Abstract
Background
Over 100 million Americans have chronic pain and most obtain their treatment in primary care clinics. However, evidence-based behavioral treatments targeting pain-related disability are not typically provided in these settings. Therefore, this study sought to: 1) evaluate implementation of a brief evidence-based treatment, Focused Acceptance and Commitment Therapy (FACT-CP), delivered by an integrated behavioral health consultant (BHC) in primary care; and 2) preliminarily explore primary (self-reported physical disability) and secondary treatment outcomes (chronic pain acceptance and engagement in valued activities).
Methods
This mixed-methods pilot randomized controlled trial included twenty-six participants with non-cancer chronic pain being treated in primary care (54% women; 46% Hispanic/Latino). Active participants completed a 30-min individual FACT-CP visit followed by 3 weekly 60-min group visits and a booster visit 2 months later. An enhanced treatment as usual (ETAU) control group received 4 handouts about pain management based in cognitive-behavioral science. Follow-up research visits occurred during and after treatment, at 12 weeks (booster visit), and at 6 months. Semi-structured interviews were conducted to collect qualitative data after the last research visit. General linear mixed regression models with repeated measures explored primary and secondary outcomes.
Results
The study design and FACT-CP intervention were feasible and acceptable. Quantitative analyses indicate at 6-month follow-up, self-reported physical disability significantly improved pre-post within the FACT-CP arm (d = 0.64); engagement in valued activities significantly improved within both the FACT-CP (d = 0.70) and ETAU arms (d = 0.51); and chronic pain acceptance was the only outcome significantly different between arms (d = 1.04), increased in the FACT-CP arm and decreased in the ETAU arm. Qualitative data analyses reflected that FACT-CP participants reported acquiring skills for learning to live with pain, consistent with increased chronic pain acceptance.
Conclusion
Findings support that FACT-CP was acceptable for patients with chronic pain and feasible for delivery in a primary care setting by a BHC. Results provide preliminary evidence for improved physical functioning after FACT-CP treatment. A larger pragmatic trial is warranted, with a design based on data gathered in this pilot.
Trial registration
clinicaltrials.gov, NCT04978961 (27/07/2021).
Similar content being viewed by others
Introduction
Most of the 100 million Americans with chronic pain receive treatment in primary care settings [1, 2]. Despite effective behavioral/nonpharmacologic interventions for chronic pain, patients continue to be offered primarily biomedical treatments, [3] rather than the full range of treatments that work [4].
Delivering behavioral treatments in primary care settings improves access to care for chronic pain. The Primary Care Behavioral Health model [5,6,7] increases availability of behavioral treatments by utilizing trained Behavioral Health Consultants (BHCs)—usually clinical psychologists or clinical social workers—who serve as members of the primary care team and deliver evidence-based nonpharmacologic treatments to patients with medical and/or psychological concerns. BHCs draw from a variety of evidence-based treatments to deliver brief, focused interventions to improve patient functioning [8,9,10].
One study to date examined BHC-delivered pain treatment using Brief Cognitive Behavioral Therapy for Chronic Pain (Brief CBT-CP) [11] and found that patients with musculoskeletal pain reported significantly less pain intensity, fewer functional limitations and improved self-efficacy [12]. While Brief CBT-CP is promising, more nonpharmacologic treatments are needed to increase options for pain treatment in primary care.
Another form of CBT is Acceptance and Commitment Therapy (“ACT”), [13] which has a strong scientific basis for improving chronic pain outcomes [14,15,16,17]. In contrast to Brief CBT-CP and traditional CBT approaches, ACT emphasizes patient values and focuses on improving overall quality of life, eschewing traditional targets of pain intensity and pain control [17, 18]. ACT uses a variety of experiential techniques to help patients improve their acceptance of chronic pain, a core component of psychological flexibility, involving willingness to engage in meaningful activities while in the presence of difficult experiences (i.e., unwanted physical and emotional aspects of chronic pain) [19].
Although substantial evidence for the general efficacy and effectiveness of ACT abounds, only one study has examined ACT for chronic pain provided in a primary care setting [20]; study findings were promising, but the duration of the group-based treatment protocol (16 sessions) is likely to make it infeasible in many settings. However, ACT in a brief format is known as “Focused” ACT (FACT), and it may be particularly suited for delivery by BHCs due to its succinct and flexible approach [21, 22]. However, no studies have examined FACT for chronic pain delivered by BHCs in a primary care setting.
Therefore, we developed a brief (5-visit) FACT for chronic pain (“FACT-CP”) -based group protocol and conducted a pilot randomized controlled trial (RCT) in a “real world” primary care setting. The objectives of this paper are to report on findings from our study and to further describe our methods and analyses. Details of the rationale, methods, and intervention are published elsewhere [23]. In preparation for a larger pragmatic trial in the future [24, 25], the aims of this pilot study were to:
-
1.
