Abstract
Background and aims
Severe acute pancreatitis (SAP) is potentially lethal. Considering the role of inflammation in the progression of acute pancreatitis (AP), this study aims to develop a model based on inflammatory indexes for identifying the presence of SAP.
Methods
Overall, 253 patients with AP who were consecutively admitted between July 2018 and November 2020 were screened, of whom 60 had SAP. Systemic immune-inflammation index (SII), neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), lymphocyte-to-monocyte ratio (LMR), neutrophil-to-platelet ratio (NPR), systemic inflammation response index (SIRI), platelet-to-albumin ratio (PAR), C-reactive protein-to-albumin ratio (CAR), C-reactive protein-to-lymphocyte ratio (CLR), and triglyceride glucose (TyG) index were calculated. Multivariate logistic regression analyses were performed to identify independent risk factors of SAP. Then, inflammation-based models were established. Receiver operating characteristics (ROC) curve analyses were performed. Area under ROC curve (AUROC) was calculated.
Results
Diabetes mellitus, fatty liver, high white blood cell count (WBC), C-reactive protein (CRP), red blood cell distribution width (RDW), procalcitonin (PCT), SII, NLR, NPR, CAR, CLR, and TyG index, and a low LMR were significantly associated with SAP. Considering the collinearity among these variables, 10 multivariate logistic regression analyses were separately performed. Finally, four independent inflammation-based models were established. Of them, the best one, which was calculated as follows: 1.204*fatty liver (yes = 1; no = 0) + 0.419*PCT + 0.005*CLR - 2.629, had an AUROC of 0.795 with a specificity of 73.4% and a sensitivity of 71.7%.
Conclusion
The inflammation-based model consisting of fatty liver, PCT, and CLR has a good diagnostic performance for SAP.
Similar content being viewed by others
Introduction
Acute pancreatitis (AP) is an inflammatory disorder triggered by pancreatic enzyme infiltration [1] with an incidence of 34 cases per 100,000 person-years around the world [2, 3]. Its main clinical presentation includes severe abdominal pain with or without nausea, vomiting, and fever [4]. It is mostly mild and may resolve within a few days [5]. However, about 20% of the patients progress to severe acute pancreatitis (SAP), which is characterized by systemic inflammatory response syndrome (SIRS) with single or multiple organ failure [5, 6], with a mortality of up to 50% [7, 8]. Identification of the patients who are at a high risk of developing SAP is necessary for predicting their outcomes and guiding the treatment strategy [9].
Inflammation is critical for the development and progression of SAP [10]. During the course of SAP, excessive inflammatory mediators are released, inducing inflammatory cascade reaction, ultimately causing bacterial translocation and secondary injuries of distant tissues and organs [11, 12]. Thus, it seems to be reasonable that inflammatory indexes, such as neutrophil to lymphocyte ratio (NLR) and lymphocyte to monocyte ratio (LMR), can predict the probability of SAP [13, 14]. But their performance was very limited, probably because only a single index was employed in previous studies. Tanoğlu et al. also suggested that NLR alone may not truly reflect the severity of AP due to the possible influencing factors, such as other diseases [15]. Subsequently, some researchers have attempted to combine various inflammatory indexes for the prediction of SAP. Kaplan et al. found a similar predictive performance of the platelet to lymphocyte ratio (PLR)-NLR combination with other scoring systems for determining the prognosis of AP patients. But there is collinearity between PLR and NLR [16]. Zhu et al. also reported a good predictive value of a combination of NLR, procalcitonin (PCT), and modified computerized tomography severity index (MCTSI) for infected pancreatic necrosis, a form of SAP [17]. But it requires the results of imaging examinations except for inflammatory indexes. Herein, we aimed to develop a model for identifying SAP by combining various inflammatory indexes.
