supplementation with synbiotics had an effective impact on reducing the TNF-α concentration, neither prebiotics nor probiotics had the same effect and further investigation is required to determine whether this is the result of a small sample effect.
There are several limitations to this study. First, there are no high-quality large RCTs, and there are currently few large-scale clinical trials of microbial therapy. Second, the microbiological distinctions between men and women may be influenced by sex hormones and chromosomes [87]. An earlier study hypothesized that the prevalence of NAFLD and obesity may be significantly influenced by sex-specific microbiomes [88]. However, the association between microbiota therapy and sexuality was not analyzed as too few studies were included; Third, due to the limited number of subgroup studies on inflammation factors that do not allow for verified method analysis, some indicators call for caution when concluding.
Conclusion
The meta-analysis revealed probiotics, prebiotics, and synbiotics supplementation may reduce BMI, liver injury, lipid profiles, and inflammatory factors in NAFLD patients. Of these, probiotics supplementation had an improving effect on liver injury, including hepatic fibrosis, AST, ALT, and ALP, and prebiotics supplementation had lower effect on TC, TG, and LDL-C. In the future, more researches, considering patients’ sex and stresses, should be undertaken on NAFLD patients under RCT design at numerous centers.
Data availability
All data generated or analysed during this study are included in this published article and its supplementary information files.
Abbreviations
- ALP:
-
Alkaline phosphatase
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- BMI:
-
Body mass index
- GGT:
-
Gamma-glutamyl transpeptidase
- GLP:
-
Glucagon like peptides
- HDL:
-
High-density lipoprotein
- hs-CRP:
-
High-sensitivity C-reactive protein
- IL-6:
-
Interleukin 6
- LDL:
-
Low-density lipoprotein
- NAFLD:
-
Nonalcoholic fatty liver disease
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analysis
- RCTs:
-
Randomized controlled trials
- ROS:
-
Reactive oxygen species
- SCFAs:
-
Short chain fatty acids
- SMD:
-
Standardized mean difference
- TC:
-
Total cholesterol
- TG:
-
Triglycerides
- TNF-α:
-
Tumor necrosis factor alpha
References
Burt AD, Lackner C, Tiniakos DG. Diagnosis and Assessment of NAFLD: definitions and histopathological classification. Semin Liver Dis. 2015;35(3):207–20.
Parker HM, Johnson NA, Burdon CA, Cohn JS, O’Connor HT, George J. Omega-3 supplementation and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Hepatol. 2012;56(4):944–51.
Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, George J. Bugianesi EJNrG, hepatology: global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Gastroenterol Hepatol. 2018;15(1):11–20.
Wong MCS, Huang JLW, George J, Huang J, Leung C, Eslam M, Chan HLY, Ng SC. The changing epidemiology of liver diseases in the Asia-Pacific region. Nat Rev Gastroenterol Hepatol. 2019;16(1):57–73.
Hardy T, Oakley F, Anstee QM, Day CP. Nonalcoholic fatty liver disease: pathogenesis and disease spectrum. Annu Rev Pathol. 2016;11:451–96.
Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metab Clin Exp. 2016;65(8):1038–48.
Arora T, Bäckhed F. The gut microbiota and metabolic disease: current understanding and future perspectives. J Intern Med. 2016;280(4):339–49.
Chen J, Vitetta L. Gut microbiota metabolites in NAFLD pathogenesis and therapeutic implications. Int J Mol Sci. 2020;21(15):5214.
Jia W, Xie G, Jia W. Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nat Rev Gastroenterol Hepatol. 2018;15(2):111–28.
Liu Y, Chen K, Li F, Gu Z, Liu Q, He L, Shao T, Song Q, Zhu F, Zhang LJH. Probiotic Lactobacillus rhamnosus GG prevents liver fibrosis through inhibiting hepatic bile acid synthesis and enhancing bile acid excretion in mice. Hepatology (Baltimore MD). 2020;71(6):2050–66.