Determine feasibility, acceptability and preliminary effectiveness of a FACT-CP treatment protocol for chronic pain delivered by a BHC in primary care, assessing patients pre- and post-treatment (at booster visit/12 weeks) and at 6-month follow-up.
-
2.
Explore underlying secondary outcomes, including chronic pain acceptance and engagement in values-based activity, in FACT-CP participants.
-
3.
Gather qualitative data to understand experiences of study participants and perceived benefits of the intervention in order to inform a larger trial and future implementation efforts.
Methods
Study design
This study was a mixed-methods sequential explanatory [26] pilot RCT with 6-month follow-up. The complete protocol, including description of the study design and methods was published prospectively [23] and registered retrospectively (27/07/2021) at ClinicalTrials.gov, #NCT04978961. All procedures were approved by the Institutional Review Board at The University of Texas Health Science Center at San Antonio (HSC20160512H) in accordance with federal codes for the conduct and protection of human subjects.
Setting and participants
Our study site was a primary care clinic affiliated with The University of Texas Health Science Center at San Antonio using the Primary Care Behavioral Health model [7] of integrated care; the census in the clinic at the time was approximately 6500 unique patients. The study site had one clinical half-time BHC and one study-funded BHC (approximately 0.1 FTE) who conducted all of the clinical procedures detailed below. Patient inclusion criteria required (a) age 18 and older; (b) at least one non-cancer pain condition persisting for 12 weeks or more; (c) a current primary care clinician at the study clinic; and (d) ongoing treatment for a non-cancer chronic pain condition. Exclusion criteria were minimized for generalizability: (a) social anxiety or unwillingness to participate in a class setting; (b) presence of symptoms of psychosis and/or delirium; (c) a medical condition or life circumstance that would contraindicate or prevent participating (e.g. upcoming surgery); and (d) inability to comprehend the informed consent process or study instructions.
Recruitment
Patients were recruited via: advertisements about the study posted in the clinic; referral by clinic personnel; and direct invitation based on pre-screening eligibility identified in the electronic health record. Recruitment and treatment were rolling, such that participants were enrolled as they completed pre-screening and consent procedures. Potential participants who indicated interest were pre-screened for eligibility by study staff then scheduled for their first visit where they completed informed consent procedures and baseline assessments. Participants were compensated for attending assessment visits. We also held a raffle for $10 gift card at each FACT-CP group visit to enhance retention. FACT-CP treatment was provided at no cost. This study started enrollment in October, 2017, and 6-month follow-up assessments concluded in December, 2018; exit interviews concluded in April, 2019.
Randomization
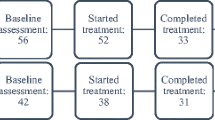
Following prescreening, consent, and baseline assessment, participants were randomized to receive either FACT-CP or Enhanced Treatment as Usual (ETAU). Because pain severity is a factor that could affect outcomes and/or responsiveness to intervention [27], we stratified to avoid misattributing effects of the intervention (i.e., type 1 error), which is especially important in small trials [28]. At baseline, participants rated their level of pain severity using the Numeric Rating Scale (NRS) for Pain, a frequently-used single-item measure consisting of a horizontal line anchored with numeric labels (0 = no pain and 10 = worst possible pain). We developed 3 levels of stratification based on clinical consensus in our team and previously-recommended cut-points [29, 30]: 1–3 (mild), 4–7 (moderate), and 8–10 (severe). Using SAS software (Cary, NC) we created randomized block sizes ranging from 4 to 12. Varying sizes were used to balance groups while blinding study staff/PI to assignment, with NRS score category entered into a custom web-based application to facilitate and mask the randomization process (see Fig. 1 for our CONSORT diagram) [31].
Data collection
Self-report measures described below were administered at 6 assessment visits: baseline, weeks 2, 3, 4, 12 (booster) and 24 (6-month follow-up; see Fig. 2). The study-appointed BHC was not involved in these visits and was blind to assessment results throughout. The study BHC did administer screening measures at each FACT-CP visit, including the NRS, in keeping with usual practice and standards of care in the clinic. Additional data were collected after the 6-month assessment visit by the PI (KEK), who contacted participants via phone and conducted semi-structured “exit interviews.”
Interventions
Focused Acceptance and Commitment Therapy treatment
Approximately 1 week after baseline quantitative assessment, participants in the FACT-CP arm attended a 30-min individual visit with the BHC consisting of typical initial visit activities [7], including role clarification of the BHC; contextual interview for functional analysis of the pain problem; biopsychosocial case conceptualization; the brief FACT-CP intervention; between-visit exercise recommendations; and collaboratively-developed goals.
Over the next 3 weeks, participants attended weekly 1-h group visits focused on increasing acceptance of chronic pain towards greater psychological flexibility in responding to and coping with chronic pain, followed by a “booster” visit approximately 2 months later [23]. The BHC communicated with participants’ primary care clinicians throughout the course of FACT-CP care (individual BHC visit through booster visit) and kept clinical notes in their electronic health records, consistent with usual practice.
