Abstract
Background
Probiotics are effective for treating acute infectious diarrhoea caused by bacteria, but there are inconsistent results for the effectiveness of probiotics for diarrhoea caused by viruses. In this article we want to determine whether Sb supplementation has an effect on acute inflammatory viral diarrhoea diagnosed with the multiplex panel PCR test. The aim of this study was to evaluate the efficacy of Saccharomyces boulardii (Sb) as a treatment in patients diagnosed with viral acute diarrhoea.
Methods
From February 2021 to December 2021, 46 patients with a confirmed diagnosis of viral acute diarrhoea diagnosed with the polymerase chain reaction multiplex assay were enrolled in a double-blind, randomized placebo-controlled trial. Patients received paracetamol 500 mg as a standard analgesic and 200 mg of Trimebutine as an antispasmodic treatment plus 600 mg of Sb (n = 23, 1 × 109/100 mL Colony forming unit) or a placebo (n = 23) orally once daily for eight days. The improvement in and severity of symptoms were measured using a symptom diary, the Patient Global Impression and the Patient Global Impression of Change scales (days 4 and 8), both answered and recorded by the patient.
Results
Of the 46 patients who completed treatment, 24 (52%) were men and 22 (48%) were women. The average age was 35.6 ± 12.28 years (range 18 to 61 years). The average duration of the evolution of illness at the time of diagnosis was 0.85 ± 0.73 days (maximum 2 days). On day 4 after the diagnosis, 20% reported pain and 2% reported fever, but on day 8, no patient reported pain or fever. On day 4, 70% of patients in the Sb group and 26% in the placebo group reported improvement (P = 0.03), based on the Patients’ Global Impression of Change scale, which assesses patient’s rating of overall improvement. These findings suggest that 3 to 4 days of treatment with Sb helped to improve symptoms of diarrhoea caused by a virus.
Conclusion
Treatment with Sb on acute inflammatory diarrhoea of viral aetiology shows no changes regarding the severity of the symptoms; nevertheless, it seems to impact improvement positively.
Trial registration
22CEI00320171130 dated on 16/12/2020, NCT05226052 dated on 07/02/2022.
Similar content being viewed by others
Background
Gastrointestinal infections represent a public health problem in developing and industrialized countries [1]. Despite advances and modifications in policies, food safety regulation, and immunization, these diseases affect millions of people each year. Rapid and accurate diagnosis is important to the management and epidemiological surveillance of these infections. The major challenges in the diagnosis of gastrointestinal infections include the wide diversity of associated viral, bacterial, and parasitic pathogens, as well as cultural factors and the identification of the aetiological agents [2].
Acute diarrhoeal disease (ADD) is defined as the expulsion of 3 or more liquid stools, with or without blood, within 24 h, that take the shape of the container in which they are placed. A diarrhoeal episode is one that meets the above criteria and ends when the last day of diarrhoea is followed by at least 48 h of normal stools [3]. Acute diarrhoea lasts less than 14 days, diarrhoea lasting more than 14 days is called persistent diarrhoea, and diarrhoea lasting more than 1 month is called chronic diarrhoea. Severe acute diarrhoea warrants immediate medical evaluation and hospitalization [4].The clinical presentation of viral gastroenteritis ranges from an asymptomatic state to diarrhoea with severe dehydration [2].
Enteric bacterial gastroenteritis can be difficult to differentiate from that of viral aetiology solely based on the clinical presentation mainly because of the presence of leukocytes in stool. In the past, the presence of leukocytes was a specific indication of diarrhoea of bacterial aetiology and was the basis for the diagnosis of acute inflammatory diarrhoea. Hence, laboratory studies are needed to make a specific diagnosis [5].
The methylene blue test is traditionally performed to identify the presence of leukocytes in stool [6]. The multiplex polymerase chain reaction (PCR) test uses an automated system in which the extraction, amplification, and detection of nucleic acid occurs in a single closed pouch. The test panel includes the aetiological identification of bacteria, parasites, and viruses [7].