Loman BR, Hernández-Saavedra D, An R, Rector RS. Prebiotic and probiotic treatment of nonalcoholic fatty liver disease: a systematic review and meta-analysis. Nutr Rev. 2018;76(11):822–39.
Sáez-Lara MJ, Robles-Sanchez C, Ruiz-Ojeda FJ, Plaza-Diaz J, Gil A. Effects of Probiotics and Synbiotics on obesity, insulin resistance syndrome, type 2 diabetes and non-alcoholic fatty liver disease: a review of human clinical trials. Int J Mol Sci. 2016;17(6):928.
Buss C, Valle-Tovo C, Miozzo S, Alves de Mattos A. Probiotics and synbiotics may improve liver aminotransferases levels in non-alcoholic fatty liver disease patients. Ann Hepatol. 2014;13(5):482–8.
Lynch SV, Pedersen O. The human intestinal microbiome in Health and Disease. N Engl J Med. 2016;375(24):2369–79.
den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud DJ, Bakker BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. 2013;54(9):2325–40.
Donohoe DR, Garge N, Zhang X, Sun W, O’Connell TM, Bunger MK, Bultman SJ. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 2011;13(5):517–26.
Yang R, Shang J, Zhou Y, Liu W, Tian Y, Shang H. Effects of probiotics on nonalcoholic fatty liver disease: a systematic review and meta-analysis. Expert Rev Gastroenterol Hepatol. 2021;15(12):1401–9.
Cho YA, Kim J. Effect of Probiotics on blood lipid concentrations: a Meta-analysis of Randomized controlled trials. Medicine. 2015;94(43):e1714.
Dong Y, Xu M, Chen L, Bhochhibhoya A. Probiotic foods and supplements interventions for metabolic syndromes: a systematic review and Meta-analysis of recent clinical trials. Ann Nutr Metab. 2019;74(3):224–41.
Wang C, Zhang C, Li S, Yu L, Tian F, Zhao J, Zhang H, Chen W, Zhai Q. Effects of Probiotic supplementation on Dyslipidemia in type 2 diabetes Mellitus: a Meta-analysis of Randomized controlled trials. Foods 2020, 9(11).
Stachowska E, Portincasa P, Jamiol-Milc D, Maciejewska-Markiewicz D, Skonieczna-Zydecka K. The relationship between prebiotic supplementation and anthropometric and biochemical parameters in patients with NAFLD-A systematic review and Meta-analysis of Randomized controlled trials. Nutrients. 2020;12(11):3460.
Ebrahimi ZS, Nasli-Esfahani E, Nadjarzade A, Mozaffari-Khosravi H. Effect of symbiotic supplementation on glycemic control, lipid profiles and microalbuminuria in patients with non-obese type 2 diabetes: a randomized, double-blind, clinical trial. J Diabetes Metab Disord. 2017;16:23.
Khan MY, Mihali AB, Rawala MS, Aslam A, Siddiqui WJ. The promising role of probiotic and synbiotic therapy in aminotransferase levels and inflammatory markers in patients with nonalcoholic fatty liver disease - a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2019;31(6):703–15.
Hadi A, Mohammadi H, Miraghajani M, Ghaedi E. Efficacy of synbiotic supplementation in patients with nonalcoholic fatty liver disease: a systematic review and meta-analysis of clinical trials: synbiotic supplementation and NAFLD. Crit Rev Food Sci Nutr. 2019;59(15):2494–505.
Khalesi S, Johnson DW, Campbell K, Williams S, Fenning A, Saluja S, Irwin C. Effect of probiotics and synbiotics consumption on serum concentrations of liver function test enzymes: a systematic review and meta-analysis. Eur J Nutr. 2018;57(6):2037–53.
Tang Y, Huang J, Zhang WY, Qin S, Yang YX, Ren H, Yang QB, Hu H. Effects of probiotics on nonalcoholic fatty liver disease: a systematic review and meta-analysis. Th Adv Gastroenterol. 2019;12:1756284819878046.
