Abstract
Background
Endoscopic transpapillary gallbladder stenting (EGBS) is considered for patients with contraindications to early surgery for acute calculus cholecystitis. However, evidence regarding the long-term outcomes of EGBS is insufficient to date. The aim of the study was to evaluate the feasibility of EGBS as a bridge to or alternative to surgery when there are contraindications.
Methods
We reviewed the cases of patients who underwent EGBS using a novel spiral-shaped plastic stent for acute calculus cholecystitis between January 2011 and December 2019. We retrospectively evaluated the long-term outcomes of EGBS using a novel spiral-shaped plastic stent.
Results
Forty-nine patients were included. The clinical success rate of EGBS was 97%. After EGBS, 25 patients (surgery group) underwent elective cholecystectomy and 24 patients did not (follow-up group). In the surgery group, the median period from EGBS to surgery was 93 days. There was a single late adverse event with cholecystitis recurrence. In the follow-up group, the median follow-up period was 236 days. Late adverse events were observed in eight patients, including recurrence of cholecystitis (four patients), duodenal penetration by the distal stent end (two patients), and distal stent migration (two patient). In the follow-up group, the time to recurrence of biliary obstruction was 527 days.
Conclusions
EGBS with a novel spiral-shaped plastic stent is safe and effective for long-term acute calculus cholecystitis. There is a possibility of EGBS to be a bridge to surgery and a surgical alternative for acute calculus cholecystitis in patients with contraindications to early cholecystectomy.
Similar content being viewed by others
Introduction
Acute cholecystitis is a common disease [1]. The most frequent cause of acute cholecystitis is gall stones [2]. Several treatment options are considered for acute calculus cholecystitis. The standard therapy for acute calculus cholecystitis is early laparoscopic cholecystectomy [3]. However, the number of patients who cannot undergo surgery due to high risk factors such as older age, ongoing antithrombotic therapy, and comorbidity is increasing. Therefore, gallbladder drainage is an important treatment option for the management of acute calculus cholecystitis. The standard method for gallbladder drainage is PTGBD. Although PTGBD is effective, it has not a few complications and has several problems may not be suitable for long-term treatment owing to external drainage.
Endoscopic transpapillary gallbladder stenting (EGBS) and endoscopic ultrasound-guided gallbladder drainage (EUS-GBD) are alternative therapies for patients with acute cholecystitis that do not involve an external fistula [4]. Although EUS-GBD reduced adverse events, re-intervention, and recurrent cholecystitis compared to PTGBD, EUS-GBD is still not universal [5]. On the other hands, EGBS is the procedure included in endoscopic retrograde cholangiopancreatography (ERCP), which is performed all over the world. Several reports have demonstrated the short-term efficacy and safety of EGBS [6,7,8,9,10]. However, the following problems need to be clarified in EGBS for acute cholecystitis: (1) the optimal stent, (2) the efficacy and safety as a bridge to surgery, and (3) the long-term outcomes for inoperable patients.
We developed a novel spiral-shaped plastic stent (SPS) for EGBS and previously reported satisfactory short-term outcomes [11]. However, long-term outcomes of SPS remain unclear.
In the present study, we retrospectively evaluated the long-term outcomes of EGBS using the novel SPS to clarify the feasibility of EGBS as a bridge to surgery or as an alternative treatment for inoperable patients.
Patients/material and methods
Patients
We retrospectively reviewed and included consecutive patients who underwent successful EGBS using the novel SPS for acute calculus cholecystitis between January 2011 and December 2019 at the Department of Gastroenterology and Hepatology of St. Marianna University School of Medicine. We excluded patients according to the following criteria: (1) acalculus cholecystitis, (2) use of a non-spiral-shaped stent, and (3) short follow-up period of < 30 days. All patients provided written informed consent for the procedure. This study was approved by the Institutional Review Board of the St. Marianna University School of Medicine (Approval Number: 4382).
Indications of EGBS for cholecystitis
The first line treatment for mild to moderate acute calculus cholecystitis was early cholecystectomy. In the patients with severe cholecystitis, we performed EGBS as gallbladder drainage. In the patients with mild to moderate cholecystitis, we performed EGBS when they had one of the following conditions: (1) contraindications for early cholecystectomy, (2) severe comorbidities, (3) severe symptoms, and (4) plan to perform ERCP for acute cholangitis with choledocholithiasis.