Enhanced treatment as usual
Participants assigned to ETAU attended assessment visits where they completed study measures and then received a 1-page double-sided handout from the study research assistant that was based on CBT principles for coping with stress and pain (relaxation, pacing, sleep, goal-setting). Participants in both arms continued to access usual primary and specialty care treatment throughout the study.
Sample size
Recruitment was stopped at 13 per arm, in keeping with guidance on conducting pilot and feasibility studies [24, 25] (e.g., CONSORT [31]). We initially sought a larger sample size, however, at this stage, our team determined that a smaller sample was sufficient to pilot our goals examining feasibility and acceptability of the interventions, as well as study procedures, and to obtain preliminary data on the effectiveness of the FACT-CP intervention.
Primary outcome measures (quantitative data)
Feasibility
Feasibility was evaluated using a priori established benchmarks: (a) < 25% participant attrition; (b) at least 80% of participants rating the FACT-CP program as satisfactory as measured by response of at least 5 on a 7-point Likert-scale (1 = Not Satisfied at All, 7 = Very Satisfied). The satisfaction question was asked in the context of an “exit interview”: all participants who completed the 6-month follow-up assessment were contacted by the PI to provide feedback on their experiences in the study. The semi-structured phone interview lasted approximately 10 min and the quantitative data portion included 5 Likert-scale questions to assess patient experiences with study participation, ease or difficulty of learning pain management skills, amount of information learned, and satisfaction with treatment.
Feasibility measures also included fidelity checks of the study BHC. Fidelity to FACT-CP was independently assessed by the study’s external consultant (PR). All treatment visits were audiotaped. Our consultant randomly selected and listened to 22% of these visits, assessing fidelity using a standardized checklist based on the FACT-CP treatment manual. Fidelity was evidenced by greater than 95% adherence to treatment.
Acceptability
Acceptability of the FACT-CP intervention from the participants’ perspective was measured via 3 Likert-scale questions gathered during the semi-structured interview: perceived benefit, ease of learning about pain management, and whether the participant would recommend the FACT-CP treatment to a friend or family member.
Effectiveness
The primary outcome of effectiveness was self-reported physical disability, assessed using the modified and psychometrically sound Oswestry Disability Index (ODI) [32]. The ODI is a 10-item self-report measure using 6-point Likert scales, originally developed as a measure of back pain. We used an established modified version that asked about “pain” rather than “back pain” [33, 34]. Scores are summed to create a total score (maximum 50) that is then divided by the highest possible score based on items completed, then doubled to provide a percentage of disability. Reliability in our study was high (Cronbach’s alpha, α = .85).
Secondary outcome measures
Pain acceptance was examined using the Chronic Pain Acceptance Questionnaire-Revised (CPAQ) [35]. The 20-item CPAQ assesses the degree to which chronic pain and related experiences influence behaviors and the degree of effort put in to controlling pain. Items are responded to on a 0 to 6 Likert scale. Higher scores indicate greater acceptance; scores range from 0 to 120. We measured engagement in values-based activity with the Chronic Pain Values Inventory (CPVI) [36, 18], an inventory that identifies which values are important to a patient with chronic pain, and assesses the degree of success they are having in following their values. The valued domains are family, intimate relations, friends, work, health, and growth or learning. The 12-item CPVI uses 6-point Likert scale questions to measure the discrepancy between ratings of importance of valued life areas and success in engaging in those life areas; lower scores reflect greater alignment (i.e., less discrepancy) between values and actions in one’s life. The stand-alone success scale includes ratings on the engagement items only. We chose to employ the discrepancy scale rather than the success scale because we wanted to measure success in engagement in valued activities in the context of their perceived importance. CPVI scores range from 0 to 6. Reliability was high for both the CPAQ (α = .85) and the CPVI (α = .82).
Measures of participants’ experiences (qualitative data)
Qualitative data were also gathered during the exit interviews, which included open-ended questions assessing the following domains: what participants liked most and least about their participation, and any changes in pain management or quality of life due to participation (e.g., “In what ways has your participation in our study changed the way you think about or manage pain?”). Participants were given time to discuss anything else they wanted to share with the PI. The PI took near-verbatim contemporaneous notes during the interviews.
Analyses
Aim 1 analyses
We examined acceptability and feasibility of FACT-CP and study procedures by calculating percentages and frequencies. Physical disability (primary outcome) was examined using a general linear mixed (within and between groups) regression model with repeated measures, controlling for baseline pain severity, with the primary focus on comparing pre-post change in the 2 study arms. Fixed effects in these statistical models were treatment arm, time, and the treatment-by-time interaction. Although the analysis does produce conventional ANOVA-type tests, those are non-specific. Instead, the hypothesis tests were done using planned, a priori contrasts that compare the regression-based least-square means to estimate change in a group using all subjects (intent-to-treat analysis), including those with missing data. Baseline pain severity was included as a covariate because it was used to stratify randomization [37].