Probiotics can be bacterial or yeast microbes. Yeast probiotics, such as Saccharomyces boulardii (Sb), are different from bacterial probiotics [3].Sb has several different mechanisms of action that can be classified into 3 main areas: luminal action, trophic action, and mucosal anti-inflammatory signalling effects. Sb has several benefits such as interference with pathogenic toxins and pathogen adhesion, preservation of cell physiology, interactions with the normal microbiota, and restoration of short-chain fatty acid level. Sb can also act as an immune regulator, both within the lumen and systemically [4].
The efficacy of other Saccharomyces strains has also been investigated. Unlike other Saccharomyces strains, only Sb is capable of degrading Clostridium difficile toxin, destroying endotoxins of pathogenic Escherichia coli, reducing the effects of cholera toxin, and inhibiting the growth of pathogens (such as Candida albicans, Salmonella typhimurium, Yersinia enterocolitica, Aeromonas haemolysin) [8].
Normal gut microbiota has many functions but the most pertinent is resistance to colonization, which involves the interaction of many bacterial microflorae and results in a barrier effect against the colonization of pathogens. Factors that disrupt this protective barrier (for example, the use of antibiotics or surgery) increase host susceptibility to pathogen colonization until the normal microbiota can be restored (usually within 6–8 weeks). Probiotics are uniquely qualified to act as a substitute for normal microbiota during this window of susceptibility until recovery. Sb does not affect the normal microbiota in healthy human controls, although the normal microbiota is rapidly restored when S. boulardii is administered to mice subjected to antibiotic shock or patients with diarrhoea [8].
Based on a systematic review of 27 clinical trials involving 5029 patients, 84% of the treatment groups that received S. boulardii for multiple causes of diarrhea demonstrated significant efficacy and safety. S. boulardii was significantly effective in preventing antibiotic-associated diarrhea, with a relative risk (RR) of 0.50, according to a 2010 meta-analysis of ten randomized, controlled trials in adults [8].
As previously mentioned in this paper, viral gastroenteritis is the leading cause of gastroenteritis, and the administration of probiotics may help to control viral infection. However, the interaction between probiotics and viral gastroenteritis has not been previously evaluated in the Mexican population.
Materials and methods
Main objective
The main objective was to determine whether Sb supplementation has an effect on acute inflammatory viral diarrhoea diagnosed with the multiplex panel PCR test in private practice.
Design
This was a randomized, placebo-controlled double-blind study. Initially, patients were randomized in a 1:1 ratio to receive all doses of Sb, 3 capsules of Saccharomyces boulardii CNCM I-745 200 mg/day, or placebo, 3 starch capsules of 200 mg as placebo/day. Both the placebo and the probiotic capsules had a similar presentation. CMCM I-745 has a concentration of 1 × 109/100 mL Colony forming unit (CFU) [9]. In this study, the rationale for administering 600 mg of S. boulardii to the patients was based on a clinical trial conducted by Hochter et al. [10]. This trial had reported efficacy for acute adult diarrhea in patients who received S. boulardii. Therefore, the dosage selected for this study was consistent with that used in the previously conducted trial, and it was expected to demonstrate similar efficacy.
Procedure
A total of 46 patients were included and divided into 2 groups, probiotics (n = 23) or placebo (n = 24) groups. The inclusion criteria were age 18 years or more, a positive leukocyte test in stool and a confirmed diagnosis of viral infection by FilmArray™ Multiplex PCR System from bioMérieux, Marcy l’Etoile, France. Patients were included after they provided informed consent.
The exclusion criteria were the presence of known autoimmune disease or inflammatory bowel disease, under immunosuppressive treatment for a known pathology, a confirmed diagnosis of infection by bacteria and/or parasites in the multiplex PCR test whether associated or not associated with viral etiology, previous administration of antibiotic treatment or consumption of any probiotic in the preceding 7 days, known allergy to the probiotic containing Sb, clinical positivity to the current operational definition of COVID-19, or no medical insurance. Patients with less than 80% adherence to the indicated probiotic treatment, treatment interruption, or withdrawal of informed consent were eliminated from the analysis.
