Abstract
Background
We analysed the survival of colorectal cancer (CRC) patients with lung metastasis and lung-only metastasis and determined the risk factors for lung metastasis in CRC patients.
Methods
Data from colorectal cancer patients with lung metastasis diagnosed from 2010 to 2015 were obtained from the SEER database. Survival was analysed using the Kaplan–Meier method and log-rank test, the Cox proportional hazards regression model, and a competing risk model. The predictive ability of the nomgram was assessed by the concordance index (C-index) and calibration curves. The data from the SEER database for the period 2016–2019 was used as an external validation set. The characteristics of 70 CRC patients treated at Shanghai East Hospital between 2016 and 2019 were retrospectively analysed and data from China was chosen as an external validation set.
Results
The median survival time for colorectal cancer patients with lung metastasis was 12 months, while this value was 24 months in patients with lung-only metastasis. Among all CRC patients with lung metastasis, age, grade, T stage, N stage, presence of liver, brain or bone metastasis, anatomic site and surgery were related to overall survival (OS). In CRC patients with lung-only metastasis, age, T stage, marital status, chemotherapy and surgery were independent prognostic factors affecting OS. Two nomograms predicting OS were established, with great discrimination (C-index between 0.67 and 0.81) and excellent calibration. Factors including age, race, sex, tumour grade, T stage, N stage, presence of liver, brain or bone metastasis, marital status, insurance status and anatomic location were related to the occurrence of lung metastasis in CRC patients.
Conclusion
We developed two reliable clinical prediction models among CRC patients to predict the OS rates in patients with lung metastasis and lung metastasis only.
Similar content being viewed by others
Introduction
Most people with colorectal caner (CRC) die from the disease, and it is the second leading cause of death in the United States [1]. There are approximately 1.8 million new cases of CRC and 900,000 related deaths each year [2]. Metastasis is a critical element in cancer-related deaths [3, 4]. Nearly half of patients with CRC will develop metastasis [5]. Approximately 21% of all CRC patients are diagnosed with stage IV disease. The areas that are most prone to metastasis are the liver and the lung [6]. The prognosis of CRC is related to the American Joint Committee on Cancer/International Union Against Cancer (AJCC/UICC) tumour-node-metastasis (TNM) staging system [7]. However, some literature highlights that, due to patient heterogeneity, the understanding of the prognosis of patients after lung metastasis treatment is beyond the scope of AJCC staging [8]. We need a more accurate prognostic system.
The lung is the second most common site of CRC metastasis, after the liver, accounting for approximately 10–15% of metastasis [9]. The median survival time for CRC patients with lung metastasis is 17.7 months (range, 5.9–31.2) [10]. We are also interested in the prediction of prognosis for those who have already developed lung metastasis. Prognosis evaluation experiments show that the 5-year overall survival (OS) rate of patients with lung metastasis is much better than that of patients with liver metastasis and brain metastasis (16.70, 15.99 and 5.51%, respectively) [11].
Some articles suggest that the site of the primary tumour also affects prognosis. That is, because of a more aggressive phenotype, right colon cancer has a worse prognosis [12,13,14]. In addition, there are some clinical differences between patients with left colon tumours and rectal cancers [15]. An article pointed out that R0 resection, preoperative carcinoembryonic antigen level, lymph node involvement rate and number of lesions are important factors affecting the prognosis of CRC patients with lung metastasis [16]. The nomogram is an intuitive display of a statistical prediction model that produces numerical probabilities of clinical events [17]. Nomograms are widely used to predict the survival of tumour patients.
Due to the lack of large-scale retrospective studies describing the clinical characteristics of CRC patients with lung metastasis, no prediction of cancer-specific survival (CSS) has been reported, and limited information is available to analyse the prognosis of CRC patients with lung-only metastasis. Therefore, we used information from the Surveillance, Epidemiology, and End Results (SEER) database to analyse the incidence, risk factors, and prognostic factors of CRC lung metastasis. This analysis was performed separately for CRC patients who developed lung metastasis and those who had only lung metastasis. Additionally, we reviewed the data of 70 CRC patients who were diagnosed with lung metastasis and were admitted to our hospital and analysed their clinical characteristics, treatment methods and efficacy.