EGBS procedure
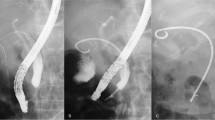
All EGBSs were performed under the supervision of an experienced ERCP expert (more than 1000 ERCP procedures). We used a duodenoscope (JF-260V, TJF-260V, or TJF-Q290V; Olympus Medical Systems, Tokyo, Japan) and performed bile duct cannulation using conventional contrast or wire-guided cannulation. After successful biliary cannulation, cholangiography was performed to assess the shape of the common bile duct (CBD) and to determine whether the cystic duct filled with contrast. We conducted a hydrophilic guidewire (e.g., Radiforcus, Terumo Co. Ltd., Tokyo, Japan, or NaviPro, Boston Scientific, Natick, MA) through the cystic duct. Once the guidewire was inserted into the gallbladder, it was replaced with a stiff type. We then inserted a 7-French (Fr)-tapered catheter with side holes (MultiFunction Catheter, Gadelius Medical, Tokyo, Japan) over the guidewire into the gall bladder to suction the infected bile. Finally, we placed the SPS (GBest-N stent; Hanaco Medical Co., Saitama, Japan) in the gallbladder fundus (Fig. 1) [11]. The top of the novel stent had a three-dimensional spiral-shape, conceived to prevent migration. The distal end of the SPS was straight, with a flap. The semicircular shaft of the stent measured 7 Fr. There were side holes within the spiral tip and shaft of the stent. SPS lengths varied, with 11, 13, 15, 17, and 19 cm. We did not perform any scheduled stent exchanges for patients after EGBS. The patients performed EGBS were followed every 6 months unless the patients refused follow-up. Elective cholecystectomy after EGBS was performed to the patients who had indication of surgery. During the cholecystectomy, the stent was removed by the following steps: (1) Dissection of the gallbladder is started from the fundus of the gallbladder and proceeds to the neck, and only the cystic artery and duct are left. (2) The gallbladder artery is ligated with a clip and dissected. (3) Assistant inserts endoscopy to duodenum, and under observation from both the abdominal cavity and the duodenum, the stent is removed by grasping forceps through the endoscope. (4) After removal of the stent, the cystic duct is ligated and separated with a clip or end loop, and the gallbladder is removed.
Measurements
We retrospectively assessed the patients’ backgrounds and EGBS treatment outcomes, including clinical success rates and adverse events. The diagnosis and severity of acute cholecystitis were assessed according to the Tokyo Guidelines 2018 (TG18) [12]. We defined the clinical success of EGBS as improvement (or tendency for improvement) in clinical symptoms and laboratory data within 3 days. Adverse events were divided into EGBS-related sequelae such as cystic duct injury or peritonitis and ERCP-related events. Cystic duct injury was defined as the dislocation of devices, such as guidewire or cannula, or the leakage of contrast media into the peritoneal cavity from the cystic duct lumen confirmed by fluoroscopic imaging [13]. ERCP-related adverse events occurred within 7 days of ERCP. The diagnosis and severity of ERCP-related adverse events included bleeding, perforation, pancreatitis, and cholangitis based on the consensus guidelines of Cotton et al. [14]. We evaluated the long-term outcomes of EGBS, including late adverse events and time to recurrent biliary obstruction (TRBO). Recurrent biliary obstruction defined as patients who were needed exchange or removal of EGBS because of late adverse event. Late adverse events were defined as recurrent cholecystitis, cholangitis, duodenal stent penetration, and migration. We evaluated patients with and without cholecystectomy separately. In this study, TRBO in patients without cholecystectomy included not only stent obstruction but also stent migration requiring endoscopic intervention. Furthermore, we assessed the pre-operative outcomes of EGBS as a bridge to surgery in patients who eventually underwent cholecystectomy. In addition, we also assessed the outcome of cholecystectomy after EGBS.
Statistical analysis
Categorical variables were compared using Fisher’s exact test. Continuous variables are presented as median (range) and compared using the Mann–Whitney U-test. TRBO was calculated using the Kaplan–Meier method. Statistical significance was set at P < 0.05. Statistical analyses were performed using R version 3.4.1 software (R Foundation, Vienna, Austria).
