Abstract
Background
Gastric cancer is often comorbid with hypertension and diabetes mellitus and increases the mortality risk.
Materials and methods
We conducted this prospective cohort study to investigate antidiabetics and antihypertensives’ impact on gastric cancer survival. 3012 patients with gastric carcinoma undergoing radical gastrectomy were enrolled since January 2000 and followed up until July 2020.
Results
Hypertension and diabetes patients had worse survival than patients without hypertension and diabetes [median survival time (MST): 48 versus 112.5 months, p < 0.001 for hypertension, MST: 32.7 versus 183+ months, p < 0.001 for diabetes]. Compared to untreated patients, treated patients had better survival (MST: 109.7 months versus 39.1 months, p < 0.001 for antihypertensives, MST: 120.9 months versus 22.3 months, p < 0.001 for antidiabetics). Antihypertensives and antidiabetics were related to 42% (HR 0.58, 95% CI 0.47–0.73, p < 0.001) and 70% (HR 0.30, 95% CI 0.24–0.38, p < 0.001) reduced mortality risk relative to those without medications. metformin and Calcium channel blockers can better-improved prognosis compared to others (p = 0.00029 and p = 0.015).
Conclusion
Post-surgical gastric cancer patients could benefit substantially from anti-diabetes and antihypertensive therapy. Metformin and Calcium channel blockers may be superior to other medications.
Similar content being viewed by others
Introduction
Gastric carcinoma (GC) is the fifth most prevalent cancer and the third leading cause of cancer death worldwide [1, 2]. Particularly in China, which accounts for nearly half of the global incidence and cancer-related deaths of gastric carcinoma, the prognosis of gastric cancer remains unfavorable [2, 3]. Consequently, finding rational and practical strategies to reduce mortality risk and prolong survival is strongly required.
Hypertension (HT) and diabetes mellitus (DM) are the most common comorbidities of GC [4, 5]. Moreover, the impact of HT and DM on cancer prognosis has been extensively studied [6]. Meta-analysis revealed that DM doubles the mortality risk of GC patients, and HT significantly increases overall mortality and cancer-related mortality [7, 8]. Likewise, our previous reports suggested that hyperglycemia and elevated blood pressure were significant predictive figures for mortality of GC patients [9].
Nevertheless, the impact of antihypertensive and antidiabetic medication on GC prognosis remains controversial. Cohort studies suggested that metformin was related to a lower recurrence and higher survival rate of GC [10, 11]. Parallelly, a recent study suggested that verapamil improved the overall survival of late-stage gastric patients under chemotherapy [12]. Conversely, several cohort studies found no extra benefit from antidiabetic therapy on cancer survival [13, 14].
We explored the long-term antihypertensive and antidiabetic therapy efficacy on GC patients’ prognosis through the detabase of the Fujian prospective investigation of cancer (FIESTA) study.
Materials and methods
The FIESTA study
The FIESTA study is a long-term prospective study to assess risk factors for death in patients with common gastrointestinal cancers (including esophagus, stomach, and colon) undergoing surgery [9, 15,16,17].
Inclusion and exclusion criteria
Since January 2000, patients with gastric carcinoma who underwent radical gastrectomy were recruited from the Department of Thoracic Surgery, Fujian Provincial Cancer Hospital. The most recent follow-up was in July 2020. Non-Han Chinese population and patients with previous radical gastrectomy, preoperative chemotherapy, or radiotherapy were excluded. Only participants who were followed up for more than one month and with complete data on HT or DM were analyzed.
Patient characteristics and diagnosis
Participants completed a self-designed questionnaire containing age, gender, tobacco and alcohol histories, and family history of tumors. Certified examiners measured blood pressure by mercury sphygmomanometers three times every 5 min. After fasting for 8–12 h, patients’ venous blood samples were collected before surgery to examine blood routine tests, blood glucose, blood lipids, and other biochemical indicators according to a standard procedure. During surgery, participants’ cancerous and normal gastric tissue samples were taken to evaluate the pathological characteristics of the tissue. Antihypertensive and antidiabetic medication use were according to self-report during the follow-up. Antihypertensives were classified as calcium channel blockers (CCB), angiotensin-converting enzyme inhibitors (ACEI), angiotensin receptor blocker (ARB), diuretics, beta-receptor antagonists (β-blocker), and unclear. Antidiabetics were classified as metformin, sulfonylurea, glucosidase inhibitor, insulin, and unclear.
