Abstract
Background
Fenofibrate is commonly used in the treatment of dyslipidemia. Fenofibrate is related to mild aminotransferase elevations and in some cases severe chronic injury such as fibrosis or cirrhosis, resulting in liver transplantation or death. The latency of disease has been reported to range between weeks to years.
Case presentation
A 63 years old male with hypertriglyceridemia developed symptoms of fatigue and anorexia 48 h after taking fenofibrate for the first time. The patient’s aminotransferase level was more than 10 times ULN. Immediately, fenofibrate was discontinued and aminotransferase level returned to normal 23 days later. To assess causality between the drug and liver damage, the standardized Roussel Uclaf Causality Assessment Method (RUCAM) was used. The patient's RUCAM score was 7, which fell in the group of “probable”. Eight months later, follow-up examination suggested the liver function was normal.
Conclusions
Weakness, fatigue and abnormal liver function during fenofibrate therapy should be closely monitored and trigger prompt withdrawal if these symptoms occur.
Similar content being viewed by others
Background
Fenofibrate is a peroxisome proliferator receptor alpha (PPAR-α) activator which is commonly used in the therapy of hypertriglyceridemia and mixed dyslipidemia. Mild, transient serum aminotransferase elevations may develop in up to 20% of patients receiving fenofibrate. Only 3–5% of patients would exhibit elevation to above 3 times of ULN. In some cases, patients may develop severe chronic injury such as fibrosis or cirrhosis, which could lead to liver transplantation or death [1]. It is widely recognized that liver damage usually occurs several weeks or months after starting of the medication [2], with some cases extending to 6 months or even years after starting of the medication [3].
Case presentation
A 63 years old male with hypertriglyceridemia [triglyceride (TG) 12.82 mmol/L], started taking fenofibrate 200 mg/day for the first time. One day before taking fenofibrate, liver function tests were performed. The laboratory result showed alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (Alk P) were all in normal range. After taking fenofibrate for 2 days, the patient developed fatigue, body aches, anorexia and dark urine, fenofibrate was discontinued immediately. There was no change to the rest of the patient’s medication. One day after withdrawal, liver function tests showed ALT 690 U/L, AST 521 U/L, the direct bilirubin (DBIL) 50.1 umol/L, the total bilirubin (TBIL) 75.8 umol/L, Alk P 202 U/L, γ-GGT 769 U/L, the International Normalized Ratio (INR) 0.93.
Patient’s body mass index (BMI) was 27.76 kg/m2 with a body surface area (BSA) of 2.21 m2. He was jaundiced with yellow sclera and skin. He presented with right upper quadrant tenderness and hepatic percussion pain was elicited. Hepatitis B surface antigen and hepatitis C antibody were negative. Anti-nuclear antibodies, anti-smooth muscle antibodies, anti-liver and kidney microsome antibodies, anti-mitochondrial antibodies, anti-myocardial antibodies, anti-parietal cell antibodies, anti-neutrophil cytoplasmic antibodies, and anti-ENA antibody were all negative. The IgG was normal (933.31 mg/dL), and the IgM was slightly decreased (36.45 mg/dL). Abdominal ultrasound showed an enlarged, fatty liver with enhanced liver parenchymal echogenicity (Fig. 1). Additionally the US showed a normal gallbladder size, normal gallbladder wall thickness, and multiple gallstones.
Six days after withdrawal bilirubin levels decreased to normal. Ten days after withdrawal, AST and ALP level decreased to normal, fatigue and anorexia was relieved. The relationship between liver function and fenofibrate is shown in the Table 1.
All evidences suggested that this patient’s liver injury were attributed to fenofibrate. Although repeated application of fenofibrate on the patient would have confirmed its pathogenicity, fenofibrate was not re-applied because of the severity of liver damage.
To assess causality between the drug and liver damage, the standardized Roussel Uclaf Causality Assessment Method (RUCAM) [4] was used. The patient's RUCAM score was 7, which fell in the group of “probable”. To categorize the pattern of liver damage the R-ratio was calculated [5]: [ALT/Upper Limit of Normal (ULN)] ÷ [Alk P/ULN]. The patient’s R-ratio was 12.8 and ALT ≥ 3 ULN, which fitted hepatocellular damage type.