Results
Between January 2011 and December 2019, 242 patients underwent endoscopic transpapillary drainage. Among them, 193 patients were excluded from the study for the following reasons: (1) acalculous cholecystitis (41 patients), (2) Technical failure of ETGBD (23 patients), (3) endoscopic nasobiliary drainage (69 patients), (4) use of a non-spiral-shaped stent (47 patients), and (5) follow-up for < 30 days (13 patients). Thus, 49 patients were enrolled in this study (Fig. 2). The baseline patient characteristics are shown in Table 1. Mild cholecystitis was diagnosed in 12 (24%), moderate in 31 (63%), and severe in 6 (12%) patients. The stones were found in the cystic duct in five patients (10%) and the gall neck in nine (16%). CBD stones were present in 17 (35%) patients. EGBS was performed instead of cholecystectomy because of CBD stones in 16 patients, antithrombotic therapy or coagulopathy in 16 patients, severe comorbidity in 10 patients, poor performance status in five patients, refused surgery by one patient, and suspected malignant gallbladder lesion in one patient.
The clinical success rate for EGBS was 97% (Table 2). Early adverse events were cystic duct injury in three patients (6%) and mild pancreatitis in two patients (4%). All early adverse events improved conservatively. No other events including bleeding, perforation, or cholangitis were observed.
In this study, 25 patients underwent elective cholecystectomy after EGBS (surgery group) and 24 underwent EGBS alone without cholecystectomy (follow-up group). In the surgery group, the median period from EGBS to cholecystectomy was 93 days (Table 3). Late adverse events were observed in only one patient (4%) with cholecystitis (due to stent obstruction). After EGBS, laparoscopic and open cholecystectomy were performed in 19 and 6 patients, respectively. The median operating time was 145 min. The median intra-operative blood loss was 10 ml.
In the follow-up group, the median follow-up period was 236 days (Table 4). Late adverse events were observed in eight patients (33%), comprising four cases of recurrent cholecystitis, two cases of duodenal stent penetration, and two case of migration. The cause of recurrent cholecystitis with stent dysfunction was obstruction in three patients and migration in one. The stent migrated toward gallbladder side in one, and toward duodenum in another. TRBO was 527 days in the follow-up group (Fig. 3).
Discussion
In this study, we reviewed the cases of 49 patients who underwent EGBS using the novel SPS for acute calculus cholecystitis to evaluate long-term results. There are few reports available that assess long-term outcomes after EGBS for acute cholecystitis. Conway et al., Schlenker et al., and Lee et al. reported the long-term outcomes of EGBS in patients who are poor surgical candidates [15,16,17]. Although these studies showed the long-term efficacy and safety of the EGBS procedure, the number of patients studied was fewer than 30, which may not be sufficient for accurate analysis. In contrast, Mutignani et al. and Inoue et al. reported the long-term outcomes of EGBS in more than 30 patients. These studies have the following limitations: (1) they included both calculous and acalculous cholecystitis and (2) they used different types of stents [18, 19]. Maekawa et al. and Kim et al. reported the long-term effectiveness of EGBS using a single type of 7Fr double-pigtail biliary stent for calculous cholecystitis in 31 patients and 83 patients, respectively [20, 21]. However, all previous studies have evaluated the long-term outcomes of EGBS with biliary stents. To the best of our knowledge, ours is the first study to evaluate the long-term outcomes of EGBS for calculous cholecystitis using an SPS.
The novel SPS has several peculiarities compare with traditional stents such as double-pigtail stents and straight stents. The SPS had side holes within the spiral tip and shaft of the stent to improve drainage both gallbladder and CBD. Additionally, the three-dimensional spiral-shaped top and the straight distal end of the SPS might be effective to prevent migration. In our study, the clinical success rate of EGBS with the SPS was 97%. In a prior study, the clinical success rate of EGBS using a double-pigtail stent was 83% [18]. Stent migration rate was 4% in the current study and 9% in a previous study using a double-pigtail stent [17]. These results suggest that the SPS might offer superior outcomes than the double-pigtail stent for EGBS.
On the other hand, the late adverse event of duodenal penetration by the stent was observed in our study. This has never been reported in another EGBS paper [15,16,17,18,19,20,21]. Duodenal stent penetration occurred on days 269 and 527 post-EGBS. The reason for penetration could have been the long placement of the stent with a typical straight tip on the duodenal side. Fortunately, the patients with penetration were asymptomatic and required only stent exchange and preventive endoscopic clipping. An EGBS stent comparison study assessing clinical success, adverse events, and long-term outcomes is warranted to determine the most appropriate stent shape.
The outcomes of pre-operative EGBS have not previously been evaluated. In our study, the median period between EGBS and surgery was 93 days. Only one patient needed re-intervention due to stent obstruction. A previous report revealed similar early adverse events occurring in EGBS and PTGBD [22]. Although further study is needed, we think that EGBS could serve as a bridge to surgery in patients who cannot undergo early cholecystectomy, especially because of stent patency and low adverse event rate of EGBS.