Materials and methods
Data extraction
The SEER database is a comprehensive cancer statistics database that contains 17 population-based cancer registries that account for 28% of the US population and record information on individuals with malignant tumours in the United States. We first submitted the data consent form to the SEER administration and then collected the relevant data using SEER*Stat version 8.3.5. The information presented in this study is based on the most recent follow-up (May 30, 2020) available in the SEER database. Informed consent was not necessary in our investigation since the data gathered from the SEER database were anonymized prior to release.
Data arrangement
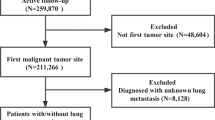
New CRC cases were collected from the SEER database between January 2010 and December 2015. Based on these data, we chose patients using the procedure depicted in Fig. 1. The eligibility criteria were as follows: (1) no history of other tumours before CRC diagnosis; (2) a positive pathologic diagnosis; and (3) follow-up time of more than 1 year. The exclusion criteria were as follows: (1) unclear lung metastatic status; (2) previous tumour diagnosis; (3) no comprehensive tumour stage information; (4) unknown race; and (5) unknown survival time or survival time not coded. We gathered information from each patient record, including race, sex, age, original tumour site, degree of tumour differentiation, tumour size, AJCC stage, T stage, N stage, and the occurrence of distant metastasis affecting bone, brain, liver, or lung at the time of first CRC diagnosis. In addition, the patients’ survival statistics were obtained. All patients were classified into two groups: those with lung metastasis and those with lung-only metastasis but no metastasis in other organs. The two groups were then randomly divided into two cohorts (training cohorts and validation cohorts) and a 7:3 population was obtained. To further evaluate the performance of the predictive model, the CRC data from the SEER database for the period 2016–2019 was used as the external validation set (Supplemental Table 1).
Patient data from our hospital
The study included 70 CRC patients with lung metastasis who underwent resection at Shanghai East Hospital between May 2016 and March 2019. The following criteria were used to determine inclusion: stage III-IV CRC; lung metastasis discovered after CRC resection in our institution; CT utilized to confirm lung metastasis; and more than 2 years of follow-up. Sex, age, site, T and N stage, survival months, organ metastasis, lymph node involvement, classification of lung metastasis, interval between operation and lung metastasis, treatment after lung metastasis, interval between lung metastasis and death or last follow-up, carcinoembryonic antigen (CEA) level (normal range, 0–5 ng/mL), and status were collected from each record. Due to the limited sample size, the cause of death for 70 patients at our hospital could not be determined. The 2016 ESMO handbook defines oligometastasis as “the presence of metastasis at up to two or three locations and five or more lesions.” This study followed the Declaration of Helsinki and the ethical principles of Shanghai East Hospital.
Statistical analysis
For the description of baseline attributes, descriptive statistics are utilized. The x2-test was performed to analyse lung metastasis incidence between subgroups for categorical variables to identify risk factors for CRC patients with lung metastasis, and variables with statistical significance were chosen for multiple logistic regression. The Kaplan–Meier technique and log-rank test were used in the training cohort to determine factors that impacted OS; competitive risk analysis was utilized to identify significant variables that influenced CSS; and a multivariate analysis was performed using a Cox regression model. The concordance index (C-index) was used to examine the internal validity of the nomogram, which was calculated by comparing the nomogram-predicted probability with the observed probability. The nomogram was then verified further by comparing the nomogram-predicted likelihood of patients in the validation cohort to their actual survival. SPSS 24.0 (IBM Corp, Armonk, NY, USA) and the ggplot and rms packages in R 3.4.3 (https://www.r-project.org/) were used for statistical analyses. All statistical tests were two-tailed, with p < 0.05 deemed statistically significant.
Results
Demographic and clinical characteristics
As shown in Table 1, 129,170 CRC patients diagnosed between 2010 and 2015 were included. Out of 6977 patients who developed lung metastasis, 1065 had lung-only metastasis. The median age of the 6977 patients was 65 years, with pathological grade II accounting for 65.5%, and the lesions were mostly located in the right-sided colon (40.9%). The median age of the 1065 patients with only lung metastasis was 66 years. The majority of the population (58.8%) had stage II CRC, and the lesions were mostly located in the rectum (40.5%).
Risk of factors for developing LM among CRC patients
As illustrated in Supplemental Table 2, age (p < 0.001); race (p < 0.001); sex (p < 0.001); grade (p < 0.001); primary tumour site (p < 0.001); T stage (p < 0.001); N stage (p < 0.001); the presence of liver (p < 0.001), brain (p < 0.001), or bone (p < 0.001) metastasis at initial diagnosis; marital status (p = 0.004) and insurance status (p < 0.001) were independent prognostic factors that affected the risk of lung metastasis.
