Abstract
Background
Reporting quality is a critical issue in health sciences. Adopting the reporting guidelines has been approved to be an effective way of enhancing the reporting quality and transparency of clinical research. In 2012, we found that only 7 (7/1221, 0.6%) journals adopted the Consolidated Standards of Reporting Trials (CONSORT) statement in China. The aim of the study was to know the implementation status of CONSORT and other reporting guidelines about clinical studies in China.
Methods
A cross-sectional bibliometric study was conducted. Eight medical databases were systematically searched, and 1039 medical journals published in mainland China, Hong Kong, Macau, and Taiwan were included. The basic characteristics, including subject, language, publication place, journal-indexed databases, and journal impact factors were extracted. The endorsement of reporting guidelines was assessed by a modified 5-level evaluation tool, namely i) positive active, ii) positive weak, iii) passive moderate, iv) passive weak and v) none.
Results
Among included journals, 24.1% endorsed CONSORT, and 0.8% endorsed CONSORT extensions. For STROBE (STrengthening the Reporting of Observational Studies in Epidemiology), PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses), STARD (An Updated List of Essential Items for Reporting Diagnostic Accuracy Studies), CARE (CAse REport guidelines), the endorsement proportion were 17.2, 16.6, 16.4, and 14.8% respectively. The endorsement proportion for SPIRIT (Standard Protocol Items: Recommendations for Interventional Trials), TRIPOD (Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis), AGREE (Appraisal of Guidelines, Research, and Evaluation), and RIGHT (Reporting Items for Practice Guidelines in Healthcare) were below 0.7%.
Conclusions
Our results showed that the implementation of reporting guidelines was low. We suggest the following initiatives including i) enhancing the level of journal endorsement for reporting guidelines; ii) strengthening the collaboration among authors, reviewers, editors, and other stakeholders; iii) providing training courses for stakeholders; iv) establishing bases for reporting guidelines network in China; v) adopting the endorsement of reporting guidelines in the policies of the China Periodicals Association (CPA); vi) promoting Chinese medical journals into the international evaluation system and publish in English.
Similar content being viewed by others
Introduction
Quality and transparency are essential for clinical research, which can promote the results of the clinical trials as clinical evidence, thus affecting the decision-making of clinical practice. Reporting guidelines have been proven to be useful tools for enhancing the quality and transparency of clinical research [1]. Since the Consolidated Standards of Reporting Trials (CONSORT) statement was first published in 1996 [2], other reporting guidelines for clinical studies and their secondary studies, such as Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [3], STrengthening the Reporting of Observational Studies in Epidemiology (STROBE) [4], Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) [5], An Updated List of Essential Items for Reporting Diagnostic Accuracy Studies (STARD) [6], Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD) [7], CAse REport guidelines (CARE) [8], Appraisal of Guidelines, Research, and Evaluation (AGREE) [9] and Reporting Items for Practice Guidelines in Healthcare (RIGHT) [10] were developed over the last 25 years. Reporting guidelines provide researchers, peer-reviewers, editors, and other stakeholders a simple and feasible method to assess whether authors have reported on these items, which are the minimum set for the basic information of clinical research. If reporting guidelines can be adequately followed, the reporting quality of research will be effectively improved, the methodological quality of research will be easily evaluated, and the transform of the research results will be accelerated realized [11].
China makes a large contribution to clinical research. The number of Chinese clinical medicine research papers reached 44,279 in 2018 and ranked second in the world [12]. During the past 10 years, the biomedical research community is witnessing a proliferation of clinical research from China [13, 14]. Evidence-based medicine worldwide needs clinical research evidence from China [15, 16]. During the process of promoting Chinese study evidence to the world, the quality, and transparency of clinical research in China are crucial. Medical journals are acting as a gatekeeper for the dissemination of the research findings. The results of Chinese medical studies published in international peer-review medical journals only account for a small part and the vast majority of them are still published in Chinese medical journals [15].