A SL DVR, Manohar T. Role of Probiotics in the treatment of nonalcoholic fatty liver disease: a Meta-analysis. Euroasian J hepato-gastroenterology. 2017;7(2):130–7.
Gao X, Zhu Y, Wen Y, Liu G, Wan C. Efficacy of probiotics in non-alcoholic fatty liver disease in adult and children: a meta-analysis of randomized controlled trials. Hepatol Research: Official J Japan Soc Hepatol. 2016;46(12):1226–33.
Ma YY, Li L, Yu CH, Shen Z, Chen LH, Li YM. Effects of probiotics on nonalcoholic fatty liver disease: a meta-analysis. World J Gastroenterol. 2013;19(40):6911–8.
Abdel Monem SM. Probiotic therapy in patients with nonalcoholic steatohepatitis in Zagazig University Hospitals. Euroasian J Hepatogastroenterol. 2017;7(1):101–6.
Abhari K, Saadati S, Yari Z, Hosseini H, Hedayati M, Abhari S, Alavian SM, Hekmatdoost A. The effects of Bacillus coagulans supplementation in patients with non-alcoholic fatty liver disease: a randomized, placebo-controlled, clinical trial. Clin Nutr ESPEN. 2020;39:53–60.
Ahn SB, Jun DW, Kang B-K, Lim JH, Lim S, Chung M-J, Randomized. Double-blind, placebo-controlled study of a Multispecies Probiotic mixture in nonalcoholic fatty liver disease. Sci Rep. 2019;9(1):5688.
Alisi A, Bedogni G, Baviera G, Giorgio V, Porro E, Paris C, Giammaria P, Reali L, Anania F, Nobili V. Randomised clinical trial: the beneficial effects of VSL#3 in obese children with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2014;39(11):1276–85.
Asgharian A, Mohammadi V, Gholi Z, Esmaillzade A, Feizi A, Askari GJIRCMJ. The Effect of Synbiotic supplementation on body composition and lipid Profile in patients with NAFLD: a Randomized, double blind, placebo-controlled clinical Trial Study. 2017, 19.
Ayob N, Muhammad Nawawi KN, Mohamad Nor MH, Raja Ali RA, Ahmad HF, Oon SF, Mohd Mokhtar N. The effects of Probiotics on small intestinal microbiota composition, inflammatory cytokines and intestinal permeability in patients with non-alcoholic fatty liver disease. Biomedicines 2023, 11(2).
Barcelos STA, Silva-Sperb AS, Moraes HA, Longo L, de Moura BC, Michalczuk MT, Uribe-Cruz C, Cerski CTS, da Silveira TR, Dall’Alba V, et al. Oral 24-week probiotics supplementation did not decrease cardiovascular risk markers in patients with biopsy proven NASH: a double-blind placebo-controlled randomized study. Ann Hepatol. 2023;28(1):100769.
Behrouz V, Aryaeian N, Zahedi MJ, Jazayeri S. Effects of probiotic and prebiotic supplementation on metabolic parameters, liver aminotransferases, and systemic inflammation in nonalcoholic fatty liver disease: a randomized clinical trial. J Food Sci. 2020;85(10):3611–7.
Bomhof MR, Parnell JA, Ramay HR, Crotty P, Rioux KP, Probert CS, Jayakumar S, Raman M, Reimer RA. Histological improvement of non-alcoholic steatohepatitis with a prebiotic: a pilot clinical trial. Eur J Nutr. 2019;58(4):1735–45.
Cai G-S, Su H, Zhang J. Protective effect of probiotics in patients with non-alcoholic fatty liver disease. Medicine. 2020;99(32):e21464.
Chong PL, Laight D, Aspinall RJ, Higginson A, Cummings MH. A randomised placebo controlled trial of VSL#3® probiotic on biomarkers of cardiovascular risk and liver injury in non-alcoholic fatty liver disease. BMC Gastroenterol. 2021;21(1):144.