Aim 2 analyses
We examined secondary outcomes, acceptance of chronic pain (CPAQ) and engagement in values-based activity (CPVI) between and within groups using general linear mixed regression models with repeated measures, again with the primary focus on comparing pre-post change in the 2 study arms. Baseline pain severity was again included as a covariate.
Missing data analyses.
Across the 6 weeks of assessments, between 0 and 15.4% of data were missing from each measure. Missing data were handled using maximum likelihood estimation. This yields valid parameters given the usual assumption that data are missing at random. Additionally, Little’s MCAR test was non-significant for all variables, [ODI: X2 (15, N = 26) = 8.34, p = .909; CPAQ: X2 (11, N = 26) = 8.13, p = .702; CPVI: X2 (15, N = 26) = 14.08, p = .520]. All data were analyzed using SPSS 26.0 [38] and/or SAS v9.4 (Cary, NC).
Aim 3 qualitative analyses
We analyzed qualitative interview data using rapid qualitative analysis [39, 40]. This approach, compared with in-depth analyses, is particularly useful in studies with resource constraints (i.e., a pilot study) [41] and in research conducted in clinical settings, to aid in timely dissemination of patient feedback [39,40,41]. Rapid qualitative analyses has been compared directly with more traditional thematic analysis and found to produce closely aligned results, and is thus considered to have comparable rigor [40]. Participant responses were organized by question (domains for both groups: Best/Most Likeable Features; Worst/Most Disliked Features; Changes in Perception of Pain; Changes in Quality of Life; and Other Feedback). Analyses were structured to identify similarities and differences within and between groups. Interview notes were consolidated into a matrix, with rows for each domain and columns for individual respondents. Themes emerging within each domain and exemplar quotes were then identified by 2 co-authors who met in person to reach consensus (KEK and EPF); 3 other independent raters (BH, LSK, CM) reviewed the table of consolidated themes and quotes via email and/or in-person review and provided iterative feedback until consensus was achieved [40].
Results
Sample characteristics
Participants’ average age was 52 years, more than half were women (53%), and most identified their race as white (85.8%), followed by “other” (11.5%) and Asian (3.8%); Hispanic ethnicity was reported by 46.2% of the participants (Table 1). On average, participants had experienced chronic pain for 11.9 years, most commonly musculoskeletal pain in multiple sites (50%) or throughout the body (46%). There were no meaningful statistical differences between groups at baseline on demographic variables; physical disability level; or pain severity, duration or acceptance; but the ETAU group reported significantly greater discrepancies between ratings of importance of valued life areas and success in engaging in activities (CPVI).
Primary analyses
Feasibility
A priori benchmarks for feasibility were met for measures of retention, satisfaction, and fidelity. Retention in the study was demonstrated by absence of any treatment drop-outs during the initial FACT-CP intervention and 77% retention through the booster visit (see Fig. 1). 75% of patients reported satisfaction (rated at least 5 on the 7-point scale) with the FACT-CP intervention.
Acceptability
There were no statistically significant differences between groups in the 3 indicators of acceptability: perceived benefit, ease of learning, and recommendation of treatment (Table 2).
Effectiveness of FACT-CP intervention
Physical disability significantly improved in the FACT-CP arm from baseline to booster visit at 12 weeks (p = 0.002) with a large effect size, d = 0.89, and at 6 months (p = 0.023) with a medium effect size, d = 0.64. The ETAU group also showed significant improvement from baseline to booster at 12 weeks (p = 0.003) with a large effect size, d = 0.84, but the improvement was no longer significant at 6-month follow-up (p = 0.546), d = 0.17. Differences between groups were not statistically significant after treatment at 12 weeks (p = 0.675), d = 0.16, or at 6-month follow-up (p = 0.196), d = 0.51 (see Fig. 3 and Table 3).
Exploratory analyses
Secondary outcomes
Chronic pain acceptance
Findings indicated that chronic pain acceptance (CPAQ) increased from baseline to 12 weeks (booster) for the FACT-CP group, and decreased for the control group, but these changes were not statistically significant (p = 0.51 and p = 0.147); there was a medium effect size for this difference, d = 0.58. However, by follow-up at 6 months, the FACT-CP arm had a significant increase in chronic pain acceptance from baseline (p = 0.049) with medium effect size, d = 0.55; and the ETAU arm experienced a significant decrease in chronic pain acceptance (p = 0.082) with a medium effect size d = − 0.49. This difference between groups was significant (p = 0.009) with a large effect size, d = 1.04 (see Fig. 4 and Table 3).
Valued activities
The discrepancy between importance and success in engaging in valued activities significantly decreased in the FACT-CP arm from pre- to post-treatment at 12 weeks (p = 0.0003) with a large effect size, d = 1.05, and at 6-month follow-up (p = 0.013) with a moderate-large effect size, d = 0.76. At 12-weeks post-treatment, the ETAU group also evidenced significantly decreased discrepancy scores (p = 0.0009) with a large effect size, d = 0.94; and at 6-month follow-up (p = 0.044), with a medium effect size, d = 0.51. The difference between groups in valued activities was not significant at post-treatment (p = 0.63), d = 0.19, or follow-up (p = 0.54), d = 0.24 (see Fig. 5 and Table 3).