Methods
Study design
The retrospective study was performed according to the Declaration of Helsinki and was approved by the Medical Ethical Committee of the General Hospital of Northern Theater Command (approval number: Y2023–120). Written informed consent was waived by the Medical Ethical Committee of the General Hospital of Northern Theater Command due to its retrospective nature. We reviewed the medical records of all patients who were diagnosed with AP and consecutively admitted to the General Hospital of Northern Theater Command between July 4, 2018 and November 20, 2020 from the Information Section of Medical Security Center. The exclusion criteria were as follows: (1) age < 18 or > 80 years; (2) medical records cannot be reviewed in detail; (3) the interval between onset of symptoms and admission was more than seven days; (4) the hospital stay was less than 5 days; (5) co-existing severe trauma or pregnancy; (6) co-existing chronic pancreatitis; and (7) co-existing viral infection or rheumatic diseases.
Data collection
Patients’ demographics (i.e., age and gender), comorbidities (i.e., diabetes mellitus, hypertension, hypertriglyceridemia, and fatty liver), history of smoking and alcohol drinking, history of AP, blood tests at admission (i.e., white blood cell count [WBC], lymphocyte count, platelet count, neutrophil count, monocyte count, C-reactive protein [CRP], red blood cell distribution width [RDW], platelet distribution width [PDW], and PCT), interval between onset of symptom and admission, length of hospital stay, and hospitalization expenses were retrieved from the inpatients’ electronic medical records. Several inflammatory indexes, including systemic immune inflammation index (SII), NLR, PLR, LMR, neutrophil to platelet ratio (NPR), systemic inflammation response index (SIRI), platelet to albumin ratio (PAR), CRP to albumin ratio (CAR), CRP to lymphocyte ratio (CLR), and triglyceride glucose (TyG) index were calculated. SII was calculated as the neutrophil counts (109/L) multiplied by the platelet counts (109/L) and divided by the lymphocyte counts (109/L) [18]. NLR was calculated as the neutrophil counts (109/L) divided by the lymphocyte counts (109/L) [19]. PLR was calculated as the platelet counts (109/L) divided by the lymphocyte counts (109/L) [20]. LMR was calculated as the lymphocyte counts (109/L) divided by the monocyte counts (109/L) [21]. NPR was calculated as the neutrophil counts (109/L) multiplied by 1000 and divided by the platelet counts (109/L) [22]. SIRI was calculated as the neutrophil counts (109/L) multiplied by the monocyte counts (109/L) and divided by the lymphocyte counts (109/L) [23]. PAR was calculated as the platelet counts (109/L) divided by the albumin levels (g/L) [24]. CAR was calculated as the CRP levels (mg/L) divided by the albumin levels (g/L) [25]. CLR was calculated as the CRP levels (mg/L) divided by the lymphocyte counts (109/L) [26]. TyG index was calculated as Ln (the triglycerides [mg/dL] multiplied by the blood glucose [mg/dL]/2) [27].
Group and definition
In the present study, the patients were classified into SAP and N-SAP group according to the revised Atlanta criteria and Ranson and BISAP scoring systems. According to the revised Atlanta criteria, AP is classified as follows [28]: (1) mild acute pancreatitis (MAP), which is defined if patients have neither local complications nor organ failure; (2) moderately acute pancreatitis (MSAP), which is defined if patients have transient organ failure (< 48 h) and/or local complications; (3) SAP, which is defined if patients have persistent organ failure (≥ 48 h) with or without local complications. Ranson score is calculated based on 11 variables: age > 55 years, WBC > 16,000/μL, lactate dehydrogenase> 350 U/L, aspartate transaminase> 250 U/L, and blood glucose> 200 mg/dL at admission, and fall in hematocrit> 10%, increase in blood urea nitrogen (BUN) > 5 mg/dL, calcium< 8 mg/dL, PaO2 < 60 mmHg, base deficit> 4 mEq/L, and fluid loss> 6 L within 48 h after admission [29]. BISAP score is calculated based on five variables: BUN> 25 mg/dL, impaired mental status, SIRS, age > 60 years, and radiographic evidence of pleural effusion within the first 24 hours after admission [30]. Ranson or BISAP score ≥ 3 is defined as SAP; otherwise, N-SAP is considered. Because not all of our patients had the data at 24 h or 48 h, we selected the value reflecting the most severe clinical condition during their hospitalizations.