Diagnose of HT and DM were based on physical examination and venous blood test preoperative and during the follow-up. HT was defined as systolic blood pressure ≥ 140 mmHg and/or diastolic blood pressure ≥ 90 mmHg, antihypertensive medication use, or former medication records. DM was defined as fasting plasma glucose ≥ 7.0 mmol/L, positive OGTT test (2 h glucose ≥ 11.1 mmol/L), antidiabetic medication use, or former medication records. GC was diagnosed through pathological examination.
Follow-up and outcome assessments
The annually follow-up assessment of GC patients, mainly through outpatient check-ups, telephone, or postal mails. We acquired death dates through family members or medical reports. The primary outcome was overall mortality. Survival time was calculated from the date of receiving radical resection until death or last follow-up.
Statistical analysis
Baseline characteristics were compared by chi-square test for Categorical/dichotomic variables and ANOVA or Kruskal–Wallis test for continuous variables. Kaplan–Meier curves and Log-rank tests were used to present and test the differences in cumulative survival rates. The effect of antihypertensive and antidiabetic medication on mortality risk was applied by Cox proportional hazards regression. Risk prediction assessment was expressed by hazard ratio (HR) and 95% confidence interval (95% CI). All statistical analyses were done by the STATA 16.0 (StataCorp, College Station, TX, USA) and R 4.1.1.
Results
Baseline characteristics
A total of 3012 participants have completed the follow-up ranging from 1.1 months to 183.3 months (median: 44.05 months). Baseline characteristics are summarized in Tables 1 and 2 (additional comparisons between baseline characteristics are in Additional file 1: Table S1 and Table S2). Among them, 312 and 497 patients had treated and untreated hypertension. Meanwhile, 243 and 733 patients had treated and untreated diabetes. Patients without HT and DM were significantly younger and with lower blood pressure, fasting plasma glucose (FPG), blood lipids, and body mass index (BMI) than hypertensive and diabetic patients. Plus, untreated HT patients have higher systolic blood pressure, and untreated DM patients have significantly higher systolic blood pressure and FPG than treated patients. Besides that, the untreated DM group had more stage IV and underwent chemotherapy patients than the other two groups. No other characteristic significance was found across the three groups.
Survival comparison
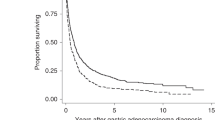
The comparison of cumulative overall survival rates across groups was provided in Fig. 1. Not unexpectedly, patients with DM and HT had remarkably poorer survival duration than non-hypertension and non-diabetic patients (MST: 48 versus 112.5 months, Log-rank test p < 0.001 for HT, MST: 32.7 versus 183+ months, p < 0.001 for DM).
Moreover, antihypertensive and antidiabetic therapies were significantly associated with improved prognosis and prolonged survival time (MST: 109.7 versus 39.1 months, p < 0.001 for antihypertensive, MST: 120.9 versus 22.3 months, p < 0.001 for antidiabetics). The combined effect of hypoglycemic and antihypertensive on gastric cancer survival is presented in Additional file 1: Fig. S1.
Mortality risk estimation
To further explore the effect size of hypoglycemic and antihypertensive therapy on GC patients’ prognosis. After the proportional-hazards assumption was satisfied, overall and stratified Cox regression was conducted to derive survival estimates (Additional file 1: Fig. S2).
As presented in Figs. 2, 3, and Additional file 1: Table S3, after adjusting for age, gender, tobacco and alcohol use history, and family history of tumor, the overall mortality risk of patients with antihypertensive and hypoglycemic therapies were lower by 42% (HR 0.58, 95% CI 0.47–0.73, p < 0.001) and 70% (HR 0.30, 95% CI 0.24–0.38, p < 0.001).