Survival analysis
Table 2 shows that 6278 of the 6977 patients were on the training cohort list. The median survival time in the training cohort was 12 months (range, 11.4–12.6 months), with a 1-year OS of 49.2% ± 0.6% and a 3-year OS of 15.9% ± 0.5%. Another 745 of the 1065 CRC patients with isolated lung metastasis were enrolled as the training set. The median survival time in the training set was 24 months (range, 11.8–26.1 months), with 1-year and 3-year OS rates of 68.5 ± 1.4 and 36.1% ± 1.6%, respectively. Kaplan–Meier survival analysis was applied to compare the difference in OS between the two groups of CRC patients with lung metastasis and lung-only metastasis (Fig. 2).
In the colorectal cancer lung metastasis group, the Kaplan–Meier analysis results are delineated in Fig. 3, which demonstrates that age (p < 0.001); race (p = 0.027); tumour grade (p < 0.001); T stage (p < 0.001); N stage (p < 0.001); M stage (p < 0.001); bone metastasis (p < 0.001); brain metastasis (p < 0.001); liver metastasis (p < 0.001); marital status (p < 0.001); insurance status (p < 0.001); chemotherapy (p < 0.001); grade (p < 0.001); radiation therapy (p < 0.001); surgery (p < 0.001); and primary tumour site (p < 0.001) were significant prognostic factors. Moreover, competing risk models were constructed, and the CSS curves of the patients were parsed (Supplemental Fig. 1).
1 Overall Kaplan-Meier survival curves for CRC patients with lung metastasis in training cohort according to A Age, B Bone metastasis, C Liver metastasis, D Brain metastasis, E Marital status, F Chemotherapy, G Insurance status, H Grade. 2 Overall Kaplan-Meier survival curves for CRC patients with lung metastasis in training cohort according to I T stage, J N stage, K Race, L Radiotherapy, M Site, N Surgery
As shown in Table 3, several variables were independently associated with OS, as follows: age (p < 0.001); pathological grade (p < 0.001); T stage (p < 0.001); N stage (p < 0.001); bone metastasis (p < 0.001); brain metastasis (p < 0.001); liver metastasis (p < 0.001); primary tumour site (p < 0.001) and surgery (p < 0.001). Additionally, the survival outcomes among CRC patients with isolated lung metastasis were as follows. The results of the Kaplan–Meier analysis are presented in Fig. 4. In Table 4, we screened the following independent prognostic factors affecting the prognosis of CRC patients with only lung metastasis: age (p < 0.001); T stage (p < 0.001); marital status (p < 0.001); chemotherapy (p < 0.001) and surgery (p < 0.001).
1 Overall Kaplan-Meier survival curves for CRC patients with lung-only metastasis in training cohort according to A Age, B Gender, C Marital status, D Race, E Chemotherapy, F Radiotherapy, G Insurance status, H Surgery. 2 Overall Kaplan-Meier survival curves for CRC patients with lung-only metastasis in training cohort according to I Grade, J T stage, K Site, L N stage
Construction and validation of the nomogram
As shown in Fig. 5, the final nomogram was developed for all colorectal cancer lung metastasis cases, depicting the 1- and 3-year OS by weighting the score of each variable. The accuracy of the nomogram was validated internally and externally using the identification and calibration method, and the calculated C-index was 0.67 for each. Further external validation was performed by applying data from SEER data for the years 2016–2019, involving a total of 2365 cases, and the C-index was 0.71(Supplemental Fig. 2). The nomogram was created incorporating six variables for CRC patients with lung metastasis only, as shown in Fig. 6. The nomogram was validated internally and externally, and the C-index of the resulting ROC curve was 0.81 for each. Further external validation was performed involving a total of 565 cases with the C-index of 0.76 for both, as shown in Supplemental Fig. 2.
