Abstract
Background
To investigate the predictive values of cytokeratin 18 for liver fibrosis in hepatitis C virus (HCV) infected patients with type 2 diabetes mellitus (T2DM).
Methods
252 HCV-infected patients with T2DM between January 2012 and August 2017 were retrospectively reviewed. Pearson/spearman correlation analysis was used to detect the correlation in the entire cohort. Multivariate linear regression was used to identify independent predictors and logistic regression was for establishing models. Combination models that incorporated CK18 and other methods (i.e. transient elastography, aspartate transaminase-to-platelet ratio index (APRI) and fibrosis-4 index (FIB-4)] were developed in a training cohort of 132 patients. Performance of models was evaluated through discrimination ability and clinical benefits. An internal validation was conducted in 120 consecutive patients.
Results
CK18 was found significantly associated with fibrosis scores (r = 0.452, P < .001). CK18 and albumin were confirmed as independent predictors for fibrosis. For predicting significant fibrosis in the validation cohort, the observed AUC values of APRI + CK18 (AUC 0.83) and FIB-4 + CK18 (AUC 0.84) were higher than those of APRI (AUC 0.61) and FIB-4 (AUC 0.65). For predicting advanced fibrosis and cirrhosis, the AUC values of FIB-4 + CK18 (AUC 0.74 and 0.77, respectively) were significantly higher than those of FIB-4 (AUC 0.61 of both). Decision curve analysis confirmed the more clinical benefits can be provided by being combined with CK18.
Conclusions
CK18 is an independent predictor of liver fibrosis for HCV-infected patients with T2DM. Noninvasive methods incorporate CK18 and other biomarker indices can have better performance for diagnosing fibrosis and help clinical decision-making.
Similar content being viewed by others
Introduction
As estimated by world health organization (WHO) in 2015, 71 million patients were infected with hepatitis C virus (HCV) and 399,000 died because of cirrhosis or hepatocellular carcinoma cause by HCV in the worldwide [1]. About 422 million people worldwide have diabetes mellitus, which have become one of the leading causes of death [2, 3]. Both HCV and diabetes mellitus contribute to the global burden of disease.
Chronic hepatitis C (CHC) has been found associated with a four-fold increased risk of insulin resistance and type 2 diabetes mellitus (T2DM) [4]. Meanwhile, hyperglycemia and insulin which can stimulate hepatic stellate cells mitogenesis and collagen synthesis, are key factors in the progression of fibrosis [5, 6]. A previous study [7] conducted by Wieckowska et al. showed that cytokeratin 18 (CK18) is at high levels and positively correlated with liver fibrosis stage in patients with non-alcoholic fatty liver disease (NAFLD). Moreover, CK18 has been proposed for direct measures of inflammation in NAFLD patients [8, 9].
However, only a few studies investigated the correlations about CK18 in patients infected with HCV [10, 11], and limited data confirmed the strong relationship between serum CK18 and HCV-related fibrosis. Sanyal et al. [10] also found CK18 associated with insulins in CHC complicated with T2DM. Additionally, there have been many developed noninvasive serum biomarkers for predicting liver fibrosis in CHC patients without any other metabolic diseases [12], but no marker was specially developed for CHC-T2DM patients. The aim of this study was to investigate the association between the level of CK18 and liver fibrosis and evaluate the predictive value of CK18 in CHC patients with T2DM.
Materials and methods
This retrospective study was approved by the institutional review board of the Fourth People’s Hospital of Huai’an. The requirement for written informed consent was waived by the institutional review board of the Fourth People’s Hospital of Huai’an due to its retrospective nature. All methods were performed in accordance with the 1975 Helsinki declaration and its later amendments.
Patients
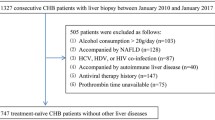
The flow chart is shown in Fig. 1. 397 patients diagnosed with CHC and T2DM, who were admitted to the Fourth People’s Hospital of Huai’an between January 2012 and August 2017 were retrospectively reviewed. The inclusion criteria were shown as follows: (a) diagnosed with CHC (positive anti-HCV antibodies for over 6 months and HCV RNA > 1.0 × 103 IU/ml); (b) clinically determined diabetes; (c) treat-naïve before hospitalization in this hospital. The exclusion criteria were: (a) focal hepatic lesion (i.e. tumor, hepatic tuberculosis and any other); (b) coinfected with other virus such as hepatitis B virus (HBV), hepatitis D virus (HDV) and human immunodeficiency virus (HIV); (c) significant alcohol intake (> 20 g/day); (d) severe hepatic failure (jaundice and ascites or transaminases level more than 10 times the upper limit of normal); (e) inadequate clinical data. Patients were allocated to the training and validation cohort according to the time of hospitalization (Training: January 2012-September 2015; Validation: October 2015-August 2017).