Virus identification
The multiplex PCR test was performed using the Gastrointestinal Panel for FilmArray™ Multiplex PCR System. This panel is an automated system in which nucleic acid extraction, amplification, and detection occur in a single closed pouch [11]. The panel includes a total of 22 targets, including bacteria (Campylobacter jejuni, Campylobacter coli, Campylobacter upsaliensis, Clostridium difficile (toxin A/B), Plesiomonas shigelloides, Salmonella, Yersinia enterocolitica, Vibrio parahaemolyticus, Vibrio vulnificus, Vibrio cholerae); diarrheagenic E. coli/Shigella (E. coli O157, enteroaggregative E. coli, enteropathogenic E. coli, enterotoxigenic E. coli lt/st, Shiga-like toxin-producing E. coli stx1/stx-2, E. coli O157, Shigella/enteroinvasive E. coli; parasites (Cryptosporidium, Cyclospora cayetanensis, Entamoeba histolytica, Giardia lamblia); and viruses (Adenovirus F 40/41, Astrovirus, Norovirus GI/GII, Rotavirus A, Sapovirus I, II, IV, and V) [12, 13].
Questionnaires
The Patient Global Impression scale (PGIs) is the patient-reported outcomes counterpart to the Clinical Global Impressions scale. The PGIs is a 1-item questionnaire that asks the patient to rate the severity of a specific condition. The Patient Global Impression of Change (PGIC) measures the patient’s change in clinical status [15]. Each participant in the study was provided with a patient diary in which they recorded the number of stools using the Bristol stool scale, the presence of pain or fever, and treatment adherence on a daily basis. Patients also self-reported their symptoms using the PGIC and PGI scales in the diary. The patient diaries and scales were evaluated on days 4 and 8 of treatment. The methods used to measure pain in the participants was the visual analogue scale (VAS) for pain. Patients were instructed on how to respond to the VAS. The guidelines for completing the form were followed. A value between 0 and 4 indicates the absence of pain. For this study, pain was considered present at a score greater than 4 [14].
The improvement was measured in two ways: first, on days 4 and 8, using the PGIC and PGIS scales. The patient was deemed to have improved when he or she reported feeling significantly and slightly better. No improvement was considered: unchanged, slightly worse, and significantly worse. With the analysis of the patient’s diary, an improvement was deemed to have occurred at a value of 0 Bristol evacuations grade 6 and 7, as well as the absence of pain or fever reported by the patient.
Statistical analysis
Means and standard deviations were calculated for the quantitative variables, and frequencies and proportions were calculated for the qualitative variables. Qualitative variables were compared using the chi square test. The Shapiro–Wilk test was used to determine the distribution of the data. The Cochran–Mantel–Hansel statistical test was used to identify the intervening variables. Relative risk and 95% confidence intervals (CIs) were calculated for symptom persistence after the beginning of the treatment and improvement of diarrhoea during treatment. P < 0.05 was considered to be significant. The data were transferred to an Excel file, and the statistical analysis was performed using the IBM SPSS Statistics (version 20.0, IBM, Corp., Armonk, NY, USA).
Results
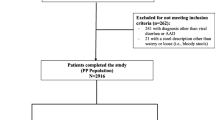
Patient recruitment began on 15 February 2021 and concluded on 27 December 2021. A total of 232 multiplex PCR tests were performed on samples from patients who met the criteria for methylene blue positivity; 52 met the inclusion criteria. Of these patients identified, 47 agreed to continue in the study, but 46 completed the treatment regimen after 1 patient was withdrawn because of lack of treatment adherence (Fig. 1).
The patient characteristics are shown in Table 1. Of the 46 patients who completed treatment, 24 (52%) were men and 22 (48%) were women. The average age was 35.6 ± 12.28 years (range 18 to 61 years). The average duration of the evolution of illness at the time of diagnosis was 0.85 ± 0.73 days (maximum 2 days).
The average number of daily bowel movements at the time of diagnosis was 8.02 ± 2.39 (range 3 to 15); 65% of the patients reported feeling pain at the beginning of treatment and 17% of the patients presented with fever. The baseline characteristics did not differ significantly between the Sb and placebo groups.
The type of viral pathogens found in patients diagnosed with acute inflammatory diarrhoea of viral aetiology seen in the gastroenterology and coloproctology clinics did not differ between the 2 groups: 65% of the patients in the Sb group and 74% in the placebo group presented with Norovirus GI/GII, 26.1% of patients in both groups had a positive result for Rotavirus A, and 9% in the Sb group and no patients in the control group presented with Sapovirus. One patient had a combination of Rotavirus A and Adenovirus F40, and this patient was included in the group with Rotavirus A. No patients were shown to be positive for Adenovirus F40/41 or Astrovirus.