We found that only 7 Chinese medical journals adopted the CONSORT statement in 2012 [17]. In the previous study, we only searched the China Academic Journals (CAJ) Full Database which included the main medical journals in mainland China. The aim of the study is to know the current status of reporting guidelines endorsement in Chinese medical journals.
Methods
A cross-sectional study was conducted. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) for cross-sectional checklist was followed.
Inclusion criteria
All medical journals that published clinical studies, systematic reviews/meta-analyses, and clinical practice guidelines, which were published in China including Mainland China, Hong Kong, Macau, and Taiwan were included. There was no language of publication restrictions.
Exclusion criteria
Journals that have ceased publication were excluded. Journals lacking official websites were also excluded.
Identification of Chinese medical journals
The Chinese Biomedical Literature Service System (CBM) [18], China National Knowledge Infrastructure (CNKI) [19], Wanfang Data [20], and VIP Chinese Medical Journal Databases [21] were systematically searched for the medical journals from mainland China. The Hong Kong Macau Periodicals Network [22], HKInChip [23], Macau Periodical Index [24], and Airiti Library [25] were systematically searched for the medical journals from Hong Kong and Macau. The Airiti Library was used to search for medical journals from Taiwan. All medical journals in the databases and networks listed under the heading “Journal Navigation” were examined. We determined whether the journal included clinical studies, systematic reviews/meta-analyses, and clinical practice guidelines by i) the classification of journals in the databases, ii) the texts on the introduction of journals and submission guidelines for authors, and iii) whether clinical studies, systematic reviews/meta-analyses, and clinical practice guidelines were included in last year’s issues. If the journal had a searchable table of contents, we used the keywords “case report”, “case series”, “observational study”, “cohort study”, “cross-sectional study”, “case–control study”, “controlled trial”, “clinical trial”, “clinical study”, “systematic review”, “meta-analysis”, and “clinical practice guideline” to search for the publication of clinical studies. The journals were searched and screened by two researchers (LZ and JC), confirmed by a third researcher (YD) from December 2020 to January 2021.
Extracting data
First, the basic characteristics of the eligible journals were extracted; these included the Chinese and English names of the journal, publication place, publication institution/publisher, subjects, languages, the journal impact factor (JIF), and the official website address of each journal. Subjects were classified by referring to the journal discipline navigation within the CNKI [19]. The JIF from Science Citation Index (SCI)/Science Citation Index Expanded (SCIE) through the Web of Science database [26] were extracted for the medical journals which are included in SCI/SCIE. The JIF from the journal citation reports in the Chinese Science Citation Index [27], which is currently the most complete citation database of Chinese journal articles on mainland China, was selected as complementary data.
Second, the endorsement of reporting guidelines [3,4,5,6,7,8,9,10, 28] was extracted by systematically searching the official websites of eligible journals. For example, whether the CONSORT statement and its extensions were mentioned in the instruction for authors, author guidelines, peer reviewer guidance, editorial policies, or other relevant directions for authors of a journal and its recommendation level were extracted using a standardized form. We assessed the level of reporting guideline endorsement with a modified 5-level evaluation tool, namely i) active strong, ii) active weak, iii) passive moderate, iv) passive weak, and v) none. For the evaluation tool, we added two conditions to “active weak” and “passive moderate” based on a reference [28].
A completed CONSORT [28, 29] checklist and/or a flow diagram with article submission was assessed as “active strong”. “Active weak” was assessed as the journal “encourages” or “should” reference or follow a specific guideline; priority publication if the manuscript follows a specific guideline. “Passive moderate” were assessed as adhering to “relevant” RGs; abstracts are required to follow a specific guideline. Preparing the manuscripts according to the International Committee of Medical Journal Editors (ICMJE) was assessed as “passive weak”. No mention of any reporting guidelines was assessed as “none”.