A nomogram for prediction of 1- and 3-year OS rates of CRC patients with lung metastasis (A); Calibration curve of the nomogram predicting 1- and 3-year OS rates of CRC patients with lung metastasis in training cohort (B); Calibration curve of the nomogram predicting 1- and 3-year OS rates of CRC patients with lung metastasis in the validation cohort (C)
A nomogram for prediction of 1- and 3-year OS rates of CRC patients with lung-only metastasis (A); Calibration curve of the nomogram predicting 1- and 3-year OS rates of CRC patients with lung-only metastasis in training cohort (B); Calibration curve of the nomogram predicting 1- and 3-year OS rates of CRC patients with lung-only metastasis in the validation cohort (C)
Clinical characteristics of patients with lung metastasis in our centre
Supplemental Table 3 presents detailed clinical data from 70 CRC patients with lung metastasis who underwent radical surgery at our centre. The mean CEA concentration (ng/mL) was 34 (range: 1–220). The median time between the start of therapy and the onset of lung metastasis was 14 months (range 1 to 106 months). From diagnosis to lung metastasis, the median period was 17 months. Within 2 years of surgery, metastasis developed in 47 instances. Twenty-three of the 70 individuals had only one pulmonary metastasis, whereas the remainder had numerous pulmonary metastasis. Thirteen of the 70 patients died during the follow-up period. The median time between pulmonary metastasis and death or final follow-up was comparable in patients with metastasis to multiple locations and individuals with only pulmonary metastasis (28 months vs. 29 months). In terms of treatment regimens, surgical resection was chosen for 8 patients, radiotherapy was chosen for 47 patients, chemotherapy was chosen for 54 patients. As illustrated in Fig. 7, data from this group of patients were entered into the existing nomogram that predicts OS as an external verification set to validate the 3-year OS rate and the C-index was 0.69.
Discussion
This study assessed the independent prognostic factors affecting survival in 6278 CRC patients with lung metastasis and 1065 patients with only lung metastasis from the SEER database records of CRC diagnosed from 2010 to 2015. Two nomograms were separately established, which performed well in predicting survival. The data of 70 hospitalized patients diagnosed between May 2016 and March 2019 were collected and analysed using the nomogram. Data from patients with colorectal cancer lung metastasis and lung-only metastasis diagnosed between 2016 and 2019 selected from the SEER database were used as external validation cohorts.
Previous studies have predicted the 1- and 3-year total survival in patients with lung metastasis from CRC, but the variables included in these equations are different due to a lack of univariate analysis [18]. Furthermore, there is an important problem that has not received attention, which is that patients with lung metastasis from colorectal cancer are prone to complicated metastasis from other sites, such as the liver, brain, and bone. If only colorectal cancer pulmonary metastasis is analysed without excluding the combined metastasis of other organs, it will affect the judgement of survival of people with colorectal cancer pulmonary metastasis. In our study, of the 6278 patients with lung metastasis from CRC, liver metastasis accounted for 71.9%, bone metastasis accounted for 9.7% and brain metastasis accounted for 2.7%. To further explore the factors independently affecting the prognosis of patients with pulmonary metastasis, we examined 1065 patients with only pulmonary metastasis to fill this gap. In addition, most articles have focused on analysing OS and CSS, but 6.2% of patients who died did not die of cancer. We focused on this population and performed survival analyses.
In clinical practice, any organ metastasis (such as brain or bone metastasis) corresponds with a relatively poor prognosis [19]. According to previous reports, liver metastasis is a useful independent parameter for predicting the survival of patients with stage IV CRC [20]. According to the results of our study, it was clearly observed that the occurrence of liver, brain, and bone metastasis were all independent prognostic factors. In the nomogram, any organ metastasis led to a higher total score and lower long-term OS.
Different primary sites of tumours determine the different biological behaviours of tumours. In our study, the right colon was the most common site of colon cancer (40.9%), while rectal cancer accounted for 22.6% of cases. In terms of lung metastasis, the incidence of lung metastasis and the incidence of lung-only metastasis in the right colon compared to rectal cancer was 31.1% vs. 31 and 28.5% vs. 40.5%, respectively, which indicates a higher risk of lung metastasis in rectal cancer. This result is similar to findings from other studies [21]. Previous studies have shown shorter disease-free survival in patients with rectal cancer due to direct spread to the systemic circulation through the haemorrhoidal vein [22, 23]. Regarding the prognosis of patients, the results showed that the median survival times for patients with lung metastasis and lung-only metastasis in the rectal, left-sided colon, and right-sided colon were 15 months vs. 13 months vs. 8.0 months and 31 months vs. 27 months vs. 19 months, respectively. Patients with metastatic rectal cancer showed better survival outcomes than patients with metastatic colon cancer, and the conclusion are also obtained in a previous study [24]. Primary CRC tumours located in the rectum often lead to lung metastasis, but their OS is better than that of CRC tumours located in other parts of the colon, and in turn, the prognosis is better in metastatic left-sided colon than in right-sided colon. There is increasing evidence that right colon cancer has a more aggressive phenotype and leads to a worse prognosis than other cancers [12, 13]. The clinical difference between patients with right- and left-sided colon cancers has been observed, and the patients with right-sided colon cancer were significantly older, mostly females, and had a higher incidence of complications [14]. This may explain the poor survival of patients with right-sided colon cancer.