As shown in Table 2, on day 4, 16 (70%) of patients in the Sb group and 6 (26%) patients in the placebo group reported improvement (P < 0.03). For the associated symptoms on day 4, 9 patients (20%) reported pain, and 1 patient (2%) reported fever. On day 8, no patient reported pain or fever. On days 4 and 8, the severity of symptoms, as assessed using the PGIs and PGIC, did not differ between groups. According to the patient dairy, patients reported improvement after 4 ± 1 days in the S. boulardii group and after 5 ± 0.95 days in the placebo group (P = 0.001).
Discussion
Similar to findings reported in the international literature [15, 16], the most frequent viral aetiology found in this adult population with acute viral diarrhoea was Norovirus GI/GII followed by Rotavirus. Despite evidence in Mexico that the paediatric population younger than 2 years usually presents with viral illness caused by Rotavirus [2], and the immune response generated favours less severe disease caused by this virus, our country continues to experience a high incidence of Rotavirus infection that requires hospital medical attention. Gonzalez et al. [17], identified Rotavirus in 14 of 100 faecal samples collected in children with gastroenteritis in the state of Sonora, Mexico; in our study, 20% of samples exhibited different viruses and 5% were specific for Rotavirus.
The mean duration of infection in our study was 4.5 days. The literature reports that the duration of Norovirus infection is shorter than the general average, which is 2 to 5 days [2, 18]. Although Rotavirus was the second most frequent aetiological agent in our study, the average duration of symptoms was 2 to 7 days, which is similar to that in the general population.
For the symptom severity in our study, no significant difference was obtained after 4 and 8 days of treatment. This finding differs from that reported in a meta-analysis in which the diarrhoea severity score on day 3 of treatment was significantly lower in Sb-treated patients (5.5 ± 6.8) than in the placebo group (6.7 ± 8.7) (P = 0.04) [11].
Although self-reported improvement on days 4 and 8 did not differ between the Sb and placebo groups, most patients in the Sb group (65%) reported improvement on days 3 and 4, whereas the highest percentage of patients in the placebo group (52%) reported improvement on day 5. This finding is similar to that reported for pooled data from 17 trials that found that Sb reduced the mean duration of diarrhoea by 19.7 h [19].
This decrease in the duration of symptomatology may be relate to the mechanisms of action of probiotics, such as an increase in the production of short-chain fatty acids in colonocytes, reduction in the permeability of the intestinal barrier, or a decrease in the invasion of microorganisms [20]. For example, Sb induces high levels of IgA and interleukin 10 in the bowel, and these participate in the immunomodulatory response to infection. Specific beneficial effects have been reported in the treatment of children with ADD, prevention of C. difficile infections, and prevention of diarrhoea associated with the use of antibiotics, including anti-toxin and anti-inflammatory effects, trophic effects on enterocytes, stimulation of the immune response, increase in disaccharidase levels, elimination of toxins and pathogens, and interference with the bacterial signalling pathways [3].
Most patients in the present study (65%) presented with pain as the primary symptom. It is relevant that only 17% presented fever, which is the most common symptom reported in the literature [16, 18]. Pain is usually the second most common particularly for patients with Norovirus as the causal agent.
The primary limitations of this study are the small sample size and the underrepresentation of the pediatric population. The used questionnaires do not collect data on some standard endpoints typically evaluated in diarrhea studies, such as hydration status and associated symptoms such as nausea and vomiting. As a result, it may not provide a complete picture of treatment response and may hinder the ability to interpret study results.
The simple stool examination continues to be the primary test at the time of diagnosis. However, we included only those patients who obtained a positive methylene blue test result, which in clinical practice would suggest an infection caused by toxin-producing bacteria. It is therefore essential to identify specifically the aetiology of the disease.