Clinical data included age, sex, body mass index (BMI), plate count (PLT), prothrombin time (PT), international normalized ratio (INR), fasting blood glucose (FBG), fasting insulin (FINS), alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), glutamyl transpeptidase (GGT), lactate dehydrogenase (LDH), total cholesterol (TC), triacylglycerol (TG), high density lipoprotein cholesterol (HDL-C) and low density lipoprotein cholesterol (LDL-C) at the first diagnosis of CHB and T2DM. Homeostatic model assessment for insulin resistance (HOMA-IR) score was calculated using the formula: HOMA-IR = FINS (mIU/L) × FBG (mmol/L)/22.5.
Liver biopsy
Liver biopsy (LB) was performed by experienced ultrasonologists and liver samples were formalin-fixed and paraffin-embedded for analysis. Two senior pathologists, who were blinded to the clinical information, determined final histological results in consensus. Liver fibrosis was scored according to the Metavir system [13]. F ≥ 2 was regarded as significant fibrosis, F ≥ 3 as advanced fibrosis and F4 as cirrhosis.
Transient elastography (Fibroscan)
United Fibroscan devices (FS402, Echosens, France) were used for measuring liver stiffness according to the manufacturer’s protocol [14]. The transient elastography (TE) results were expressed in kilopascal (kPa) and the final measuring result was the median value of 10 successful acquisitions.
Serum index calculation
Because of simple formulas and easily available parameters, aspartate transaminase (AST)-to-platelet ratio index (APRI) and the fibrosis-4 (FIB-4) were frequently used [15, 16]. Formulas were as follows:
Measurement of CK18 in patients
During liver biopsy, a blood sample was obtained from each patient and processed to plasma (stored at − 80 °C). CK18 was quantitatively measured using the M30-Apoptisense ELISA kit (PEVIVA; Alexis, Grünwald, Germany) from the plasma. All measurements were performed in triplicate and the absorbance was determined using a microplate reader (Molecular Devices M2, Sunnyvale, CA).
Sample size calculation
The sample size was calculated according to 10 events per variable (EPV). The calculation was done based on the assumption that the possible significant variables including ALT, AST, GGT, PLT and CK18 according to previous studies about non-invasive tests for evaluation of liver disease severity and prognosis. A total of 5 variables were considered as possibly significant. Then, the estimated minimum sample size was 100.
Statistical analysis
Continuous data were presented as means with standard deviations or medians with percentiles (25th and 75th), and the comparisons between two groups were analyzed by the Mann–Whitney U test. Count data were presented as percentages and the comparison was performed by the χ2 test. Pearson and spearman correlation analysis were used for categorical and continuous data, respectively. Forward conditional linear multivariate analysis was used to capture independent correlations for multivariate data. All combination models were established using logistic regression in the training cohort. The optimal cutoff value was determined due to the receiver operating characteristic (ROC) curve by maximizing the Youden index (sensitivity + specificity − 1). The validation cohort tested the model using the formula and optimal cutoff values derived from the primary cohort. The discrimination ability of models was quantified by the ROC curve and area under the curve (AUC) value. Delong test was used to compare AUC values. Decision curve analysis (DCA) was used to calculate the net benefit from the models at different threshold probabilities. A two-sided P value less than 0.05 was considered statistically significant.
Results
Main characteristics for CHC-T2DM patients
In total, 252 patients between January 2012 and August 2017 were finally included: 132 (between January 2012 and September 2015) were allocated for training and 120 (between October 2015 and August 2017) for internal validation. As summarized in Table 1, no differences were observed in CK18 and other clinical-pathological characteristics between the training and validation cohorts. The overall rate of significant fibrosis, advanced fibrosis and cirrhosis was 77.0% (194 of 252), 58.7% (148 of 252) and 38.5% (97 of 252).