Discussion and conclusion
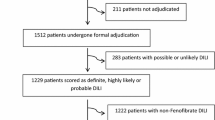
Recent literature reported that among 1229 patients with confirmed drug induced liver injury (DILI), 7 cases (0.6%) were attributed to fenofibrate. The latency was short (5–8 weeks) in 4 patients but much longer (18–56 weeks) among the rest [1]. Previously, it was reported that a 53 year old female developed severe abdominal pain 36 h after re-application of fenofibrate [6]. But the uniqueness of the very patient mentioned in this article is that he was a first-time fenofibrate user, and liver damage occurred within 2 days of taking the drug.
This patient was not examined for hepatitis A IgM antibody and hepatitis E IgM antibody as he was living in Beijing, which was not the epidemic area of the above mentioned diseases. His aminotransferase level was normal before commencing fenofibrate. Aminotransferase levels returned to normal 23 days after withdrawal, which was also not in accordance with the course of hepatitis A and E as well.
Patient’s past medical history included dyslipidemia, recurrent chylemia, hypertension, hyperuricemia, Type 2 diabetes, sleep apnea hypopnea syndrome and neurodermatitis. He took telmisartan, felodipine, arotinolol hydrochloride, allopurinol, and metformin as usual medications. These drugs rarely induce liver damage.
The mechanism of hepatotoxicity of fenofibrate is not known but appears to be immunological [7] In case the patient visits again, low-frequency HLA type HLA-A*33:01 shall be tested, which is associated with DILI [8].
The symptoms of liver injury occurring 48 h after taking fenofibrate, suggests that liver injury may have occurred promptly after the initial use. Examination of liver function should be taken before taking medicine. Any symptom of fatigue, poor appetite and yellow stained skin and sclera during fenofibrate therapy should trigger prompt withdrawal. Severe chronic injury and mortality may occur if drug discontinuation is delayed.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- PPAR-α:
-
peroxisome proliferator receptor alpha
- TG:
-
triglyceride
- ALT:
-
alanine aminotransferase
- AST:
-
aspartate aminotransferase
- AlkP:
-
alkaline phosphatase
- γ-GGT:
-
γ-glutamyltransferase
- DBIL:
-
direct bilirubin
- TBIL:
-
total bilirubin
- INR:
-
the International Normalized Ratio
- BMI:
-
body mass index
- BSA:
-
body surface area
- DILI:
-
drug induced liver injury
References
Ahmad J, et al. Identification and characterization of fenofibrate-induced liver injury. Dig Dis Sci. 2017;62(12):3596–604.
Ho CY, et al. Fenofibrate-induced acute cholestatic hepatitis. J Chin Med Assoc. 2004;67(5):245–7.
Rigal J, et al. Severe mixed hepatitis caused by fenofibrate? A review of the literature apropos of a case. Rev Med Interne. 1989;10(1):65–7.
Benichou C, Danan G, Flahault A. Causality assessment of adverse reactions to drugs—II. An original model for validation of drug causality assessment methods: case reports with positive rechallenge. J Clin Epidemiol. 1993;46(11):1331–6.
Chalasani NP, et al. ACG clinical guideline: the diagnosis and management of idiosyncratic drug-induced liver injury. Am J Gastroenterol. 2014;109(7):950–66.
Ahmed F, Rouhier ML, et al. Painful acute liver involvement related to ingestion of fenofibrate. Gastroenterol Clin Biol. 1996;12(20):1137–8.
Ahmed F, et al. Fenofibrate-induced cirrhosis. Dig Dis Sci. 2005;50(2):312–3.
Nicoletti P, et al. Association of liver injury from specific drugs, or groups of drugs, with polymorphisms in HLA and other genes in a genome-wide association study. Gastroenterology. 2017;152(5):1078–89.
Acknowledgements
Thanks to Chen Zhang, School of Clinical Medicine, University of Cambridge, for her language corrections.
Funding
No.
Author information
Authors and Affiliations
Contributions
YH made substantial contributions to the acquisition, analysis, and interpretation of data. MQ and YC made substantial contributions to the analysis and interpretation of data. All authors have approved the submitted version. All authors have agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethics Committee of Beijing Tongren Hospital, Capital Medical University, TRECKY2020-122.
Consent for publication
The patient gave written consent form for his personal and clinical details to be published in this study.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
He, Y., Qin, Mz. & Chen, Yw. Liver injury caused by fenofibrate within 48 h after first administration: a case report. BMC Gastroenterol 21, 298 (2021). https://doi.org/10.1186/s12876-021-01874-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12876-021-01874-7