Qualitative analysis of participant experiences
The FACT-CP participants appreciated acquiring skills for learning to live with pain (see Table 4). They also appreciated practicing mindfulness and meditation, and receiving treatment in a small group setting. Some of the ETAU participants reported that their handouts provided helpful reminders about how to manage chronic pain. Features of the treatment that were most disliked by the FACT-CP group included the perceived small “dose” of the program; participants were interested in additional monthly classes to facilitate deeper learning and connection with others. Some ETAU participants reported that the handouts were ineffective in helping to manage pain.
When asked about changes in thinking about or managing pain, FACT-CP participants described a changed relationship with their pain and acquisition of different strategies for handling pain. Participants described a more hopeful outlook after treatment, with one participant noting “there is a next chapter.” The reference to a chapter is directly related to a metaphor and exercise used in the FACT-CP class. FACT-CP participants described positive changes in their quality of life as a result of participating in this study, including changed perspectives on their abilities, life and pain; one participant noted, “I was able to look at things clearer.”
FACT-CP participants also mentioned developing more coping skills or tools to live with pain, such as being able to observe thoughts and not get stuck in them (i.e., through cognitive defusion exercises and mindfulness practices). Interestingly, some ETAU participants also experienced a positive impact on their quality of life, reported as a result of both the intervention handouts and the regular assessments. Some felt motivated or more accountable to engage in healthy pain management strategies because they had to report on many aspects of functioning via our assessment battery, with one participant reporting she would motivate herself by asking, “Do you want to be a 7 or a 1?!” Others said their quality of life improved to some degree because of increased awareness about how to care for themselves (e.g., handouts on sleep, relaxation).
Discussion
This mixed-methods RCT pilot study examined brief BHC-delivered FACT-CP for chronic pain in an integrated primary care setting. We found that our study design, as well as mode of delivery of FACT-CP, were both feasible and acceptable. Furthermore, this pilot study provides preliminary evidence for improved self-reported physical disability. Regarding secondary outcomes, values-based activities improved for participants in both arms but remained significantly better only in the FACT-CP arm at 6-month follow-up. Furthermore, acceptance of chronic pain significantly increased in the FACT-CP arm, but decreased in the control group, resulting in a statistically significant difference between arms by 6-month follow up despite the small sample size, with a correspondingly large effect size.
FACT-CP participants reported finding the FACT-CP classes to be beneficial, easy to learn, and would recommend FACT-CP treatment to a family or friend. All but 1 FACT-CP participant gave ratings of at least 5 on a 7-point scale on items about perceived benefit and ease of learning. The FACT-CP intervention also met criteria for feasibility, based on success in retention, fidelity, and satisfaction.
Another notable finding is that 4.5 h of FACT-CP delivered by a BHC in primary care was promising in reducing self-reported disability. This finding is consistent with other studies of ACT interventions for people with chronic pain [14, 42]. Our secondary outcomes, especially chronic pain acceptance, were found to be influenced by FACT-CP treatment. Evidence abounds that acceptance is a powerful mechanism in improving functioning and emotional health in people with chronic pain [43]; future research on FACT-CP should examine this further in studies that are more adequately powered to examine that hypothesis. FACT-CP participants reported appreciating skills for learning to live with pain, consistent with increased chronic pain acceptance.
It is encouraging that a brief intervention produced a large between-group effect size in chronic pain acceptance, comparable to medium-large effect sizes identified in much longer ACT interventions [43]. Our effect size for acceptance at 6-month follow-up was also much larger than the reported effect in the 16-h ACT intervention in primary care at 3-month follow-up.
Interestingly, the Brief CBT-PC demonstration project (the only study of BHC-delivered treatment to date) reported medium effect size (d = 0.65) at the third appointment in pain intensity and functional limitations, but improvements diminished over subsequent visits [12]. In contrast, FACT-CP produced a large effect size by the fourth visit (d = 0.89) with reduced, but sustained improvements at 6 months (d = 0.69). Both of these modalities for chronic pain in Primary Care Behavioral Health settings require additional study, but it is favorable that 2 low-intensity interventions may be effective in improving functioning for patients with chronic pain.
The primary limitation of our study is the small sample size. Therefore, findings about effect of the FACT-CP intervention on acceptance need to be interpreted cautiously. While it is encouraging that an underpowered pilot study detected a large effect size, that result needs to be replicated in a larger sample. However, as Moore and colleagues [25] recommend, our study exceeded recommendations for N = 12/arm as the optimal sample size in a pilot RCT, and meets sample size recommendations to assess feasibility and acceptability of the intervention [24, 25]. Additionally, findings may not be generalizable due to the sample size, as well as specific geographic location.