Statistical analyses
Continuous variables were presented as mean ± standard deviation (SD) and median with range. If the variables followed normal distribution, their differences between groups would be evaluated by independent sample T-test; otherwise, their differences between groups would be evaluated by Mann-Whitney U test. Categorical variables were presented as frequency with percentage. Differences between groups were evaluated by Chi-squared test or Fisher’s exact test. Statistically significant factors in the univariate logistic regression analyses were included in the multivariate logistic regression analyses. Multivariate logistic regression models were established after eliminating the factors with collinearity. The discrimination of the models was evaluated and compared by calculating the area under the receiver operating characteristic curve (AUROC). The concordance index (c-index) was calculated, and the calibration curve was plotted by bootstrapping with 1000 resamples to evaluate the accuracy and conformity of the models. SPSS 26.0, R 4.2.2, and GraphPad Prism 8.0.1 statistical software were used for the data analyses. A two-tailed P < 0.05 was statistically significant.
Results
Characteristics of patients
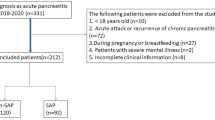
Initially, 336 AP patients were screened. Finally, 253 patients were included, of whom 60 and 193 were assigned to SAP and N-SAP group, respectively (Fig. 1). Baseline characteristics of the patients were shown in Table 1. The mean age was 45.98 ± 0.89 years, and 64.0% of the patients were male. The mean length of hospital stay was 12.02 ± 0.35 days, the mean interval between onset of symptom and admission was 1.50 ± 0.11 days, and the mean hospitalization expense was 27,160.88 ± 1279.54 yuan.
Difference between SAP and N-SAP groups
Gender, diabetes mellitus, and fatty liver were significantly different between the two groups. WBC, CRP, RDW, PDW, PCT, SII, NLR, PLR, NPR, SIRI, PAR, CAR, CLR, TyG index, length of hospital stay, and hospitalization expense were significantly higher in the SAP group than the N-SAP group. LMR was significantly lower in the SAP group than the N-SAP group. Age, history of smoking, drinking, and AP, hypertriglyceridemia, hypertension, and interval between onset of symptom and admission were statistically similar between them (Table 2).
Inflammatory index models
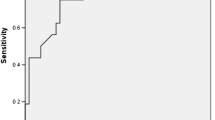
Univariate logistic regression analyses demonstrated that fatty liver, high WBC, CRP, RDW, PCT, SII, NLR, NPR, CAR, CLR, and TyG index, and a low LMR were significantly associated with the presence of SAP (Table 3). Considering the collinearity among these inflammatory indexes, 10 multivariate logistic regression analyses were performed (Supplementary Figs. 1–10). Finally, four models for the identification of SAP were established (Table 4). Their AUROCs were 0.771–0.795 (Fig. 2). Of them, the model 4 had the best discrimination ability (Fig. 2). The c-index was 0.795, and the calibration curve was close to the ideal diagonal line, indicating a good fit (Fig. 3).
Discussion
In the present study, four inflammation-based models for identifying the presence of SAP have been established, of which one, consisting of fatty liver, PCT, and CLR, has a superior diagnostic performance with an accuracy of 79.5%. Notably, the three components are easily obtained through routine blood tests, allowing to assess the severity of AP rapidly.
CLR derives from CRP and lymphocyte. It was widely used to predict the prognosis in many diseases, such as oral cavity squamous cell carcinoma, pancreatic cancer, and colorectal cancer [26, 31, 32]. In our patients, CLR was positively associated with the risk of SAP. The possible reasons are as follows. First, CRP, an acute-phase protein, elevates dramatically during inflammation [33]. This is because that transcriptional induction of the CRP gene mainly occurs in response to an increase of inflammatory cytokines, especially IL-6 [34]. In SAP patients, IL-6 was excessively released [35, 36]. Besides, CRP is deposited at inflammatory sites and amplifies a pro-inflammatory response by a positive feedback loop [37]. Taken together, CRP rises in SAP patients. Second, lymphocyte counts are significantly reduced in SAP [38]. This may be due to the effects of endotoxins released from bacteria and cytokines on T lymphocyte reduction [39] and cytokines released from monocytes or endothelial cells on the apoptosis of peripheral lymphocytes [40].