Crommen S, Rheinwalt KP, Plamper A, Simon MC, Rösler D, Fimmers R, Egert S, Metzner C. A specifically tailored Multistrain Probiotic and Micronutrient Mixture affects nonalcoholic fatty liver disease-related markers in patients with obesity after Mini gastric bypass surgery. J Nutr. 2022;152(2):408–18.
Derosa G, Guasti L, D’Angelo A, Martinotti C, Valentino MC, Di Matteo S, Bruno GM, Maresca AM, Gaudio GV, Maffioli P. Probiotic therapy with VSL#3® in patients with NAFLD: a Randomized Clinical Trial. Front Nutr. 2022;9:846873.
Duseja A, Acharya SK, Mehta M, Chhabra S, Rana S, Das A, Dattagupta S, Dhiman RK, Chawla YK. High potency multistrain probiotic improves liver histology in non-alcoholic fatty liver disease (NAFLD): a randomised, double-blind, proof of concept study. BMJ open Gastroenterol. 2019;6(1):e000315.
Ekhlasi G, Zarrati M, Agah S, Hosseini AF, Hosseini S, Shidfar S, Soltani Aarbshahi SS, Razmpoosh E, Shidfar F. Effects of symbiotic and vitamin E supplementation on blood pressure, nitric oxide and inflammatory factors in non-alcoholic fatty liver disease. EXCLI J. 2017;16:278–90.
Escouto GS, Port GZ, Tovo CV, Fernandes SA, Peres A, Dorneles GP, Houde VP, Varin TV, Pilon G, Marette A, et al. Probiotic supplementation, hepatic fibrosis, and the Microbiota Profile in patients with nonalcoholic steatohepatitis: a Randomized Controlled Trial. J Nutr. 2023;153(7):1984–93.
Famouri F, Shariat Z, Hashemipour M, Keikha M, Kelishadi R. Effects of Probiotics on nonalcoholic fatty liver disease in obese children and adolescents. J Pediatr Gastroenterol Nutr. 2017;64(3):413–7.
Farhangi MA, Dehghan P, Musazadeh V, Kavyani M, Maleki P. Effectiveness of omega-3 and prebiotics on adiponectin, leptin, liver enzymes lipid profile and anthropometric indices in patients with non-alcoholic fatty liver disease: a randomized controlled trial. J Funct Foods. 2022;92:9.
Ferolla SM, Couto CA, Costa-Silva L, Armiliato GNA, Pereira CAS, Martins FS, Ferrari MLA, Vilela EG, Torres HOG, Cunha AS et al. Beneficial effect of Synbiotic supplementation on hepatic steatosis and anthropometric parameters, but not on gut permeability in a Population with Nonalcoholic Steatohepatitis. Nutrients 2016, 8(7).
Javadi L, Ghavami M, Khoshbaten M, Safaiyan A, Barzegari A, Gargari BPJIRCMJ. The Effect of Probiotic and/or prebiotic on liver function tests in patients with nonalcoholic fatty liver disease: a double Blind Randomized Clinical Trial. 2017, 19.
Javadi L, Ghavami M, Khoshbaten M, Safaiyan A, Barzegari A, Gargari BPJIRCMJ. The Effect of Probiotic and/or prebiotic on liver function tests in patients with nonalcoholic fatty liver disease: a double Blind Randomized Clinical Trial. 2018, 19.
Kavyani M, Saleh-Ghadimi S, Dehghan P, Abbasalizad Farhangi M, Khoshbaten M. Co-supplementation of camelina oil and a prebiotic is more effective for in improving cardiometabolic risk factors and mental health in patients with NAFLD: a randomized clinical trial. Food Funct. 2021;12(18):8594–604.
Kobyliak N, Abenavoli L, Mykhalchyshyn G, Kononenko L, Boccuto L, Kyriienko D, Dynnyk O. A multi-strain Probiotic reduces the fatty liver index, cytokines and aminotransferase levels in NAFLD patients: evidence from a Randomized Clinical Trial. J Gastrointest Liver Diseases: JGLD. 2018;27(1):41–9.