The endorsement types of other reporting guidelines, including PRISMA, STROBE, SPIRIT, STARD, TRIPOD, CARE, AGREE, and RIGHT, which are the basic reporting guidelines related to clinical studies, systematic reviews/meta-analyses, and clinical practice guidelines were also extracted according to the above evaluation criteria.
All the above information was extracted and assessed by two researchers (LZ and JC) during the period from January to August 2021. After the first extraction, 10% of records were double-checked by two researchers (YD and YM). When disagreements happened, the judgment was made by senior researchers (ZB and DM). Primary data sources (i.e., website pages) were recorded; relevant text describing guideline endorsement was extracted and coded into a standard data extraction sheet in Excel. All the original data has been submitted as an open-source data set on the Open Science Framework platform.
Statistical analyses
All data were collected and recorded in Microsoft Office Excel (Version 2016). Basic characteristics of included journals (clinical contents, language, publication place, journals indexed databases, and journal impact factor), endorsement type of reporting guidelines, were presented using descriptive statistics such as counts (n) and percentages (%). The bar and pie charts made by Excel 2016 were used to show the results of subjects, language, and journals indexed databases. A heat map was generated using Tableau (Version 2018.3.2) to present the number of journals in different publication places. Logistic regression was used to analyze the influencing factors of reporting guidelines endorsement. The factors associated with the endorsement of reporting guidelines were analyzed by logistic regression using SPSS (Version 25.0). The endorsement types of CONSORT statement including “active strong”, “active weak”, “passive moderate”, and “passive weak” were used as a positive outcome. The endorsement type of CONSORT, “none” was considered as a negative outcome. Whether the journal is included in the SCI/SCIE database, whether the journal is for traditional Chinese medicine (TCM), and whether the publication language of the journal includes English were included as the independent variables.
Results
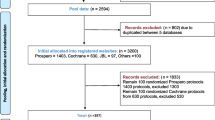
We initially identified 7806 journals. Of the remaining 3761 journals after removing the duplicates, 1473 had ceased publication; 869 did not include clinical studies, systematic reviews/meta-analyses, and clinical practice guidelines; 150 were not published in mainland China, Hong Kong, Macau, nor Taiwan; and 230 lacking official websites: all of these were excluded. A total of 1039 journals (Additional file 1: Appendix 1) met our inclusion criteria. Figure 1 presents the flow chart of the review process.
Study characteristics
As reported in Fig. 2A, for subjects, many journals focused on Medical and Health Integration (24.4%), followed by TCM (9.4%), Clinical Medicine (9.0%), and Surgery (9.0%). For publication language (Fig. 2B), 881(84.8%) journals were published in simplified Chinese, 81 (7.8%) in English, 33 (3.2%) in traditional Chinese and English, 32 (3.1%) in traditional Chinese, 10 (1.0%) in simplified Chinese, and English, 2 (0.2%) in traditional Chinese, simplified Chinese, and English. For geographic distribution (Fig. 2C), most journals (278, 26.8%) were published in Beijing, followed by Taiwan (73, 7.0%), and Shanghai (71, 6.8%). For journals index (Fig. 2D), 231(22.2%) were indexed in SCI/SCIE, 60 (5.8%) in the International Comprehensive Biomedical Information Bibliographic Database produced by the National Library of Medicine (MEDLINE), and 31 (3.0%) in Chinese Science Citation Database (CSCD). For journals included in SCI/SCIE (Fig. 2D), only three journals (0.1%), namely Bone Research, Cellular & Molecular Immunology, and Acta Pharmaceutica Sinica B had a JIF of more than 10 in 2020. The JIFs of 21 (67.7%) journals were below 5, and 7 (22.6%) journals were between 5 to 10.
Basic characteristics of 1039 Chinese medical journals. A Clinical contents of 1039 Chinese medical journals. B: Publication language of 1039 Chinese medical journals. C Geographic distribution of journals in China. *The figure was made according to the study results using the Tableau (Version 2018.3.2). D Journals indexed by SCI/SCIE, Medline, and/or CSCD. ‡ 2020 Journal Impact Factor of SCI/SCIE
Endorsement of reporting guidelines
The endorsement types were identified, and the examples of each endorsement type were shown in Table 1. The endorsement proportion of the CONSORT extensions and other reporting guidelines were shown in Table 2.