PCT, a protein of 116 amino acids, is coded for by the calcitonin I (CALC-I) gene [41]. Serum PCT level is elevated in infectious diseases or conditions [42]. PCT was first found to be associated with the severity of infection in 1993 [43]. Later, it was demonstrated that PCT was a good predictor of short-term survival in patients with sepsis and pneumonia [44, 45]. Besides, high PCT level could predict the probability of SAP [46,47,48]. Similarly, our study found that PCT was an independent risk factor of SAP. This finding may be attributed to the fact that an increase of cytokines in SAP patients causes endotoxemia, inducing CALC-I expression in pancreas [49].
Previous studies also demonstrated the importance of other inflammatory indexes and prognostic scores for predicting the AP severity. Jain et al. reported that inflammatory indexes, including NLR, LMR, RDW, and PNI, were comparable to gold standard scoring systems for predicting the severity and mortality of AP [50]. Besides, CAR also has good predictive value in AP severity. It derives from CRP and albumin, which can be calculated rapidly and easily. Kiyak et al. compared CAR with traditional scores, and showed that CAR values were positively correlated with Balthazar score, and its AUC was higher than that of NLR and PLR in mortality prediction of AP [51]. However, their predictive values have not been confirmed in our study.
There were some limitations in our study. First, due to the retrospective nature of this study, some data could not be sufficiently extracted. Thus, we had to define SAP by meeting any of the three following criteria: the revised Atlanta criteria, BISAP score, or Ranson score. Besides, the extreme data obtained during hospitalizations, but not the data at baseline, was used to establish the model. Second, it should be noted that the interval between the onset of symptoms and laboratory assessment was different among our patients, but it could not be controlled due to the retrospective nature of our study. Third, this study was only performed at a single center. Therefore, the findings should be externally validated at other affiliations. Forth, only a relatively small number of AP patients were included. Thus, the findings should be validated in large-scale studies.
Conclusion
Our study suggested an association of SAP with higher levels of SII, NLR, NPR, CAR, CLR, RDW, PDW, PCT, and TyG index, and a lower level of LMR. We developed an inflammation-based model comprising fatty liver, PCT, and CLR for identifying the presence of SAP with a good diagnostic ability. In future, multi-center studies should be conducted to validate these findings.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from.
Abbreviations
- AP:
-
Acute pancreatitis
- MAP:
-
Mild acute pancreatitis
- MSAP:
-
Moderately severe acute pancreatitis
- SAP:
-
Severe acute pancreatitis
- SIRS:
-
Systemic inflammatory response syndrome
- RDW:
-
Red blood cell distribution width
- PDW:
-
Platelet distribution width
- PCT:
-
Procalcitonin
- MCTSI:
-
Modified computerized tomography severity index
- BUN:
-
Blood urea nitrogen
- SII:
-
Systemic immune inflammation index
- NLR:
-
Neutrophil to lymphocyte ratio
- PLR:
-
Platelet to lymphocyte ratio
- LMR:
-
Lymphocyte to monocyte ratio
- NPR:
-
Neutrophil to platelet ratio
- SIRI:
-
Systemic inflammation response index
- PAR:
-
Platelet to albumin ratio
- CAR:
-
C-reactive protein to albumin ratio
- CLR:
-
C-reactive protein to lymphocyte ratio
- TyG index:
-
Triglyceride glucose index
- ROC curve:
-
Receiver operating characteristics curve
- AUROC:
-
Area under receiver operating characteristics curve
- c-index:
-
Concordance index
- SD:
-
Standard deviation
- MCV:
-
Mean corpuscular volume
- RBC:
-
Red blood cells
- CALC-I gene:
-
calcitonin I gene
References
Saluja A, Dudeja V, Dawra R, Sah RP. Early intra-acinar events in pathogenesis of pancreatitis. Gastroenterology. 2019;156(7):1979–93.
Petrov MS, Yadav D. Global epidemiology and holistic prevention of pancreatitis. Nat Rev Gastroenterol Hepatol. 2019;16(3):175–84.
Zilio MB, Eyff TF, Azeredo-Da-Silva ALF, Bersch VP, Osvaldt AB. A systematic review and meta-analysis of the aetiology of acute pancreatitis. HPB. 2019;21(3):259–67.
Gardner TB. Acute Pancreatitis. Ann Intern Med. 2021;174(2):Itc17-itc32.