Correlation between CK18 and Fibrosis score in the entire cohort
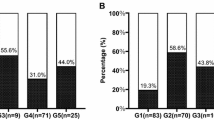
As is shown in Fig. 2, the level of CK18 was found significantly positively associated with fibrosis scores (Spearman correlation analysis, r = 0.452, P < 0.001). The CK18 of patients in F0, F1, F2, F3 and F4 fibrosis group was 76.6 ± 10.9 (ng/L), 87.1 ± 9.7 (ng/L), 103.0 ± 13.3 (ng/L), 101.8 ± 18.4 (ng/L) and 111.3 ± 32.2 (ng/L), respectively. TE had the ability of reflecting the actual progression of liver fibrosis (Pearson correlation analysis, r = 0.855, P < 0.001). The significant positive correlation between CK18 and TE (Pearson correlation analysis, r = 0.325, P < 0.001) also provided additional evidence of the relation between CK18 and histologic fibrosis score. In addition, the level CK18 is positively associated with HOMA-IR (Pearson correlation analysis, r = 0.160, P = 0.025).
Correlation analyses. a One-way ANOVA test of CK18 due to fibrosis stages; b Boxplots of TE due to fibrosis stages; c Pearson correlation analysis of CK18 and TE; d Pearson correlation analysis of CK18 and HOMA-IR. CK18, cytokeratin 18; HOMA-IR, homeostasis model assessment of insulin resistance; TE, transient elastography
Independent predictors for liver fibrosis
In initial spearman correlation analysis (Table 2), ALB, WBC, PLT, PT, INR, TE and CK18 were observed significantly related to fibrosis stage (P < 0.005 for all). Through the forward conditional linear multivariable analysis, ALB, CK18 and TE were confirmed as independent predictors for the prediction of fibrosis stage (P = 0.008, < 0.001, < 0.001, respectively). As summarized in Table 3, the cutoff values of isolated CK18 for the prediction of significant fibrosis, advanced fibrosis and cirrhosis were 90.5 (ng/L), 90.5 (ng/L) and 104 (ng/L), respectively.
Predictive values of CK18
According to the cutoff values, the input values of CK18 were set as ordered categorical data (0, ≤ 90.5 ng/L; 1, > 90.5 & ≤ 104 ng/L; 2, > 104 ng/L). The formula of combination models for predicting significant fibrosis, advanced fibrosis and cirrhosis are developed from the training cohort and summarized in Table 4.
All ROC curves from the validation cohort are provided in Fig. 3. All AUC values and comparison results are summarized in Table 5. For predicting significant fibrosis, the observed AUC values of APRI + CK18 (AUC 0.83 [95% CI 0.75, 0.92]) and FIB-4 + CK18 (AUC 0.84 [95% CI 0.77, 0.92]) were higher than those of APRI (AUC 0.61 [95% CI 0.50, 0.73]; P < 0.001) and FIB-4 (AUC 0.65 [95% CI 0.53, 0.76]; P < 0.001). For predicting advanced fibrosis and cirrhosis, the AUC values of FIB-4 + CK18 (AUC 0.74 [95% CI 0.65, 0.83], 0.77 [95% CI 0.69, 0.86], respectively) were significantly higher than those of FIB-4 (AUC 0.61 [95% CI 0.51, 0.71], P = 0.02; 0.61 [95% CI 0.51, 0.71], P = 0.03). No significant differences between TE + CK18 and TE were observed.
The decision curve analysis for the diagnostic methods are presented in Fig. 4. Compared with scenarios in which no prediction model would be used (i.e. treat-all or treat-none scheme), the TE or TE + CK18 provides a better net benefit to predict fibrosis than the other models for threshold probabilities of more than 10%. Compared with isolated serum biomarker indices (APRI or FIB-4), APRI + CK18 or FIB-4 + CK18 provides more benefits for threshold probabilities of more than 40%.
Discussion
In this study, we found the positive correlation between the level of CK18 and liver fibrosis stage (Spearman correlation analysis, r = 0.452, P < 0.001) in CHC patients with T2DM. CK18, TE and ALB were confirmed as independent predictors for liver fibrosis. In addition, combination Models that incorporate the previously proposed methods (APRI, FIB-4 and TE) and CK were established and validated for the prediction of significant fibrosis, advanced fibrosis and cirrhosis. Promisingly, the corresponding combination models of serum biomarker indices (APRI and FIB-4) showed better discrimination ability and more clinical benefits, thereby providing important information for medical decision support.
Our results demonstrated that CK18 has the predictive value for liver fibrosis in CHC patients with T2DM. For serum biomarker indices, CK18 can contribute to diagnosing the fibrosis stage. For liver stiffness measurement, CK18 cannot significantly improve the diagnostic performance due to enough favorable performance of TE [17, 18]. However, the Fibroscan device is still expensive and requires annual maintenance (€34,000 for a portable device and €5000 for its annual maintenance). In China, the machine is often accessible in the main hospitals [19]. Therefore, CK18 as an inexpensive alternative biomarker, can be combined with serum indices as inexpensive alternative methods for identifying patients with CHC and T2DM who need treatment.