In stratified analysis by personal and family history, only patients without drinking and smoking history and age more than 50 years reduce significant risk in the antihypertensive group. By blood routine test and biochemical indicators, only patients with dyslipidemia, and blood type A/O, white blood cell counts between 4 and 10*10^9/L, and platelet under 300*10^9/L reduced significant mortality risks. All stratified groups with antidiabetic reduced significant risks despite stratification of blood routine tests and biochemical indicators, personal and family history. Noteworthily, gastric cancer patients with distant metastasis, tumor size under 4 cm, non-adenocarcinoma type, sited in the gastric antrum and whole gastric did not receive significant advantage undertaken antihypertensives. By contrast, there was significant risk reduction for antidiabetic therapy in all patients despite TNM stages, tumor sites, pathological types, and tumor size, except for patients with T1/T2 stages.
Survival comparison of medications
Figure 4 and Additional file 1: Table S4 represent the efficacy of each antidiabetic and antihypertensive medication on cumulative survival rates. After being stratified by antihypertensives, CCB users had a significantly better survival rate after surgery than those who received other medications: the HR for CCB is 0.33, the HR for other antihypertensives was 0.71 and the log-rank test among groups was 0.015. Similarly, after stratification by antidiabetic medications, gastric cancer patients who received metformin had a better survival rate than patients who received other antidiabetics: the HR for metformin is 0.16, the HR for other antidiabetics was 0.78 and the log-rank test among groups were 0.00029. Moreover, in co-existing DM and HT patients, CCB and metformin better reduced mortality risk compared to other medications (HR = 0.41and 0.18, respectively).
Discussion
In this present study, we found that co-existing DM or HT are related to significant mortality risk in post-surgical gastric cancer patients. Whereas antidiabetic and antihypertensive therapy can remarkably improve GC patient outcomes. Our finding supports the needing for enhanced screening and targeted intervention of HT and DM in all GC patients.
Numerous epidemiological evidences support that DM and HT could increase cancer mortality [6, 8, 18]. In supporting our findings, a meta-analysis including 66 studies indicated that HT was associated with a 20% overall mortality risk and a 12% cancer-specific mortality risk increased [7]; our findings suggested that antihypertensive intervention can prolong survival time and reduce overall mortality risk by 42% of GC patients. Meta-analysis demonstrated that DM could significantly increase GC risk and mortality [19]; our results further revealed that antidiabetic intervention could reduce the 70% mortality risk of GC patients. Antihypertensive and antidiabetic medication may improve GC prognosis by several means. First of all, decreased glucose suppresses tumor growth and metastasis [20, 21]. Additionally, hypertension and hyperglycemia correlated to more anti-cancer cardiovascular complications [22, 23]. Furthermore, studies have suggested that antidiabetics and antihypertensives may increase the efficacy of chemotherapy [24].
Another important finding is that metformin and CCB can better-improved prognosis compared to other medications. A recent study revealed that metformin could activate adenosine 5’-monophosphate-activated protein kinase (AMPK) by inhibiting the lysosomal proton pump vacuolar-type ATPase [25], and the AMPK pathway has been found to promote apoptosis in tumor cells [26]. Metformin can regulate the expression of programmed cell death 1 ligand 1 (PD-L1) to enhance the immune response against cancer [27]. Some scholars support that metformin can reduce the helicobacter pylori infection to inhibit gastric cancer invasion and migration [28]. Therefore, metformin users may obtain more benefits other than blood glucose reduction. Merely a few studies have focused on the antitumor effect of CCB. Though, recent studies have indicated that CCB could specifically repress voltage-gated Ca+ channels (VGCCs) and other pathways to suppress gastric cancer cell growth [29]. Besides that, observation studies demonstrated that CCB is also associated with lower risk and better prognosis of cancer [12, 30]. Nevertheless, Further investigation is awaited.
Several limitations of our study need to be considered. Foremost, this study spans more than ten years; in the meantime, profoundly surgical techniques in advance might introduce a possible bias, which may underestimate the influence of antihypertensives or antidiabetics. Secondly, patients with untreated diabetes tend to have more progressed tumors, which may overestimate the impact of antidiabetics. Finally, this study merely included post-operation patients from a single-center, which made the finding lack external authenticity among other gastric patients.