Leppäniemi A, Tolonen M, Tarasconi A, et al. 2019 WSES guidelines for the management of severe acute pancreatitis. World J of Emerg Surg. 2019;14:27.
Gliem N, Ammer-Herrmenau C, Ellenrieder V, Neesse A. Management of Severe Acute Pancreatitis: an update. Digestion. 2021;102(4):503–7.
Trikudanathan G, Wolbrink DRJ, van Santvoort HC, Mallery S, Freeman M, Besselink MG. Current concepts in severe acute and necrotizing pancreatitis: an evidence-based approach. Gastroenterology. 2019;156(7):1994–2007.e3.
Iannuzzi JP, King JA, Leong JH, et al. Global incidence of acute pancreatitis is increasing over time: a systematic review and Meta-analysis. Gastroenterology. 2022;162(1):122–34.
Jaber S, Garnier M, Asehnoune K, et al. Guidelines for the management of patients with severe acute pancreatitis, 2021. Anaesth Crit Care Pain Med. 2022;41(3):101060.
Rao CY, Fu LY, Hu CL, et al. H2S mitigates severe acute pancreatitis through the PI3K/AKT-NF-κB pathway in vivo. World J Gastroenterol. 2015;21(15):4555–63.
Xu J, Cui Y, Tian X. Early continuous Veno-venous hemofiltration is effective in decreasing intra-abdominal pressure and serum Interleukin-8 level in severe acute pancreatitis patients with abdominal compartment syndrome. Blood Purif. 2017;44(4):276–82.
Bhatia M. Novel therapeutic targets for acute pancreatitis and associated multiple organ dysfunction syndrome. Curr Drug Targets Inflamm Allergy. 2002;1(4):343–51.
Li Y, Zhao Y, Feng L, Guo R. Comparison of the prognostic values of inflammation markers in patients with acute pancreatitis: a retrospective cohort study. BMJ Open. 2017;7(3):e013206.
Mubder M, Dhindsa B, Nguyen D, et al. Utility of inflammatory markers to predict adverse outcome in acute pancreatitis: a retrospective study in a single academic center. Saudi J Gastroenterol. 2020;26(4):216–21.
Tanoğlu A, Düzenli T. Neutrophil-to-lymphocyte ratio alone may not be a true indicator of the severity of acute pancreatitis. Turk J Gastroenterol. 2019;30(10):937.
Kaplan M, Ates I, Oztas E, et al. A new marker to determine prognosis of acute pancreatitis: PLR and NLR combination. J Med Biochem. 2018;37(1):21–30.
Zhu QY, Li RM, Zhu YP, et al. Early predictors of infected pancreatic necrosis in acute severe pancreatitis - implications of neutrophil to lymphocyte ratio, blood procalcitonin concentration and modified CT severity index. Dig Dis; 2023;41(4):677–84.
Chen JH, Zhai ET, Yuan YJ, et al. Systemic immune-inflammation index for predicting prognosis of colorectal cancer. World J Gastroenterol. 2017;23(34):6261–72.
Ojerholm E, Smith A, Hwang WT, et al. Neutrophil-to-lymphocyte ratio as a bladder cancer biomarker: assessing prognostic and predictive value in SWOG 8710. Cancer. 2017;123(5):794–801.
Bong TSH, Tan GHC, Chia C, Soo KC, Teo MCC. Preoperative platelet-lymphocyte ratio is an independent prognostic marker and superior to carcinoembryonic antigen in colorectal peritoneal carcinomatosis patients undergoing cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Int J Clin Oncol. 2017;22(3):511–8.
Rodríguez-Padilla Á, Morales-Martín G, Pérez-Quintero R, Gómez-Salgado J, Ruiz-Frutos C. Serological biomarkers and diversion colitis: changes after stimulation with probiotics. Biomolecules. 2021;11(5)
Lin Y, Dai W, Chen Y, He X, Xu Y. Neutrophil-to-platelet ratio predicts mortality following percutaneous coronary intervention in patients with acute ST-elevation myocardial infarction. Front Physiol. 2022;13:1011048.