Another study limitation is that the BHC interventionist was the PI (KEK), which could bias results, as she has expertise and 15+ years of experience delivering acceptance-based treatments. However, the PI is not likely to be a source of bias, as she was blind to research outcome assessments through the study. Additionally, study staff, not the PI, delivered the ETAU handouts to control participants. Due to the PI’s expertise, it is possible our findings would not generalize if less-experienced BHCs were delivering the intervention. As such, we developed the FACT-CP manual so that future BHCs could adopt the protocol without extensive training or experience; in our planned upcoming trial, we will examine clinical experience as potential factor affecting implementation, including adoption and effectiveness. Having the PI deliver the intervention was also beneficial in facilitating refinement of the treatment protocol and study design in preparation for a larger trial. Future dissemination and implementation efforts will focus on facilitating adoption of FACT-CP by a wide range of BHCs in diverse primary care clinics.
An additional limitation involves qualitative data collection, as the interviews were not recorded and transcribed. However, the interviewer (PI) took contemporaneous notes with an effort to capture statements verbatim, as in a real-time transcription. Although this means our data may not be as refined, employing such an approach is common in pragmatic implementation studies [44], and in this pilot study, the procedure reduced costs and resources required to record and professionally transcribe these interviews [41].
A final limitation to consider is that all measures in this study were self-reported, and are thus subjective. Yet, patient perception of their own disability status, as gathered in this study with the ODI, is highly correlated with objective ratings of pain behavior and physical functioning [45]. Nonetheless, incorporating objective measures of outcomes, including behavioral measures (e.g., daily diary ratings, app use, family-observed data on engagement in valued activity), physical functioning measures (e.g., 6-min walk test, sit-to-stand tests), or other objective measures (e.g., fitness tracker data, prescribed opioid use reduction, healthcare utilization related to pain exacerbations) in future studies would provide additional information regarding the impact of the FACT-CP protocol.
We sought to balance scientific rigor with demands of a “real-world” primary care setting by including both pragmatic and explanatory trial elements [46], publishing our protocol a priori [23] and setting up opportunities for a future, larger trial. Although we did not formally gather primary care clinician feedback, there were multiple avenues for study personnel, clinic leadership, clinicians, and staff to provide feedback before, during, and after the study. Still, future research should prioritize stakeholder perspectives throughout the study.
Testing on a larger scale, across multiple sites, will allow for fully powered evaluation of the effectiveness of this brief FACT-CP intervention. Future research should also study this intervention in underserved, more diverse primary care populations. Feedback from ETAU participants highlights the need to consider alternatives to traditional RCTs when conducting clinical research, such as using a waitlist-control design so that all participants can experience the active treatment. It may also be useful to study adaptation of this group intervention to individual BHC visits, which may be more feasible in some clinical settings and was requested by some participants.
Conclusions
Our mixed-methods pilot RCT demonstrates that FACT-CP delivered by a BHC in primary care is feasible and acceptable and may improve physical disability. Acceptance of chronic pain emerged as a strong treatment outcome and should be examined as a mechanism of change in adequately powered future research. A well-powered pragmatic trial is warranted, with modifications based on data gathered in this pilot study.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ACT:
-
Acceptance and Commitment Therapy
- CBT:
-
Cognitive Behavioral Therapy
- BCBT-CP:
-
Brief Cognitive Behavioral Therapy for Chronic Pain
- BHC:
-
Behavioral Health Consultant
- CPAQ:
-
Chronic Pain Acceptance Questionnaire-Revised
- CPVI:
-
Chronic Pain Values Inventory
- ETAU:
-
Enhanced Treatment as Usual
- ODI:
-
Oswestry Disability Index
- NRS:
-
Numeric Rating Scale for Pain
- RCT:
-
Randomized controlled trial
References
Fortney L, Abraham NR. Managing noncancer-related chronic pain without opioids. Prim Care Rep. 2012;18(11):137–51.
Gureje O, Von Korff M, Simon GE, Gater R. Persistent pain and well-being: a World Health Organization study in primary care. JAMA. 1998;280(2):147–51.
Edmond SN, Heapy AA, Kerns RD. Engaging mental health professionals in addressing pain. JAMA Psychiatry. 2019;76(6):565–6.
Janke EA, Cheatle M, Keefe FJ, Dhingra L, Committee SoBMHP. Society of Behavioral Medicine (SBM) position statement: improving access to psychosocial care for individuals with persistent pain: supporting the National Pain Strategy’s call for interdisciplinary pain care. Transl Behav Med. 2018;8(2):305–8.
Strosahl K. Integrating behavioral health and primary care services: the primary mental health care model. In: Blount A, editor. Integrated primary care: the future of medical and mental health collaboration. New York: W W Norton & Co; 1998. p. 139–66.
Robinson P, Reiter J. Behavioral consultation and primary care. New York: Springer; 2007.
Robinson PJ, Reiter JT. Behavioral consultation and primary care: a guide to integrating services. 2nd ed. New York: Springer; 2016.