Of the 1039 journals, 24% endorsed CONSORT. Among 157 (15.1%) journals that actively endorsed CONSORT, 108 (10.4%) journals required the use of CONSORT (active strong), while 49 (4.7%) journals encouraged the use of CONSORT (active weak). The endorsement of remaining journals was assessed as passive moderate and passive weak, representing 64 (6.2%) and 29 (2.8%) journals, respectively. Only 8 (0.8%) journals required the use of the CONSORT extensions.
Of the other reporting guidelines, the endorsement proportion of STROBE, PRISMA, STARD, and CARE were 17.2, 16.6, 16.4, and 14.8% respectively. Only a few journals (14.0%-14.6%) required submitting a completed checklist along with the manuscript. The remaining reporting guidelines, such as SPIRIT, TRIPOD, AGREE, and RIGHT were only mentioned in a few included journals, below 0.7%.
Factors associated with the endorsement of reporting guidelines
Regression analysis found that i) whether the journal belongs to SCI/SCIE has an association with on the endorsement of CONSORT (OR = 3.164, 95%CI = [1.313, 7.620], P = 0.010); ii) whether the journal published in English has an association with the endorsement of CONSORT (OR = 1.987, 95%CI = [1.127, 3.503], P = 0.018); iii) there is no evidence to support whether the journal belongs to TCM has an association with the endorsement of CONSORT (OR = 0.656, 95%CI = [0.294, 1.461], P = 0.302). The details of the regression results are shown in Table 3.
Discussion
Our study provided a comprehensive overview of how many and to what extent Chinese medical journals adopt reporting guidelines. Taking CONSORT as an example, our previous study has shown that the number of Chinese medical journals which endorsed CONSORT consisted of less than 0.6% (7/1221) in 2012 [17]. In this study, we found that the endorsement proportion of CONSORT was 24.1% (250/1039). There is still much work that needs to be done to enhance the uptake of CONSORT and other reporting guidelines in Chinese medical journals.
The first reporting guideline CONSORT statement, which also found the development path of reporting guidelines [15]. According to CONSORT group statistics, there are currently 585 journals and over 50% of the core medical journals listed in the Abridged Index Medicus on PubMed that endorse CONSORT [29]. The CONSORT statement was first introduced to China in 2001 [30], followed by SPIRIT, PRISMA, and other reporting guidelines. After the introduction, many studies found that the positive function of reporting guidelines in improving the reporting quality of Chinese clinical research [31,32,33,34,35,36]. Meanwhile, Chinese medical journals began to endorse CONSORT and other reporting guidelines. However, according to our study results, there existed a big gap between Chinese medical journals and core medical journals in the world.
As for clinical contents of Chinese medical journals, “medical and health integration”, which is like comprehensive medical journals accounted for the largest type, followed by TCM journals. The big proportion of TCM journals reflect the feature for the medical subject in China. Therefore, the quality and transparency of TCM research can represent an important part of the level of Chinese clinical research. Since 2001, the reporting guidelines system of TCM has gradually been established [37]. The existing TCM reporting guidelines have included major study designs and main TCM interventions [38]. Although the results of this study do not show that journal endorsement in TCM is better than in other fields, given the efforts of the Chinese scholars in TCM reporting guidelines and the finding that other studies have shown that TCM reporting guidelines do improve the quality of reporting of TCM research [39, 40], it is foreseeable that both the endorsement of reporting guidelines and the quality of TCM research in TCM will improve if the implementation of reporting guidelines continue to be promoted in the future.