In terms of treatment, surgery is also a key factor affecting the prognosis of cancer. The choice of primary tumour surgery remains controversial. In fact, more than two-thirds of elderly patients with stage IV CRC are known to have undergone primary tumour surgery [25]. The reason for this is that primary tumours may promote the development of metastasis and have serious complications that can significantly reduce the survival time of patients [26,27,28]. Moreover, resection of the primary tumour may contribute to the recovery of autoimmunity [27]. Some articles have confirmed that primary tumour surgery is associated with an increase in OS [29]. However, considering that complications from surgery delay the overall treatment plan [30] and that only a small proportion of people develop severe primary tumour-related complications [31, 32], it has been suggested that palliative resection does not prolong survival [33]. In our study, surgical resection was proved beneficial for survival. For CRC patients with lung metastasis, the 1- and 3-year OS rates were 61.7% vs. 41.7 and 25.7% vs. 9.7%, respectively. For those with and without primary tumour resection, the rates were 79.4% vs. 55.3 and 50.1% vs. 17.9%, respectively, among patients with lung metastasis only. In addition, evidence from population-based cancer survival analyses and clinical trial reviews suggests that chemotherapy improves survival in patients with CRC, particularly in stage IV patients [34]; similarly, in the current study, chemotherapy was shown to be beneficial to OS in both patient groups (either patients with lung metastasis from CRC or patients with lung metastasis only). The results also revealed that chemotherapy reduced the risk of cancer-specific death in patients with lung metastasis from colorectal cancer (Supplemental Fig. 1–1), and chemotherapy was also effective in reducing the risk of noncancer-specific death in this population (Table 3).
The data for 70 patients with colorectal cancer lung metastasis in our hospital were evaluated. The incidence of lung metastasis 1 year after primary resection was 40.7%, and the probability of lung metastasis after 2 years was 20.0%, which suggests that we should identify lung metastasis as soon as possible, especially for patients with high-risk factors, and regular CT reviews should be conducted. In comparison with a previous study, Facciorusso A. et al. [35]. defined the features of advanced colorectal adenomas associated with recurrence and classified the population according to their clinical characteristics in terms of the risk of recurrence to assist doctors in generating more individualized surveillance recommendations. In our study, we identified that age, race, grade, T stage, N stage, metastasis to other organs (including the liver, brain and/or bone), insurance status, and site of tumour growth were associated with the risk of lung metastasis from CRC. However, there are no risk classes to guide the clinical identification of specific high-risk patients among CRC patients, so further research is warranted in the future.
Our study has clear limitations. First, the manuscript lacks of granularity in the SEER database as it relates to the therapy sequencing and timing, patient comorbidities, and timing of clinical progression. Numerous data were missing in the SEER registry. Second, the differences in race, treatment methods and some baseline data could lead to bias when using data from 70 patients in China to verify the nomogram established from the SEER database in the US. Third, in addition to clinical baseline data, many biomarkers are associated with prognosis in CRC patients, such as RAS, BRAF, and MMR/MSI [36,37,38]. Several inflammatory biomarkers have been evaluated in patients with metastatic colorectal cancer. There is an article suggesting that the lymphocyte to monocyte ratio can be used to predict survival of colorectal liver metastasis [39]. In future research, more emphasis will be placed on conducting a prospective multicentre research project for enrolling large sample cases and complementing data related to molecular markers and inflammatory biomarkers to explore independent prognostic factors affecting Chinese patients with colorectal cancer lung metastasis.
Conclusion
In summary, we developed two nomograms to predict OS in CRC patients with lung metastasis and lung metastasis only. It is recommended to use nomograms as a useful tool to predict prognosis at different time points in CRC patients with lung metastasis and to help clinicians choose appropriate treatment options for different populations. In addition, advanced age, nonwhite race, female sex, poor differentiation, T4 stage, lymph node metastasis, liver metastasis, brain metastasis, bone metastasis, being unmarried, and rectal cancer are associated with an increased risk of lung metastasis.