CK18 is the predominant intermediate filament protein in the liver and contribute to substrates of caspases during hepatocyte apoptosis [20]. Levels of CK18 have been shown to be elevated in hepatocellular carcinoma, viral hepatitis, alcoholic hepatitis, NAFLD and cholestatic liver disease [21]. George et al. [22] reported that serum apoptotic caspase activity is associated with the severity of liver histologic lesions in both CHC and NAFLD.
In addition, HCV infection leads to a defect in insulin receptor substrate (IRS)-1 association with the IR and insulin signaling defects in hepatic IRS-1 tyrosine phosphorylation and phosphatidylinositol 3-kinase (PI3-kinase) association/activation, which contribute to insulin resistance [23]. Moreover, insulin resistance accompanied with the type 2 diabetes mellitus is positively associated with hepatic steatosis, causing an increased risk of liver fibrosis [24]. In this study, although the significant correlations between IR and CK18 (r = 0.160, P = 0.025) or liver fibrosis (r = 0.110, P = 0.026) were observed through correlation analysis, HOMA-IR is not confirmed as an independent predictor for liver fibrosis in CHC with T2DM. No significant correlation was found between CK18 and histologic liver steatosis. These might indicate that the insulin resistance might partly account for (not via liver steatosis) the correlation between CK18 and liver fibrosis. A study conducted by Jazwinski et al. [25] also demonstrated the similar conclusion that CK18 in CHC is related to advanced fibrosis but not steatosis. CK18 might be a indicator not only for liver fibrosis but also for insulin resistance in CHC with T2DM, which requires more large sample-size studies to investigate.
Several limitations should be noted in this study. First, inherent selection biases cannot be avoided due to the retrospective nature of this study. For instance, patients with unclear or unsatisfied results of noninvasive tests would accept liver biopsy. Absence of control group is HCV patients without T2DM is also a major limitation. We cannot conclude that the predictive value of CK18 is only special for CHC with T2DM. Further study should add the control group for more analyses. Finally, the combination models were established and validated on the basis of data obtained from a single center. Multi-institutional studies are required for further validations.
In conclusion, CK18 is an independent predictor of liver fibrosis for CHC patients with T2DM. It can provide added values to noninvasive methods for diagnosing fibrosis and help clinical decision-making.
Availability of data and materials
The datasets analyzed during the current study are available from the corresponding authors on reasonable request.
References
World Health Organization. Guidelines for the care and treatment of persons diagnosed with chronic hepatitis C virus infection. 2018. https://www.who.int/publications-detail/guide-care-treat-persons-diagnosed-chronic-hepatitis-c-978-92-4-155034-5.
World Health Organization. Global report diabetes. 2016. https://www.who.int/health‐topics/diabetes.
Noubiap JJ, Nansseu JR, Nyaga UF, et al. Global prevalence of diabetes in active tuberculosis: a systematic review and meta-analysis of data from 2·3 million patients with tuberculosis. Lancet Glob Health. 2019;7(4):e448–60.
Gill K, Ghazinian H, Manch R, Gish R. Hepatitis C virus as a systemic disease: reaching beyond the liver. Hepatol Int. 2016;10(3):415–23.
Svegliati-Baroni G, Ridolfi F, Di Sario A, et al. Insulin and insulin-like growth factor-1 stimulate proliferation and type I collagen accumulation by human hepatic stellate cells: differential effects on signal transduction pathways. Hepatology. 1999;29(6):1743–51.
Paradis V, Perlemuter G, Bonvoust F, et al. High glucose and hyperinsulinemia stimulate connective tissue growth factor expression: a potential mechanism involved in progression to fibrosis in nonalcoholic steatohepatitis. Hepatology. 2001;34(4 Pt 1):738–44.
Wieckowska A, Zein NN, Yerian LM, Lopez AR, McCullough AJ, Feldstein AE. In vivo assessment of liver cell apoptosis as a novel biomarker of disease severity in nonalcoholic fatty liver disease. Hepatology. 2006;44(1):27–33.
Wong VW, Adams LA, de Lédinghen V, Wong GL, Sookoian S. Noninvasive biomarkers in NAFLD and NASH - current progress and future promise. Nat Rev Gastroenterol Hepatol. 2018;15(8):461–78.