Hunter CL, Goodie JL, Oordt MS, Dobmeyer AC. Integrated behavioral health in primary care: Step-by-step guidance for assessment and intervention. Washington, DC: American Psychological Association; 2017.
Bryan CJ, Corso ML, Corso KA, Morrow CE, Kanzler KE, Ray-Sannerud B. Severity of mental health impairment and trajectories of improvement in an integrated primary care clinic. J Consult Clin Psychol. 2012;80(3):396–403.
Hunter CL, Funderburk JS, Polaha J, Bauman D, Goodie JL, Hunter CM. Primary Care Behavioral Health (PCBH) model research: Current state of the science and a call toaction. J Clin Psychol Med Settings. 2018;25(2):127–56.
Beehler GP, Dobmeyer AC, Hunter CL, Funderburk JS. In: Agency DH, editor. Brief cognitive behavioral therapy for chronic pain: BHC manual. Silver Spring; 2018.
Beehler GP, Murphy JL, King PR, Dollar KM, Kearney LK, Haslam A, et al. Brief cognitive behavioral therapy for chronic pain: results from a clinical demonstration project in primary care behavioral health. Clin J Pain. 2019;35(10):809–17.
Hayes SC, Strosahl KD, Wilson KG. Acceptance and commitment therapy: the process and practice of mindful change. 2nd ed. New York: Guilford Press; 2011 August 29; 2016. p. 402.
Hann KE, McCracken LM. A systematic review of randomized controlled trials of acceptance and commitment therapy for adults with chronic pain: outcome domains, design quality, and efficacy. J Contextual Behav Sci. 2014;3(4):217–27.
McCracken LM, Vowles KE. Acceptance and commitment therapy and mindfulness for chronic pain: model, process, and progress. Am Psychol. 2014;69(2):178–87.
Vowles KE, McCracken LM, O'Brien JZ. Acceptance and values-based action in chronic pain: a three-year follow-up analysis of treatment effectiveness and process. Behav Res Ther. 2011;49(11):748–55.
Hayes SC, Levin ME, Plumb-Vilardaga J, Villatte JL, Pistorello J. Acceptance and commitment therapy and contextual behavioral science: examining the progress of a distinctive model of behavioral and cognitive therapy. Behav Ther. 2013;44(2):180–98.
Vowles KE, McCracken LM. Acceptance and values-based action in chronic pain: a study of treatment effectiveness and process. J Consult Clin Psychol. 2008;76(3):397–407.
McCracken LM, Morley S. The psychological flexibility model: a basis for integration and progress in psychological approaches to chronic pain management. J Pain. 2014;15(3):221–34.
McCracken LM, Sato A, Taylor GJ. A trial of a brief group-based form of acceptance and commitment therapy (ACT) for chronic pain in general practice: pilot outcome and process results. J Pain. 2013;14(11):1398–406.
Strosahl K, Robinson P, Gustavsson T. Brief interventions for radical change: principles and practice of focused acceptance and commitment therapy. Oakland: New Harbinger Publications; 2012.
Glover NG, Sylvers PD, Shearer EM, Kane M-C, Clasen PC, Epler AJ, et al. The efficacy of focused acceptance and commitment therapy in VA primary care. Psychol Serv. 2016;13(2):156.
Kanzler KE, Robinson PJ, McGeary DD, Mintz J, Potter JS, Muñante M, et al. Rationale and design of a pilot study examining acceptance and commitment therapy for persistent pain in an integrated primary care clinic. Contemp Clin Trials. 2018;66:28–35.
Thabane L, Ma J, Chu R, Cheng J, Ismaila A, Rios LP, et al. A tutorial on pilot studies: the what, why and how. BMC Med Res Methodol. 2010;10(1):1–10.
Moore CG, Carter RE, Nietert PJ, Stewart PW. Recommendations for planning pilot studies in clinical and translational research. Clin Transl Sci. 2011;4(5):332–7.
Ivankova NV, Creswell JW, Stick SL. Using mixed-methods sequential explanatory design: from theory to practice. Field Methods. 2006;18(1):3–20.
Kernan WN, Viscoli CM, Makuch RW, Brass LM, Horwitz RI. Stratified randomization for clinical trials. J Clin Epidemiol. 1999;52(1):19–26.
Katz N. Design and conduct of confirmatory chronic pain clinical trials. Pain Rep. 2021;6(1):e845.
Krebs EE, Carey TS, Weinberger M. Accuracy of the pain numeric rating scale as a screening test in primary care. J Gen Intern Med. 2007;22(10):1453–8.
McGeary DD, Mayer TG, Gatchel RJ. High pain ratings predict treatment failure in chronic occupational musculoskeletal disorders. JBJS. 2006;88(2):317–25.
Eldridge SM, Chan CL, Campbell MJ, Bond CM, Hopewell S, Thabane L, et al. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. Pilot Feasibility Stud. 2016;2(1):64.