Availability of data and materials
Data files from SEER database were downloaded directly from the SEER website, https://seer.cancer.gov/. The data from Shanghai East hostipal that support the fndings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- CRC:
-
Colorectal cancer
- SEER:
-
Surveillance, Epidemiology and End Results
- OS:
-
Overall survival
- CSS:
-
Cancer-specific survival
- NCSS:
-
Non-cancer-specific survival
- CSD:
-
Cancer-specific death
- AJCC/UICC:
-
American Joint Committee on Cancer/International Union Against Cancer
- TNM:
-
Tumour–node–metastasis
- C-index:
-
Concordance index
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34.
Miyoshi N, Ohue M, Yasui M, Noura S, Shingai T, Sugimura K, Akita H, Gotoh K, Marubashi S, Takahashi H, et al. Novel prognostic prediction models for patients with stage IV colorectal cancer after concurrent curative resection. ESMO Open. 2016;1(3):e000052.
Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science (New York, NY). 2011;331(6024):1559–64.
Van Cutsem E, Nordlinger B, Cervantes A. Advanced colorectal cancer: ESMO clinical practice guidelines for treatment. Ann Oncol. 2010;21(Suppl 5):v93–7.
Kopetz S, Chang GJ, Overman MJ, Eng C, Sargent DJ, Larson DW, Grothey A, Vauthey JN, Nagorney DM, McWilliams RR. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol. 2009;27(22):3677–83.
Mo S, Zhou Z, Li Y, Hu X, Ma X, Zhang L, Cai S, Peng J. Establishment and validation of a novel nomogram incorporating clinicopathological parameters into the TNM staging system to predict prognosis for stage II colorectal cancer. Cancer Cell Int. 2020;20:285.
Stewart CL, Warner S, Ito K, Raoof M, Wu GX, Kessler J, Kim JY, Fong Y. Cytoreduction for colorectal metastases: liver, lung, peritoneum, lymph nodes, bone, brain. When does it palliate, prolong survival, and potentially cure? Curr Probl Surg. 2018;55(9):330–79.
Parnaby CN, Bailey W, Balasingam A, Beckert L, Eglinton T, Fife J, Frizelle FA, Jeffery M, Watson AJ. Pulmonary staging in colorectal cancer: a review. Colorectal Dis. 2012;14(6):660–70.
Cavallaro P, Bordeianou L, Stafford C, Clark J, Berger D, Cusack J, Kunitake H, Francone T, Ricciardi R. Impact of single-organ metastasis to the liver or lung and genetic mutation status on prognosis in stage IV colorectal cancer. Clin Colorectal Cancer. 2020;19(1):e8–e17.
Zhang GQ, Taylor JP, Stem M, Almaazmi H, Efron JE, Atallah C, Safar B. Aggressive multimodal treatment and metastatic colorectal cancer survival. J Am Coll Surg. 2020;230(4):689–98.
Kennecke HF, Yin Y, Davies JM, Speers CH, Cheung WY, Lee-Ying R. Prognostic effect of sidedness in early stage versus advanced colon cancer. Health Sci Rep. 2018;1(8):e54.
Meguid RA, Slidell MB, Wolfgang CL, Chang DC, Ahuja N. Is there a difference in survival between right- versus left-sided colon cancers? Ann Surg Oncol. 2008;15(9):2388–94.
Benedix F, Kube R, Meyer F, Schmidt U, Gastinger I, Lippert H. Comparison of 17,641 patients with right- and left-sided colon cancer: differences in epidemiology, perioperative course, histology, and survival. Dis Colon Rectum. 2010;53(1):57–64.
Price TJ, Beeke C, Ullah S, Padbury R, Maddern G, Roder D, Townsend AR, Moore J, Roy A, Tomita Y, et al. Does the primary site of colorectal cancer impact outcomes for patients with metastatic disease? Cancer. 2015;121(6):830–5.
Zampino MG, Maisonneuve P, Ravenda PS, Magni E, Casiraghi M, Solli P, Petrella F, Gasparri R, Galetta D, Borri A, et al. Lung metastases from colorectal cancer: analysis of prognostic factors in a single institution study. Ann Thorac Surg. 2014;98(4):1238–45.