Conclusion
After all, post-surgical gastric cancer patients could benefit substantially from anti-diabetes and antihypertensive therapy. Metformin and Calcium channel blockers may be superior to other medications in gastric cancer patients. This study emphasized that supporting screening programs and pharmacological treatment for diabetes mellitus and hypertension is critical to prolonging the survivorship of gastric cancer patients.
Availability of data and materials
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Rawla P, Barsouk A. Epidemiology of gastric cancer: global trends, risk factors and prevention. Prz Gastroenterol. 2019;14(1):26–38.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–32.
Koene RJ, Prizment AE, Blaes A, Konety SH. Shared risk factors in cardiovascular disease and cancer. Circulation. 2016;133(11):1104–14.
Lau ES, Paniagua SM, Liu E, Jovani M, Li SX, Takvorian K, et al. Cardiovascular risk factors are associated with future cancer. JACC CardioOncol. 2021;3(1):48–58.
Chen Y, Wu F, Saito E, Lin Y, Song M, Luu HN, et al. Association between type 2 diabetes and risk of cancer mortality: a pooled analysis of over 771,000 individuals in the Asia Cohort Consortium. Diabetologia. 2017;60(6):1022–32.
Petrelli F, Ghidini A, Cabiddu M, Perego G, Lonati V, Ghidini M, et al. Effects of hypertension on cancer survival: a meta-analysis. Eur J Clin Invest. 2021;51(6):e13493.
Rao Kondapally Seshasai S, Kaptoge S, Thompson A, Di Angelantonio E, Gao P, Sarwar N, et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364(9):829–41.
Hu D, Peng F, Lin X, Chen G, Zhang H, Liang B, et al. Preoperative metabolic syndrome is predictive of significant gastric cancer mortality after gastrectomy: the Fujian Prospective Investigation of Cancer (FIESTA) Study. EBioMedicine. 2017;15:73–80.
Cho MH, Yoo TG, Jeong SM, Shin DW. Association of aspirin, metformin, and statin use with gastric cancer incidence and mortality: a Nationwide Cohort Study. Cancer Prev Res (Phila). 2021;14(1):95–104.
Chung WS, Le PH, Kuo CJ, Chen TH, Kuo CF, Chiou MJ, et al. Impact of metformin use on survival in patients with gastric cancer and diabetes mellitus following gastrectomy. Cancers (Basel). 2020;12(8):2013.
Fan GF, Pan JJ, Fan PS, Zhang TY, Liu YB, Huang J, et al. The clinical observation of verapamil in combination with interventional chemotherapy in advanced gastric cancer. Eur Rev Med Pharmacol Sci. 2018;22(17):5508–18.
Dulskas A, Patasius A, Linkeviciute-Ulinskiene D, Zabuliene L, Smailyte G. A cohort study of antihyperglycemic medication exposure and survival in patients with gastric cancer. Aging (Albany NY). 2019;11(17):7197–205.
Hosio M, Urpilainen E, Hautakoski A, Marttila M, Arffman M, Sund R, et al. Association of antidiabetic medication and statins with survival from ductal and lobular breast carcinoma in women with type 2 diabetes. Sci Rep. 2021;11(1):10445.
Hu D, Peng F, Lin X, Chen G, Liang B, Li C, et al. The elevated preoperative fasting blood glucose predicts a poor prognosis in patients with esophageal squamous cell carcinoma: The Fujian prospective investigation of cancer (FIESTA) study. Oncotarget. 2016;7(40):65247–56.
Peng F, Hu D, Lin X, Chen G, Liang B, Zhang H, et al. Analysis of preoperative metabolic risk factors affecting the prognosis of patients with Esophageal squamous cell carcinoma: the Fujian Prospective Investigation of Cancer (FIESTA) Study. EBioMedicine. 2017;16:115–23.
Peng F, Hu D, Lin X, Liang B, Chen Y, Zhang H, et al. Impact of long-term antihypertensive and antidiabetic medications on the prognosis of post-surgical colorectal cancer: the Fujian prospective investigation of cancer (FIESTA) study. Aging (Albany NY). 2018;10(5):1166–81.
Tseng CH. Diabetes, insulin use, and gastric cancer: a population-based analysis of the Taiwanese. J Clin Gastroenterol. 2013;47(6):e60-64.