Conclusion
In this study, the most frequent aetiology was Norovirus GI/GII infection followed by Rotavirus, Sapovirus, and the combination of Rotavirus A and Adenovirus F40. Both the Sb and placebo groups exhibited similar symptoms on day 4 after the diagnosis (20% with pain and 2% with fever). On day 8, no patient reported any associated symptom. Treatment with Sb on acute inflammatory diarrhoea of viral aetiology shows no changes regarding the severity of the symptoms; nevertheless, it seems to impact improvement positively. Most patients (70%) in the Sb group reported improvement on days 3 and 4; by contrast, in the placebo group, the highest percentage of patients (74%) reported improvement on day 5 (P = 0.03). The severity of the symptoms did not differ significantly between the 2 groups on days 4 and 8.
Data Availability
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- Sb:
-
Saccharomyces boulardii
- ADD:
-
Acute diarrhoeal disease
- PCR:
-
Polymerase chain reaction
References
O’Ryan M, Prado V, Pickering LK. A millennium update on pediatric diarrheal illness in the developing world. Semin Pediatr Infect Dis. 2005. https://doi.org/10.1053/j.spid.2005.12.008. 16;125–136 [PMID: 15825143].
Farfán M, Piemonte P, Labra Y, Henríquez J, Candía E, Torres JP. [Filmarray GI TM panel for detection of enteric pathogens in stool samples: preliminary experience]. Rev Chil Infectol. 2016;33:89–91. https://doi.org/10.4067/S0716-10182016000100016. [PMID: 26965886].
Czerucka D, Piche T, Rampal P. Review article: yeast as probiotics –Saccharomyces boulardii. Aliment Pharmacol Ther. 2007;26:767–78. https://doi.org/10.1111/j.1365-2036.2007.03442.x. [PMID: 17767461].
Gore JI, Surawicz C. Severe acute diarrhea. Gastroenterol Clin North Am. 2003;32:1249–67. https://doi.org/10.1016/s0889-8553(03)00100-6. [PMID: 14696306].
Olaiz-Fernández GA, Gómez-Peña EG, Juález-Flores A, Vicuña-de Anda FJ, Morales-Ríos JE, Carrasco OF. [Historical overview of acute infectious diarrhea in Mexico and future preventive strategies]. Salud Publica Mex. 2020;62:25–35. https://doi.org/10.21149/10002. [PMID: 31869558].
Palacio-Mejía LS, Rojas-Botero M, Molina-Vélez D, García-Morales C, González-González L, Salgado-Salgado AL, Hernández-Ávila JE, Hernández-Ávila M. Overview of acute diarrheal disease at the dawn of the 21st century: the case of Mexico. Salud Publica Mex. 2020;62:14–24. https://doi.org/10.21149/9954. [PMID: 31314211].
Bányai K, Estes MK, Martella V, Parashar UD. Viral gastroenteritis. Lancet. 2018;392:175–86. https://doi.org/10.1016/S0140-6736(18)31128-0. [PMID: 30025810].
McFarland LV. Systematic review and meta-analysis of Saccharomyces boulardii in adult patients. World J Gastroenterol. 2010;16:2202–22. https://doi.org/10.3748/wjg.v16.i18.2202. [PMID: 20458757].
Justino PFC, Melo LFM, Nogueira AF, Costa JVG, Silva LMN, Santos CM, et al. Treatment with Saccharomyces boulardii reduces the inflammation and dysfunction of the gastrointestinal tract in 5-fluorouracil-induced intestinal mucositis in mice. Br J Nutr Camb Univ Press. 2014;111(9):1611–21.
Hochter W, Chase D, Hagenhoff G. Saccharomyces boulardii in acute adult diarrhea: efficacy and tolerability of treatment. Munch Med Wschr. 1990;132(12):188–92.
Mihalov-Kovács E, Gellért Á, Marton S, Farkas SL, Fehér E, Oldal M, Jakab F, Martella V, Bányai K. Candidate new rotavirus species in sheltered dogs, Hungary. Emerg Infect Dis. 2015;21:660–3. https://doi.org/10.3201/eid2104.141370. [PMID: 25811414].
Bányai K, Kemenesi G, Budinski I, Földes F, Zana B, Marton S, Varga-Kugler R, Oldal M, Kurucz K. Jakab F.Candidate new rotavirus species in Schreiber’s bats, Serbia. Infect Genet Evol. 2017;48:19–26. https://doi.org/10.1016/j.meegid.2016.12.002. [PMID: 27932285].