Iasonos A, Schrag D, Raj GV, Panageas KS. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol. 2008;26(8):1364–70.
Wang Y, Wu J, He H, Ma H, Hu L, Wen J, Lyu J. Correction to: nomogram predicting cancer-specific mortality in early-onset rectal cancer: a competing risk analysis. Int J Color Dis. 2020;35(6):1167–8.
Gaitanidis A, Alevizakos M, Tsaroucha A, Tsalikidis C, Pitiakoudis M. Predictive nomograms for synchronous distant metastasis in rectal cancer. J Gastrointest Surg. 2018;22(7):1268–76.
Kobayashi H, Kotake K, Sugihara K. Prognostic scoring system for stage IV colorectal cancer: is the AJCC sub-classification of stage IV colorectal cancer appropriate? Int J Clin Oncol. 2013;18(4):696–703.
Mitry E, Guiu B, Cosconea S, Jooste V, Faivre J, Bouvier AM. Epidemiology, management and prognosis of colorectal cancer with lung metastases: a 30-year population-based study. Gut. 2010;59(10):1383–8.
Tanriverdi O, Kaytan-Saglam E, Ulger S, Bayoglu IV, Turker I, Ozturk-Topcu T, Cokmert S, Turhal S, Oktay E, Karabulut B, et al. The clinical and pathological features of 133 colorectal cancer patients with brain metastasis: a multicenter retrospective analysis of the Gastrointestinal Tumors Working Committee of the Turkish Oncology Group (TOG). Med Oncol (Northwood, London, England). 2014;31(9):152.
Vatandoust S, Price TJ, Karapetis CS. Colorectal cancer: metastases to a single organ. World J Gastroenterol. 2015;21(41):11767–76.
Suthananthan AE, Bhandari M, Platell C. Influence of primary site on metastatic distribution and survival in stage IV colorectal cancer. ANZ J Surg. 2018;88(5):445–9.
Temple LK, Hsieh L, Wong WD, Saltz L, Schrag D. Use of surgery among elderly patients with stage IV colorectal cancer. J Clin Oncol. 2004;22(17):3475–84.
Holmgren L, O'Reilly MS, Folkman J. Dormancy of micrometastases: balanced proliferation and apoptosis in the presence of angiogenesis suppression. Nat Med. 1995;1(2):149–53.
Danna EA, Sinha P, Gilbert M, Clements VK, Pulaski BA, Ostrand-Rosenberg S. Surgical removal of primary tumor reverses tumor-induced immunosuppression despite the presence of metastatic disease. Cancer Res. 2004;64(6):2205–11.
van der Wal GE, Gouw AS, Kamps JA, Moorlag HE, Bulthuis ML, Molema G, de Jong KP. Angiogenesis in synchronous and metachronous colorectal liver metastases: the liver as a permissive soil. Ann Surg. 2012;255(1):86–94.
Ha GW, Kim JH, Lee MR. Meta-analysis of oncologic effect of primary tumor resection in patients with unresectable stage IV colorectal cancer in the era of modern systemic chemotherapy. Ann Surg Treat Res. 2018;95(2):64–72.
Lam VW, Laurence JM, Pang T, Johnston E, Hollands MJ, Pleass HC, Richardson AJ. A systematic review of a liver-first approach in patients with colorectal cancer and synchronous colorectal liver metastases. HPB. 2014;16(2):101–8.
Mudgway R, Chavez de Paz Villanueva C, Lin AC, Senthil M, Garberoglio CA, Lum SS. The impact of primary tumor surgery on survival in HER2 positive stage IV breast cancer patients in the current era of targeted therapy. Ann Surg Oncol. 2020;27(8):2711–20.
Niitsu H, Hinoi T, Shimomura M, Egi H, Hattori M, Ishizaki Y, Adachi T, Saito Y, Miguchi M, Sawada H, et al. Up-front systemic chemotherapy is a feasible option compared to primary tumor resection followed by chemotherapy for colorectal cancer with unresectable synchronous metastases. World J Surg Oncol. 2015;13:162.
Hu CY, Bailey CE, You YN, Skibber JM, Rodriguez-Bigas MA, Feig BW, Chang GJ. Time trend analysis of primary tumor resection for stage IV colorectal cancer: less surgery, improved survival. JAMA Surg. 2015;150(3):245–51.