Tian T, Zhang LQ, Ma XH, Zhou JN, Shen J. Diabetes mellitus and incidence and mortality of gastric cancer: a meta-analysis. Exp Clin Endocrinol Diabetes. 2012;120(4):217–23.
Li W, Liu H, Qian W, Cheng L, Yan B, Han L, et al. Hyperglycemia aggravates microenvironment hypoxia and promotes the metastatic ability of pancreatic cancer. Comput Struct Biotechnol J. 2018;16:479–87.
Kwon HJ, Park MI, Park SJ, Moon W, Kim SE, Kim JH, et al. Insulin resistance is associated with early gastric cancer: a prospective multicenter case control study. Gut Liver. 2019;13(2):154–60.
Gopal S, Miller KB, Jaffe IZ. Molecular mechanisms for vascular complications of targeted cancer therapies. Clin Sci (Lond). 2016;130(20):1763–79.
Kenzik KM, Balentine C, Richman J, Kilgore M, Bhatia S, Williams GR. New-onset cardiovascular morbidity in older adults with stage I to III colorectal cancer. J Clin Oncol. 2018;36(6):609–16.
Panneerpandian P, Rao DB, Ganesan K. Calcium channel blockers lercanidipine and amlodipine inhibit YY1/ERK/TGF-beta mediated transcription and sensitize the gastric cancer cells to doxorubicin. Toxicol In Vitro. 2021;74:105152.
Ma T, Tian X, Zhang B, Li M, Wang Y, Yang C, et al. Low-dose metformin targets the lysosomal AMPK pathway through PEN2. Nature. 2022;603(7899):159–65.
Luo Z, Zhu T, Luo W, Lv Y, Zhang L, Wang C, et al. Metformin induces apoptotic cytotoxicity depending on AMPK/PKA/GSK-3beta-mediated c-FLIPL degradation in non-small cell lung cancer. Cancer Manag Res. 2019;11:681–9.
Cha JH, Yang WH, Xia W, Wei Y, Chan LC, Lim SO, et al. Metformin promotes antitumor immunity via endoplasmic-reticulum-associated degradation of PD-L1. Mol Cell. 2018;71(4):606–620 e607.
Lu H, Han X, Ren J, Ren K, Li Z, Zhang Q. Metformin attenuates synergic effect of diabetes mellitus and Helicobacter pylori infection on gastric cancer cells proliferation by suppressing PTEN expression. J Cell Mol Med. 2021;25(10):4534–42.
Shiozaki A, Katsurahara K, Kudou M, Shimizu H, Kosuga T, Ito H, et al. Amlodipine and verapamil, voltage-gated Ca(2+) channel inhibitors, suppressed the growth of gastric cancer stem cells. Ann Surg Oncol. 2021;28(9):5400–11.
Li B, Cheung KS, Wong IY, Leung WK, Law S. Calcium channel blockers are associated with lower gastric cancer risk: a territory-wide study with propensity score analysis. Int J Cancer. 2021;148(9):2148–57.
Acknowledgements
Not applicable.
Funding
This study was supported by grants from National Natural Science Foundation of China (Grant No. 81970370); Foreign Cooperation Project of Science and Technology, Fujian Province (2021I0013); and Joint Funds for the innovation of science and Technology, Fujian province (Grant number:2018Y9113). The funder of this study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Author information
Authors and Affiliations
Contributions
FP, DH, XL planned and designed the study, and directed its implementation; FP, DH, JL drafted the protocol; LW, ZF, JY, SZ, XC, YL obtained statutory and ethics approvals; LW, DH, ZF, JY, SZ, YL, XC, YX contributed to data acquisition; FP, DH, LW, XL had access to all raw data; FP, DH, LW did the data preparation, quality control and data analysis; FP, LW wrote the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Fujian Cancer Hospital. All patients have given written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors have none conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
. Supplementary materials.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, L., Hu, D., Fan, Z. et al. Prognostic value of long-term antidiabetic and antihypertensive therapy in postoperative gastric cancer patients: the FIESTA study. BMC Gastroenterol 22, 429 (2022). https://doi.org/10.1186/s12876-022-02514-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12876-022-02514-4