Fairbank JC, Couper J, Davies JB, O'Brien JP. The Oswestry low back pain disability questionnaire. Physiotherapy. 1980;66(8):271–3.
Wittink H, Turk DC, Carr DB, Sukiennik A, Rogers W. Comparison of the redundancy, reliability, and responsiveness to change among SF-36, Oswestry disability index, and multidimensional pain inventory. Clin J Pain. 2004;20(3):133–42.
Kanzler KE, Pugh JA, McGeary DD, Hale WJ, Mathias CW, Kilpela LS, et al. Mitigating the effect of pain severity on activity and disability in patients with chronic pain: the crucial context of acceptance 2018.
McCracken LM, Vowles KE, Eccleston C. Acceptance of chronic pain: component analysis and a revised assessment method. Pain. 2004;107(1–2):159–66.
McCracken LM, Yang SY. The role of values in a contextual cognitive-behavioral approach to chronic pain. Pain. 2006;123(1–2):137–45.
Forsythe AB. Validity and power of tests when groups have been balanced for prognostic factors. Computational Statistics & Data Analysis. 1987;5(3):193–200.
Corp I. IBM SPSS statistics for windows, version 26.0. Armonk: IBM Corp; 2019.
Hamilton A. Qualitative methods in rapid turn-around health services research. In: Health Services Research & Development Cyberseminar; 2013.
Gale RC, Wu J, Erhardt T, Bounthavong M, Reardon CM, Damschroder LJ, et al. Comparison of rapid vs in-depth qualitative analytic methods from a process evaluation of academic detailing in the veterans health administration. Implement Sci. 2019;14(1):11.
Vindrola-Padros C, Johnson GA. Rapid techniques in qualitative research: a critical review of the literature. Qual Health Res. 2020;30(10):1596–604.
Veehof M, Trompetter H, Bohlmeijer ET, Schreurs KMG. Acceptance-and mindfulness-based interventions for the treatment of chronic pain: a meta-analytic review. Cogn Behav Ther. 2016;45(1):5–31.
Hughes LS, Clark J, Colclough JA, Dale E, McMillan D. Acceptance and commitment therapy (ACT) for chronic pain. Clin J Pain. 2017;33(6):552–68.
Finley EP, Huynh AK, Farmer MM, Bean-Mayberry B, Moin T, Oishi SM, et al. Periodic reflections: a method of guided discussions for documenting implementation phenomena. BMC Med Res Methodol. 2018;18(1):1–15.
Koho P, Aho S, Watson P, Hurri H. Assessment of chronic pain behaviour: reliability of the method and its relationship with perceived disability, physical impairment and function. J Rehabil Med. 2001;33(3):128–32.
Loudon K, Treweek S, Sullivan F, Donnan P, Thorpe KE, Zwarenstein M. The PRECIS-2 tool: designing trials that are fit for purpose. BMJ. 2015;350:h2147.
Acknowledgements
The researchers thank the patients who participated; Alex Carrizales, MA, for his outstanding assistance with data collection and study coordination; and all the primary care clinicians and staff who supported our study.
Funding
This work was supported in part by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant KL2 TR001118 (PI: Tsevat); National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under Award Number K23DK123398 (PI: Kanzler) and by National Institute on Aging of the National Institutes of Health grant number K76AG060003-01A1 (PI: Kilpela). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. No funding body was involved in design of the study and collection, analysis, nor interpretation of data or in writing the manuscript.
Author information
Authors and Affiliations
Contributions
KEK and PJR conceived of the overall study, with contributions to study design from DDM, JM, DV, JT, EPF, JSP, and JP. KEK and PJR wrote the intervention manuals. KEK, EJL, LSK and MM carried out the study (recruitment, intervention delivery). JM and KEK conducted quantitative analyses; KEK, JM and EJL contributed to tables/figures. KEK, LSK, EPF, CM and BH conducted qualitative analyses and developed corresponding table. KEK, PJR, DDM, CM and JP contributed to literature review and discussion. PJR, DDM, LSK, EPF, CM, CWM, DV, JSP, JT and JP provided critical review and revisions. All authors contributed intellectual content, edited the manuscript and approved the final version for submission.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study obtained ethics approval from the institutional review board at The University of Texas Health Science Center at San Antonio (HSC20160512H). All methods were performed in accordance with relevant guidelines and regulations. All participants gave written informed consent before taking part in this study.
Consent for publication
N/A.
Competing interests
PJR is a consultant for Mountainview Consulting Group, Inc. and receives royalties from New Harbinger Press and Springer Science + Media. No other authors have competing interests to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kanzler, K.E., Robinson, P.J., McGeary, D.D. et al. Addressing chronic pain with Focused Acceptance and Commitment Therapy in integrated primary care: findings from a mixed methods pilot randomized controlled trial. BMC Prim. Care 23, 77 (2022). https://doi.org/10.1186/s12875-022-01690-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12875-022-01690-2