Chen Y, Qiu Z, Kamruzzaman A, Snodgrass T, Scarfe A, Bryant HE. Survival of metastatic colorectal cancer patients treated with chemotherapy in Alberta (1995-2004). Support Care Cancer. 2010;18(2):217–24.
Facciorusso A, Di Maso M, Serviddio G, Vendemiale G, Spada C, Costamagna G, Muscatiello N. Factors associated with recurrence of advanced colorectal adenoma after endoscopic resection. Clin Gastroenterol Hepatol. 2016;14(8):1148–1154.e1144.
Osumi H, Shinozaki E, Suenaga M, Matsusaka S, Konishi T, Akiyoshi T, Fujimoto Y, Nagayama S, Fukunaga Y, Ueno M, et al. RAS mutation is a prognostic biomarker in colorectal cancer patients with metastasectomy. Int J Cancer. 2016;139(4):803–11.
Nakaji Y, Oki E, Nakanishi R, Ando K, Sugiyama M, Nakashima Y, Yamashita N, Saeki H, Oda Y, Maehara Y. Prognostic value of BRAF V600E mutation and microsatellite instability in Japanese patients with sporadic colorectal cancer. J Cancer Res Clin Oncol. 2017;143(1):151–60.
Sinicrope FA, Mahoney MR, Smyrk TC, Thibodeau SN, Warren RS, Bertagnolli MM, Nelson GD, Goldberg RM, Sargent DJ, Alberts SR. Prognostic impact of deficient DNA mismatch repair in patients with stage III colon cancer from a randomized trial of FOLFOX-based adjuvant chemotherapy. J Clin Oncol. 2013;31(29):3664–72.
Facciorusso A, Del Prete V, Crucinio N, Serviddio G, Vendemiale G, Muscatiello N. Lymphocyte-to-monocyte ratio predicts survival after radiofrequency ablation for colorectal liver metastases. World J Gastroenterol. 2016;22(16):4211–8.
Acknowledgements
We thank the staf from the Shanghai East Hospital of Tongji University School of Medicine who participated in our study.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No.81960525 and 82160591), Science and Technology Commission of Shanghai Municipality (Grant No.19DZ1930900), and Key Project of Clinical Research of Shanghai East Hospital, Tongji University (Grant No.DFLC2022012).
Author information
Authors and Affiliations
Contributions
(I) Conception and design: Zuo-Lin Xiang. (II) Administrative support: Zuo-Lin Xiang. (III) Provision of study materials or patients: All authors. (IV) Collection and assembly of data: All authors. (V) Data analysis and interpretation: Lin-Lin Liu. (VI) Manuscript writing: All authors. (VII) Final approval of manuscript: All authors.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All participants signed written informed consent. The study protocol conformed to the ethical guidelines of the Declaration of Helsinki and was approved by the Ethical Committee at Shanghai East Hospital, Tongji University School of Medicine (Shanghai, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that there are no conficts of interest regarding the publication of this article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplemental Table 1.
Clinical and demographic characteristics of the external cohort.
Additional file 2: Supplemental Table 2.
Univariate and multivariate analysis for the presence of lung metastasis in CRC patients.
Additional file 3: Supplemental Table 3.
Clinical and pathological characteristics, treatment modalities, and outcomes.
Additional file 4: Supplemental Figure 1.
1 Competing risk analyses for CRC patients with lung metastasis in training cohort according to (A) Grade, (B) Age, (C) Race, (D) Site, (E) Bone metastasis, (F) Brain metastasis, (G) Insurance status, (H) Chemotherapy. 2 Competing risk analyses for CRC patients with lung metastasis in training cohort according to (I) Liver metastasis, (J) Marital status, (K) N stage, (L) Radiotherapy, (M) Gender, (N) Surgery, (O) T stage.
Additional file 5: Supplemental Figure 2.
Calibration curve of the nomogram predicting 1- and 3-year OS rates of CRC patients with lung metastasis in the external validation cohort (A) Calibration curve of the nomogram predicting 1- and 3-year OS rates of CRC patients with lung-only metastasis in the external validation cohort (B).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liu, LL., Sun, JD. & Xiang, ZL. Survival nomograms for colorectal carcinoma patients with lung metastasis and lung-only metastasis, based on the SEER database and a single-center external validation cohort. BMC Gastroenterol 22, 446 (2022). https://doi.org/10.1186/s12876-022-02547-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12876-022-02547-9