We evaluated the long-term outcomes of EGBS in patients who did not undergo cholecystectomy due to poor PS or severe comorbidity. The previous reports showed late adverse event rate in patients who had no indication of surgery as 25–28% [23, 24]. As follow-up periods of our study were longer than previous reports, the late adverse event rate became slight high. In our study, the TRBO of EGBS was 527 days. A previous report showed superiority of EGBS over PTGBD for acute cholecystitis, with similar clinical success and low late adverse event rates [25]. Our data support the low late adverse event rate associated with EGBS. Although cholecystectomy is the best treatment for calculus cholecystitis, we believe that EGBS is feasible and has potential as an alternative therapy for acute cholecystitis in patients with contraindications for surgery.
Our study had several limitations. First, this was a nonrandomized, retrospective study conducted at a single center. Second, there were biases, such as those related to the treatment strategy and management of acute cholecystitis. Although we followed a continuous strategy for indication of EGBS, we could not eliminate the selection bias in this retrospective study. Third, the confounding effects of EGBS and antibiotic treatment on clinical outcomes were not evaluated in this study. Forth, each number of patients evaluated for follow-up group and surgery group may be small. Fifth, the median follow-up period of the patients in follow-up group was less than 1 year because they had difficulty to come continually to our hospital because they had problems such as poor PS or severe comorbidities. Finally, all EGBS procedures were performed by a specialist experienced in ERCP and EGBS. Therefore, the results are not likely to be universal.
In conclusion, EGBS using a novel SPS is safe and effective in the long-term for treatment of acute calculus cholecystitis. There is a possibility of EGBS to be a bridge to surgery and a surgical alternative for acute calculus cholecystitis in patients for whom early cholecystectomy is contraindicated.
Availability of data and materials
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- EGBS:
-
Endoscopic transpapillary gallbladder stenting
- PTGBD:
-
Percutaneous transhepatic gallbladder drainage
- ERCP:
-
Endoscopic retrograde cholangiopancreatography
- SPS:
-
Spiral-shaped plastic stent
- TRBO:
-
Time to recurrent biliary obstruction
- CBD:
-
Common bile duct
References
Eskelinen M, Ikonen J, Lipponen P. Diagnostic approaches in acute cholecystitis: a prospective study of 1333 patients with acute abdominal pain. Theor Surg. 1993;8:15–20.
Gouma DJ, Obertop H. Acute calculous cholecystitis. What is new in diagnosis and therapy? HPB Surg 1992;69:69–78.
Johansson M, Thune A, Nelvin L, et al. Randomized clinical trial of open versus laparoscopic cholecystectomy in the treatment of acute cholecystitis. Br J Surg. 2005;92:44–9.
Mori Y, Itoi T, Baron TH, et al. Tokyo Guidelines 2018: management strategies for gallbladder drainage in patients with acute cholecystitis (with videos). J Hepatobil Pancreat Sci. 2018;25:87–95.
Teoh AYB, Kitano M, Itoi T, et al. Endosonography-guided gallbladder drainage versus percutaneous cholecystostomy in very high-risk surgical patients with acute cholecystitis: an international randomized multicentre controlled superiority trial (DRAC 1). Gut. 2020;69:1085–91.
Tamada K, Seki H, Sato K, et al. Efficacy of endoscopic retrograde cholecystoendoprosthesis (ERCCE) for cholecystitis. Endoscopy. 1991;23:2–3.
Gaglil PJ, Buniak B, Leevhy CB. Primary endoscopic retrograde cholecystoendoprosthesis: a nonsurgical modality for symptomatic cholelithiasis in cirrhotic patients. Gastrointest Endosc. 1996;44:339–42.
Hatanaka T, Itoi T, Ijima M, et al. Efficacy and safety of endoscopic gallbladder stenting for acute cholecystitis in patients with concomitant unresectable cancer. Intern Med. 2016;55:1411–7.
Toyota N, Takada T, Amano H, et al. Endoscopic naso-gallbladder drainage in the treatment of acute cholecystitis: alleviates inflammation and fixes operator’s aim during early laparoscopic cholecystectomy. J Hepatobil Pancreat Surg. 2006;13:80–5.
Jandure DM, Puli SR. Efficacy and sagety of endoscopic transpapillary gallbladder drainage in acute cholecystitis: an updated meta-analysis. World J Gastrointest Endosc. 2021;13:345–55.