Malaguarnera M, Vacante M, Antic T, Giordano M, Chisari G, Acquaviva R, Mastrojeni S, Malaguarnera G, Mistretta A, Li Volti G, et al. Bifidobacterium longum with fructo-oligosaccharides in patients with non alcoholic steatohepatitis. Dig Dis Sci. 2012;57(2):545–53.
Miccheli A, Capuani G, Marini F, Tomassini A, Praticò G, Ceccarelli S, Gnani D, Baviera G, Alisi A, Putignani L, et al. Urinary (1)H-NMR-based metabolic profiling of children with NAFLD undergoing VSL#3 treatment. Int J Obes. 2015;39(7):1118–25.
Mitrović M, Dobrosavljević A, Odanović O, Knežević-Ivanovski T, Kralj Đ, Erceg S, Perućica A, Svorcan P, Stanković-Popović V. The Effects of Synbiotics on the Liver Steatosis, Inflammation, and Gut Microbiome of Metabolic Dysfunction-associated Liver Disease Patients-Randomized trial. Romanian journal of internal medicine = Revue roumaine de medecine interne 2024.
Mohamad Nor MH, Ayob N, Mokhtar NM, Raja Ali RA, Tan GC, Wong Z, Shafiee NH, Wong YP, Mustangin M, Nawawi KNM. The Effect of Probiotics (MCP® BCMC® Strains) on Hepatic Steatosis, Small Intestinal Mucosal Immune Function, and Intestinal Barrier in Patients with Non-Alcoholic Fatty Liver Disease. Nutrients 2021, 13(9).
Nabavi S, Rafraf M, Somi MH, Homayouni-Rad A, Asghari-Jafarabadi M. Effects of probiotic yogurt consumption on metabolic factors in individuals with nonalcoholic fatty liver disease. J Dairy Sci. 2014;97(12):7386–93.
Rodrigo T, Dulani S, Nimali Seneviratne S, De Silva AP, Fernando J, De Silva HJ, Jayasekera, Wickramasinghe VP. Effects of probiotics combined with dietary and lifestyle modification on clinical, biochemical, and radiological parameters in obese children with nonalcoholic fatty liver disease/nonalcoholic steatohepatitis: a randomized clinical trial. Clin Experimental Pediatr. 2022;65(6):304–11.
Sadrkabir M, Jahed S, Sadeghi Z, Isazadeh K. The Effect of GeriLact on Non-Alcoholic Fatty Liver Disease. In: 2020; 2020.
Sayari S, Neishaboori H, Jameshorani M. Combined effects of synbiotic and sitagliptin versus sitagliptin alone in patients with nonalcoholic fatty liver disease. Clin Mol Hepatol. 2018;24(3):331–8.
Scorletti E, Afolabi PR, Miles EA, Smith DE, Almehmadi A, Alshathry A, Childs CE, Del Fabbro S, Bilson J, Moyses HE et al. Synbiotics Alter Fecal microbiomes, but Not Liver Fat or Fibrosis, in a Randomized Trial of patients with nonalcoholic fatty liver disease. Gastroenterology 2020, 158(6).
Sepideh A, Karim P, Hossein A, Leila R, Hamdollah M, Mohammad EG, Mojtaba S, Mohammad S, Ghader G, Seyed Moayed A. Effects of Multistrain Probiotic supplementation on glycemic and inflammatory indices in patients with nonalcoholic fatty liver disease: a double-blind Randomized Clinical Trial. J Am Coll Nutr. 2016;35(6):500–5.
Shavakhi A, Minakari M, Firouzian H, Assali R, Hekmatdoost A, Ferns G. Effect of a Probiotic and Metformin on Liver aminotransferases in non-alcoholic steatohepatitis: a double Blind Randomized Clinical Trial. Int J Prev Med. 2013;4(5):531–7.