Zhou Y, Zhang Y, Cui M, Zhang Y, Shang X. Prognostic value of the systemic inflammation response index in patients with acute ischemic stroke. Brain Behav. 2022;12(6):e2619.
Tan J, Song G, Wang S, et al. Platelet-to-albumin ratio: a novel IgA nephropathy prognosis predictor. Front Immunol. 2022;13:842362.
Liao CK, Yu YL, Lin YC, et al. Prognostic value of the C-reactive protein to albumin ratio in colorectal cancer: an updated systematic review and meta-analysis. World J Surg Oncol. 2021;19(1):139.
Fan Z, Luo G, Gong Y, et al. Prognostic value of the C-reactive protein/lymphocyte ratio in pancreatic Cancer. Ann Surg Oncol. 2020;27(10):4017–25.
Tao LC, Xu JN, Wang TT, Hua F, Li JJ. Triglyceride-glucose index as a marker in cardiovascular diseases: landscape and limitations. Cardiovasc Diabetol. 2022;21(1):68.
Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62(1):102–11.
Ranson JH, Pasternack BS. Statistical methods for quantifying the severity of clinical acute pancreatitis. J Surg Res. 1977;22(2):79–91.
Singh VK, Wu BU, Bollen TL, et al. A prospective evaluation of the bedside index for severity in acute pancreatitis score in assessing mortality and intermediate markers of severity in acute pancreatitis. Am J Gastroenterol. 2009;104(4):966–71.
Ko CA, Fang KH, Hsu CM, et al. The preoperative C-reactive protein-lymphocyte ratio and the prognosis of oral cavity squamous cell carcinoma. Head Neck. 2021;43(9):2740–54.
Meng Y, Long C, Huang X, et al. Prognostic role and clinical significance of C-reactive protein-lymphocyte ratio in colorectal cancer. Bioengineered. 2021;12(1):5138–48.
Lapić I, Padoan A, Bozzato D, Plebani M. Erythrocyte sedimentation rate and C-reactive protein in acute inflammation. Am J Clin Pathol. 2020;153(1):14–29.
Boras E, Slevin M, Alexander MY, et al. Monomeric C-reactive protein and Notch-3 co-operatively increase angiogenesis through PI3K signalling pathway. Cytokine. 2014;69(2):165–79.
van den Berg FF, de Bruijn AC, van Santvoort HC, Issa Y, Boermeester MA. Early laboratory biomarkers for severity in acute pancreatitis; a systematic review and meta-analysis. Pancreatology. 2020;20(7):1302–11.
Gao N, Yan C, Zhang G. Changes of serum Procalcitonin (PCT), C-reactive protein (CRP), Interleukin-17 (IL-17), Interleukin-6 (IL-6), high mobility group protein-B1 (HMGB1) and D-dimer in patients with severe acute pancreatitis treated with continuous renal replacement therapy (CRRT) and its clinical significance. Med Sci Monit. 2018;24:5881–6.
Mihlan M, Blom AM, Kupreishvili K, et al. Monomeric C-reactive protein modulates classic complement activation on necrotic cells. FASEB J. 2011;25(12):4198–210.
Christophi C, McDermott F, Hughes ES. Prognostic significance of the absolute lymphocyte count in acute pancreatitis. Am J Surg. 1985;150(3):295–6.
Curley PJ, McMahon MJ, Lancaster F, et al. Reduction in circulating levels of CD4-positive lymphocytes in acute pancreatitis: relationship to endotoxin, interleukin 6 and disease severity. Br J Surg. 1993;80(10):1312–5.
Bommireddy R, Saxena V, Ormsby I, et al. TGF-beta 1 regulates lymphocyte homeostasis by preventing activation and subsequent apoptosis of peripheral lymphocytes. J Immunol. 2003;170(9):4612–22.
Wiedermann FJ, Kaneider N, Egger P, et al. Migration of human monocytes in response to procalcitonin. Crit Care Med. 2002;30(5):1112–7.
Maruna P, Nedelníková K, Gürlich R. Physiology and genetics of procalcitonin. Physiol Res. 2000;49(Suppl 1):S57–61.
Assicot M, Gendrel D, Carsin H, Raymond J, Guilbaud J, Bohuon C. High serum procalcitonin concentrations in patients with sepsis and infection. Lancet. 1993;341(8844):515–8.