Nakahara K, Michikawa Y, Morita R, et al. Endoscopic transpapillary gallbladder stenting using a newly designed plastic stent for acute cholecystitis. Endosc Int Open. 2019;7:E1105–14.
Yokoe M, Hata J, Takada T, et al. Tokyo Guidelines 2018: diagnostic criteria and severity grading of acute cholecystitis (with videos). J Hepatobil Pancreat Sci. 2018;25:41–54.
Nakahara K, Sato J, Morita R, et al. Incidence and management of cystic duct perforation during endoscopic transpapillary gallbladder drainage for acute cholecystitis. Dig Endosc. 2022;34:207–14.
Cotton PB, Lehman G, Vennes J, et al. Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointest Endosc. 1991;37:383–93.
Conway JF, Russo MW, Shrestha R. Endoscopic stent insertion into the gallbladder for symptomatic gallbladder disease in patients with end-stage liver disease. Gastrointest Endosc. 2005;61:32–6.
Schlenker C, Trotter JF, Shah RJ, et al. Endoscopic gallbladder stent placement for treatment of symptomatic cholelithiasis in patients with end-stage liver disease. Am J Gastroenterol. 2006;101:278–83.
Lee TH, Park DH, Lee SS, et al. Outcomes of endoscopic transpapillary gallbladder stenting for symptomatic gallbladder diseases: a multicenter prospective follow-up study. Endoscopy. 2011;43:702–8.
Mutignani M, Lacopini F, Perri V, et al. Endoscopic gallbladder drainage for acute cholecystitis: technical and clinical results. Endoscopy. 2009;41:539–46.
Inoue T, Okumura F, Kachi K, et al. Long-term outcomes of endoscopic gallbladder stenting in high-risk surgical patients with calculous cholecystitis (with videos). Gastrointest Endosc. 2016;83:905–13.
Maekawa S, Nomura R, Murase T, et al. Endoscopic gallbladder stenting for acute cholecystitis: a retrospective study of 46 elderly patients aged 65 years of older. BMC Gastroenterol. 2013;13:65.
Kim TH, Park DE, Chon HK. Endoscopic transpapillary gallbladder drainage for the management of acute calculus cholecystitis patients unfit for urgent cholecystectomy. PLoS ONE. 2020;15:e0240219.
Maruta A, Iwashita T, Iwata K et al. Permanent endoscopic gallbladder stenting versus removal of gallbladder drainage, long-term outcomes after management of acute cholecystitis I high-risk surgical patients for cholecystectomy: multi-center retrospective cohort study. J Hepatobiliary Pancreat Sci 2021;00:1–9.
Higa JT, Sahar N, Kozarek RA, et al. EUS-guided gallbladder drainage with a lumen-apposing metal stent versus endoscopic transpapillary gallbladder drainage for the treatment of acute cholecystitis (with videos). Gastrointest Endosc. 2019;90:483–92.
Hayes D, Lucas G, Discolo A, et al. Endoscopic transpapillary stenting for the management of acute cholecystitis. Langenbecks Arch Surg. 2020;405:191–8.
Kedia P, Sharaiha RZ, Kumta NA, et al. Endoscopic gallbladder drainage compared with percutaneous drainage. Gastrointest Endosc. 2015;82:1031–6.
Acknowledgements
We would like to thank Editage (http://www.editage.jp/) for English language editing and manuscript preparation.
Funding
This study received no funding.
Author information
Authors and Affiliations
Contributions
Conception and design of the study: J.S., K.N.; acquisiton of data: Y.M., K.S., Y.I., A.S., and Y.S.; analysis and interpretation of data J.S., K.N.; drafting the article J.S., K.N.; and revising it critically for important intellectual content: J.S., K.N., Y.M., K.S., Y.I., A.S., Y.S., S.K., T.O., and K.T.. The manuscript was approved by all authors. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of the St. Marianna University School of Medicine (Approval Number: 4382). This retrospective chart review study involving human participants was in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Data used this study was anonymized before its use.
Consent for publication
Not applicable.
Competing interests
The authors declare that the research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest. This manuscript has not been published or presented elsewhere in part or in entirety and is not under consideration by another journal.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sato, J., Nakahara, K., Michikawa, Y. et al. Long-term outcomes of endoscopic transpapillary gallbladder drainage using a novel spiral plastic stent in acute calculus cholecystitis. BMC Gastroenterol 22, 539 (2022). https://doi.org/10.1186/s12876-022-02610-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12876-022-02610-5