Gratz SW, Mykkanen H, El-Nezami HS. Probiotics and gut health: a special focus on liver diseases. World J Gastroenterol. 2010;16(4):403–10.
Xu X, Poulsen KL, Wu L, Liu S, Miyata T, Song Q, Wei Q, Zhao C, Lin C, Yang J. Targeted therapeutics and novel signaling pathways in non-alcohol-associated fatty liver/steatohepatitis (NAFL/NASH). Signal Transduct Target Ther. 2022;7(1):287.
Cao C, Shi M, Wang X, Yao Y, Zeng R. Effects of probiotics on non-alcoholic fatty liver disease: a review of human clinical trials. Front Nutr. 2023;10:1155306.
Musazadeh V, Roshanravan N, Dehghan P, Ahrabi SS. Effect of Probiotics on Liver enzymes in patients with non-alcoholic fatty liver disease: an umbrella of systematic review and Meta-analysis. Front Nutr. 2022;9:844242.
Zarezadeh M, Musazadeh V, Faghfouri AH, Sarmadi B, Jamilian P, Jamilian P, Tutunchi H, Dehghan P. Probiotic therapy, a novel and efficient adjuvant approach to improve glycemic status: an umbrella meta-analysis. Pharmacol Res. 2022;183:106397.
Anh SB, Jun DW, Kang B-K, Lim JH, Lim S, Chung M-J, Randomized. Double-blind, placebo-controlled study of a Multispecies Probiotic mixture in nonalcoholic fatty liver disease. Sci Rep 2019, 9.
Derosa G, Guasti L, D’Angelo A, Martinotti C, Valentino MC, Di Matteo S, Bruno GM, Maresca AM, Gaudio GV, Maffioli P. Probiotic therapy with VSL#3 (R) in patients with NAFLD: a Randomized Clinical Trial. Front Nutr 2022, 9.
Farhangi MA, Dehghan P, Musazadeh V, Kavyani M, Maleki P. Effectiveness of omega-3 and prebiotics on adiponectin, leptin, liver enzymes lipid profile and anthropometric indices in patients with non-alcoholic fatty liver disease: a randomized controlled trial. J Funct Food. 2022;92:105074.
Vilar-Gomez E, Chalasani N. Non-invasive assessment of non-alcoholic fatty liver disease: clinical prediction rules and blood-based biomarkers. J Hepatol. 2018;68(2):305–15.
Koutnikova H, Genser B, Monteiro-Sepulveda M, Faurie JM, Rizkalla S, Schrezenmeir J, Clément K. Impact of bacterial probiotics on obesity, diabetes and non-alcoholic fatty liver disease related variables: a systematic review and meta-analysis of randomised controlled trials. BMJ open. 2019;9(3):e017995.
Li B, Wang HY, Huang JH, Xu WF, Feng XJ, Xiong ZP, Dong YJ, Li LZ, He X, Wu HS, et al. Polysaccharide, the active component of Dendrobium officinale, ameliorates metabolic hypertension in rats via regulating Intestinal Flora-SCFAs-Vascular Axis. Front Pharmacol. 2022;13:935714.
Ma Q, Zhai R, Xie X, Chen T, Zhang Z, Liu H, Nie C, Yuan X, Tu A, Tian B, et al. Hypoglycemic effects of Lycium barbarum Polysaccharide in type 2 diabetes Mellitus mice via modulating gut microbiota. Front Nutr. 2022;9:916271.
Musazadeh V, Mohammadi Anilou M, Vajdi M, Karimi A, Sedgh Ahrabi S, Dehghan P. Effects of synbiotics supplementation on anthropometric and lipid profile parameters: finding from an umbrella meta-analysis. Front Nutr. 2023;10:1121541.
Fernandez MA, Marette A. Potential health benefits of combining yogurt and fruits based on their Probiotic and Prebiotic properties. Adv Nutr (Bethesda Md). 2017;8(1):s155–64.
Delzenne NM, Kok NN. Biochemical basis of oligofructose-induced hypolipidemia in animal models. J Nutr. 1999;129(7 Suppl):s1467–70.