Vilar-Gomez E, Chalasani N. Non-invasive assessment of non-alcoholic fatty liver disease: clinical prediction rules and blood-based biomarkers. J Hepatol. 2018;68(2):305–15.
Sanyal A, Cusi K, Hartman ML, et al. Cytokeratin-18 and enhanced liver fibrosis scores in type 1 and type 2 diabetes and effects of two different insulins. J Investig Med. 2018;66(3):661–8.
Darweesh SK, AbdElAziz RA, Abd-ElFatah DS, et al. Serum cytokeratin-18 and its relation to liver fibrosis and steatosis diagnosed by FibroScan and controlled attenuation parameter in nonalcoholic fatty liver disease and hepatitis C virus patients. Eur J Gastroenterol Hepatol. 2019;31(5):633–41.
European Association for Study of Liver, Asociacion Latinoamericana para el Estudio del Higado. EASL-ALEH Clinical Practice Guidelines: non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol. 2015;63(1):237–64.
Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR cooperative study. Group Hepatol. 1996;24(2):289–93.
Sandrin L, Fourquet B, Hasquenoph JM, Yon S, Fournier C, Mal F, Christidis C, Ziol M, Poulet B, Kazemi F, Beaugrand M, Palau R. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29(12):1705–13.
Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38(2):518–26.
Vallet-Pichard A, Mallet V, Pol S. FIB-4: a simple, inexpensive and accurate marker of fibrosis in HCV-infected patients. Hepatology. 2006;44(3):769.
Castera L, Forns X, Alberti A. Non-invasive evaluation of liver fibrosis using transient elastography. J Hepatol. 2008;48(5):835–47.
Corpechot C, Carrat F, Poujol-Robert A, et al. Noninvasive elastography-based assessment of liver fibrosis progression and prognosis in primary biliary cirrhosis. Hepatology. 2012;56(1):198–208.
Lemoine M, Shimakawa Y, Nayagam S, et al. The gamma-glutamyl transpeptidase to platelet ratio (GPR) predicts significant liver fibrosis and cirrhosis in patients with chronic HBV infection in West Africa. Gut. 2016;65(8):1369–76.
Linder S, Havelka AM, Ueno T, Shoshan MC. Determining tumor apoptosis and necrosis in patient serum using cytokeratin 18 as a biomarker. Cancer Lett. 2004;214(1):1–9.
Yilmaz Y. Systematic review: caspase-cleaved fragments of cytokeratin 18—the promises and challenges of a biomarker for chronic liver disease. Aliment Pharmacol Ther. 2009;30(11–12):1103–9.
Papatheodoridis GV, Hadziyannis E, Tsochatzis E, et al. Serum apoptotic caspase activity in chronic hepatitis C and nonalcoholic Fatty liver disease. J Clin Gastroenterol. 2010;44(4):e87–95.
Aytug S, Reich D, Sapiro LE, Bernstein D, Begum N. Impaired IRS-1/PI3-kinase signaling in patients with HCV: a mechanism for increased prevalence of type 2 diabetes. Hepatology. 2003;38(6):1384–92.
Sanyal AJ. Role of insulin resistance and hepatic steatosis in the progression of fibrosis and response to treatment in hepatitis C. Liver Int. 2011;31(Suppl 1):23–8.
Jazwinski AB, Thompson AJ, Clark PJ, Naggie S, Tillmann HL, Patel K. Elevated serum CK18 levels in chronic hepatitis C patients are associated with advanced fibrosis but not steatosis. J Viral Hepat. 2012;19(4):278–82.
Acknowledgements
We would like to express our great appreciation to the R Development Core Team and contributors for R packages used in our study.
Funding
None.
Author information
Authors and Affiliations
Contributions
Conception and design: CW and LZ; Administrative support: LZ and QG; Provision of study materials or patients: FL and FL; Collection and assembly of data: FL, FL and CW; Data analysis and interpretation: CW and QG; Manuscript writing: all authors. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This retrospective study was approved by the institutional review board of the Fourth People’s Hospital of Huai’an. The requirement for written informed consent was waived by the institutional review board of the Fourth People’s Hospital of Huai’an due to its retrospective nature.
Consent for publication
Not applicable.
Competing interests
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, F., Lei, F., Wen, C. et al. Cytokeratin 18 can help predict liver fibrosis in HCV infected patients with type 2 diabetes mellitus. BMC Gastroenterol 21, 391 (2021). https://doi.org/10.1186/s12876-021-01963-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12876-021-01963-7