Azevedo JR, Torres OJ, Czeczko NG, Tuon FF, Nassif PA, Souza GD. Procalcitonin as a prognostic biomarker of severe sepsis and septic shock. Rev Col Bras Cir. 2012;39(6):456–61.
Bloos F, Marshall JC, Dellinger RP, et al. Multinational, observational study of procalcitonin in ICU patients with pneumonia requiring mechanical ventilation: a multicenter observational study. Crit Care. 2011;15(2):R88.
Kylänpää-Bäck ML, Takala A, Kemppainen EA, et al. Procalcitonin, soluble interleukin-2 receptor, and soluble E-selectin in predicting the severity of acute pancreatitis. Crit Care Med. 2001;29(1):63–9.
Ebik B, Kacmaz H, Tuncel ET, Arpa M, Ucmak F, Kaya M. What does the Procalcitonin Level Tell us in Patients with Acute Pancreatitis? J Coll Physicians Surg Pak. 2022;32(10):1272–7.
Riché FC, Cholley BP, Laisné MJ, et al. Inflammatory cytokines, C reactive protein, and procalcitonin as early predictors of necrosis infection in acute necrotizing pancreatitis. Surgery. 2003;133(3):257–62.
Aloisio E, Dolci A, Panteghini M. Procalcitonin: between evidence and critical issues. Clin Chim Acta. 2019;496:7–12.
Jain V, Nath P, Satpathy SK, Panda B, Patro S. Comparing prognostic scores and inflammatory markers in predicting the severity and mortality of acute pancreatitis. Cureus. 2023;15(5):e39515.
Kiyak M, Tanoglu A. Comparison of the efficacy of Balthazar score and C-reactive protein-albumin ratio for determination of acute pancreatitis severity. Curr Health Sci J. 2022;48(1):81–7.
Acknowledgements
None.
Funding
None.
Author information
Authors and Affiliations
Contributions
Conceptualization: Xingshun Qi; Methodology: Xiaotong Li, Xingshun Qi; Data curation: Xiaotong Li, Yao Meng, Huimin Chen, and Guiyang Chu; Formal analysis: Xiaotong Li, Weiwei Wang, and Xingshun Qi; Data interpretation: Xiaotong Li, Yiyan Zhang, Yao Meng, Weiwei Wang, Huimin Chen, Guiyang Chu, Hongyu Li, and Xingshun Qi; Writing-original draft: Xiaotong Li, YiYan Zhang, and Xingshun Qi; Writing-review and editing: Xiaotong Li, Yiyan Zhang, Yao Meng, Weiwei Wang, Huimin Chen, Guiyang Chu, Hongyu Li, and Xingshun Qi; Supervision: Hongyu Li and Xingshun Qi. All authors have made an intellectual contribution to the manuscript and approved the submission.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The retrospective study was performed according to the Declaration of Helsinki and was approved by the Medical Ethical Committee of the General Hospital of Northern Theater Command (approval number: Y2023–120). Written informed consent was waived by the Medical Ethical Committee of the General Hospital of Northern Theater Command due to its retrospective nature.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
Forest plot showing the results of multivariate logistic regression analysis 1. Figure S2. Forest plot showing the results of multivariate logistic regression analysis 2. Figure S3. Forest plot showing the results of multivariate logistic regression analysis 3. Figure S4. Forest plot showing the results of multivariate logistic regression analysis 4. Figure S5. Forest plot showing the results of multivariate logistic regression analysis 5. Figure S6. Forest plot showing the results of multivariate logistic regression analysis 6. Figure S7. Forest plot showing the results of multivariate logistic regression analysis 7. Figure S8. Forest plot showing the results of multivariate logistic regression analysis 8. Figure S9. Forest plot showing the results of multivariate logistic regression analysis 9. Figure S10. Forest plot showing the results of multivariate logistic regression analysis 10.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, X., Zhang, Y., Wang, W. et al. An inflammation-based model for identifying severe acute pancreatitis: a single-center retrospective study. BMC Gastroenterol 24, 63 (2024). https://doi.org/10.1186/s12876-024-03148-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12876-024-03148-4