Dóro R, Farkas SL, Martella V, Bányai K. Zoonotic transmission of rotavirus: surveillance and control. Expert Rev Anti Infect Ther. 2015;13:1337–50. https://doi.org/10.1586/14787210.2015.1089171. [PMID: 26428261].
Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res (Hoboken). 2011 Nov;63 Suppl 11:S240-52. https://doi.org/10.1002/acr.20543. PMID: 22588748.
Blaclow NR, Greenberg HB. Viral gastroenteritis. N Engl J Med. 1991;325:252–64. https://doi.org/10.1056/NEJM199107253250406. [PMID: 1647494].
Robilotti E, Deresinski S, Pinsky BA, Norovirus. Clin Microbiol Rev. 2015;28:134–64. https://doi.org/10.1128/CMR.00075-14. [PMID: 25567225].
González-Ochoa G, de J G, Calleja-García PM, Rosas-Rodriguez JA, Virgen-Ortíz A, Tamez-Guerra P. Detection of emergin rotavirus G12P[8] in Sonora, Mexico. Acta Virol. 2016;60:136–42. https://doi.org/10.4149/av_2016_02_136. [PMID: 27265462].
Glass RI, Parashar UD, Estes MK. Norovirus gastroenteritis. N Engl J Med. 2009;361:1776–85. https://doi.org/10.1056/NEJMra0804575. [PMID: 19864676].
Sniffen JC, McFarland LV, Evans CT, Goldstein EJC. Choosing an appropriate probiotic product for your patient: an evidence–based practical guide. PLoS ONE 2018;13. https://doi.org/10.1371/journal.pone.0209205. [PMID: 30586435].
Pérez- Reytor D, Puebla C, Karahanian E, García K. Use of short-chain fatty acids for the recovery of the intestinal epithelial barrier affected by bacterial toxins. Front Physiol. 2021;12. https://doi.org/10.3389/fphys.2021.650313. [PMID: 34108884].
Bosch A, Pintó RM, Guix S. Human astroviruses. Clin Microbiol Rev. 2014;27:1048–74. https://doi.org/10.1128/CMR.00013-14. [PMID: 25278582].
World Medical Association. World Medical Association Declaration of Helsinski: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–4. https://doi.org/10.1001/jama.2013.281053. [PMID: 24141714].
Acknowledgements
We appreciate the support of Hospital Puerta de Hierro Sur, as well as OnLine English for editing the manuscript, including the use of language, structure, and expression of ideas for publication in the biomedical literature. We acknowledge Dr. Jonathan Matias Chejfec-Ciociano for his support throughout this research.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
M.A.G.S.P., X.A.R.T.T. and A.G.O. participated in the protocol design, collection of clinical information, statistical analysis and were involved in editing the manuscript; M.A.G.S.P., R.U.C.N., J.J.D.M., H.I.C.N., J.M.G.D., M.J.G.R., J.O.V.G., K.V.A.D., M.F.Z.C., F.Y.G.P., F.J.B.C., C.F.O., A.G.O., G.C.G., E.C.P., G.A.C.C., A.O.C.F. were involved in the protocol design, identification and inclusion of candidates, and critically revised the article for important intellectual content; all the authors reviewed and approved the final version to be published.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was performed according to the 1989 Declaration of Helsinki principles and its amendments according to the Mexican Health Guidelines and in adherence to the CONSORT 2000 criteria [21, 22]. The Integral Medical society of Queretaro Hospital Research Ethics Committee approved this study with the identifier number 22CEI00320171130 dated on 16/12/2020. The study is registered with the National Clinical Trials with the identifier number NCT05226052 (07/02/2022). Full written informed consent was obtained from all patients before their inclusion in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Supported by
This study did not receive any kind of financial support by any public or private institution. All resources were supported by the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Salazar-Parra, M.A., Cruz-Neri, R.U., Trujillo-Trujillo, X.A. et al. Effectiveness of Saccharomyces Boulardii CNCM I-745 probiotic in acute inflammatory viral diarrhoea in adults: results from a single-centre randomized trial. BMC Gastroenterol 23, 229 (2023). https://doi.org/10.1186/s12876-023-02863-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12876-023-02863-8