Borges Haubert NJ, Marchini JS, Carvalho Cunha SF, Suen VM, Padovan GJ, Jordao AAJ, Marchini Alves CM, Marchini JF, Vannucchi H. Choline and Fructooligosaccharide: non-alcoholic fatty liver Disease, Cardiac Fat Deposition, and oxidative stress markers. Nutr Metabolic Insights. 2015;8:1–6.
Rinninella E, Cintoni M, Raoul P, Lopetuso LR, Scaldaferri F, Pulcini G, Miggiano GAD, Gasbarrini A, Mele MC. Food Components and Dietary Habits: Keys for a Healthy Gut Microbiota Composition. Nutrients 2019, 11(10).
Liu L, Li P, Liu Y, Zhang Y. Efficacy of Probiotics and Synbiotics in patients with nonalcoholic fatty liver disease: a Meta-analysis. Dig Dis Sci. 2019;64(12):3402–12.
Eslamparast T, Poustchi H, Zamani F, Sharafkhah M, Malekzadeh R, Hekmatdoost A. Synbiotic supplementation in nonalcoholic fatty liver disease: a randomized, double-blind, placebo-controlled pilot study. Am J Clin Nutr. 2014;99(3):535–42.
Mann JP, Raponi M, Nobili V. Clinical implications of understanding the association between oxidative stress and pediatric NAFLD. Expert Rev Gastroenterol Hepatol. 2017;11(4):371–82.
Nabavi-Rad A, Sadeghi A, Asadzadeh Aghdaei H, Yadegar A, Smith SM, Zali MR. The double-edged sword of probiotic supplementation on gut microbiota structure in Helicobacter pylori management. Gut Microbes. 2022;14(1):2108655.
Roy S, Dhaneshwar S. Role of prebiotics, probiotics, and synbiotics in management of inflammatory bowel disease: current perspectives. World J Gastroenterol. 2023;29(14):2078–100.
Sun M, Wu W, Liu Z, Cong Y. Microbiota metabolite short chain fatty acids, GPCR, and inflammatory bowel diseases. J Gastroenterol. 2017;52(1):1–8.
Song M, Yuan F, Li X, Ma X, Yin X, Rouchka EC, Zhang X, Deng Z, Prough RA, McClain CJ. Analysis of sex differences in dietary copper-fructose interaction-induced alterations of gut microbial activity in relation to hepatic steatosis. Biology sex Differences. 2021;12(1):3.
Pafčo B, Sharma AK, Petrželková KJ, Vlčková K, Todd A, Yeoman CJ, Wilson BA, Stumpf R, White BA, Nelson KE, et al. Gut microbiome composition of wild western lowland gorillas is associated with individual age and sex factors. Am J Phys Anthropol. 2019;169(3):575–85.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural Science Foundation of China under Grant (82073551) and the Youth Fund of the Basic Scientific Research Program of Jiangnan University (JUSRP123080).
Author information
Authors and Affiliations
Contributions
Conceptualization, Y.Y., and Y.P.; Methodology, Y.Y.; Software, H.Z., and J.W.; Validation, Y.Y., C.Y. and Y.P.; Formal Analysis, Y.Y., and Y.P.; Investigation, Y.Y.; Resources, H.Z.; Writing – Original Draft Preparation, Y.Y., C.Y; Writing – Review & Editing, Y.P., and J.W.; Visualization, H.Z.; Supervision, C.Y.; Project Administration, C.Y.; Funding Acquisition, C.Y.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Pan, Y., Yang, Y., Wu, J. et al. Efficacy of probiotics, prebiotics, and synbiotics on liver enzymes, lipid profiles, and inflammation in patients with non-alcoholic fatty liver disease: a systematic review and meta-analysis of randomized controlled trials. BMC Gastroenterol 24, 283 (2024). https://doi.org/10.1186/s12876-024-03356-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12876-024-03356-y