Based on the results of regression analysis, whether the inclusion of SCI/SCIE and whether the publication language includes English is associated with the reporting guideline endorsement. On 23, June 2021, an opinion document jointly released by the Central Propaganda Department of the Communist Party of China, Ministry of Education and Ministry of Science and Technology of China proposed to strengthen the bilingual construction of Chinese journals in English and Chinese and improve the academic evaluation system of journals [41]. Combined with our findings, promoting the journals to be published in English and indexed by an international evaluation system like SCI can contribute to the endorsement of reporting guidelines in Chinese medical journals. We believe that those journals only published in Chinese should also endorse reporting guidelines to meet the same standards.
During the past ten years, the efforts of Chinese scholars in promoting the reporting guidelines, especially in the introduction and translation of reporting guidelines, the establishment of the system for TCM reporting guidelines, and the leading role of the ministries of China in promoting the internationalization of journals should be admitted. However, there is a long way to go to be optimal. This current gap is likely to make it difficult to accurately assess the quality of clinical research in China and track the raw data. It will also damage the credibility of Chinese clinical research in the international community. Knowing but not doing it will lead to research waste [41,42,43]. Some studies suggest that one of the barriers to the implementation of reporting guidelines in Chinese medical journals is the low level of awareness of reporting guidelines among stakeholders such as journal editors [44, 45]. To enhance the use of reporting guidelines in China, we proposed the following initiatives.
First, as the final guarantee to medical research publication, journals should take action to safeguard the reporting quality of medical research, for example, adopting the reporting guidelines as “active strong” [46]. Second, the authors, reviewers, editors, and other stakeholders must work together to ensure that research is reported in line with the relevant reporting guidelines. Third, the 1039 Chinese journal editors should be surveyed to find out their needs regarding implementing reporting guidelines and other issues, including implementing open science practices. Based on their need, the corresponding training courses could be provided. Forth, establishing bases of international reporting guidelines network in China. In January 2021, the Chinese EQUATOR centre launched [47]. The Chinese EQUATOR centre will implement the EQUATOR Network's vision and mission, thus promoting the reporting guidelines in China. Fifth, from the national level, the journals included by the CPA should require the use of reporting guidelines as the Chinese Medical Association (CMA). Our previous study indicated that 69 journals of the CMA used a unified submission system, all of which recommended the use of reporting guidelines [48]. Sixth, we recommend that promoting the Chinese medical journals into the world journal evaluation system, publishing in English, and endorsing the reporting guidelines could be carried out simultaneously.
Our study has limitations. First, we only searched online databases without manual search, thus may omit some medical journals published in print only. Second, there is a certain degree of information delay as the collection of journal information is a one-off and the content of journal websites are updated in real-time. Third, due to the large amount of Chinese medical journals, the researchers did not extract and assess the data independently, although we introduced other researchers to double-check the results, which may cause potential bias in the conclusion. In order to assure the accuracy and transparency of the study results, we provided links to the extracted sources for each record and permanently stored them on the OSF platform for readers to access.
Conclusions
In conclusion, the endorsement of reporting guidelines in Chinese medical journals remains far from optimal. If the Chinese scientific community wants to improve and safeguard the quality and transparency of medical research, effective implementation strategies must be taken to promote the use of reporting guidelines in China [49, 50, 51].
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request. The lead authors (the manuscript’s guarantors) affirm that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as originally planned (and, if relevant, registered) have been explained.
References
Trish G. Enhancing the quality and transparency of health research. BMJ. 2008;337(7661):66. https://doi.org/10.1136/bmj.a718.
Begg C, Cho M, Eastwood S, et al. Improving the quality of reporting of randomized controlled trials: the CONSORT statement. JAMA. 1996;276(8):637–9. https://doi.org/10.1001/jama.276.8.637.
Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009;6(7):e1000097. https://doi.org/10.1371/journal.pmed.1000097.
Von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147(8):573–7. https://doi.org/10.7326/0003-4819-147-8-200710160-00010.
Gagnier JJ, Kienle G, Altman DG, Moher D, Sox H, Riley D, the CARE Group. The CARE guidelines: consensus-based clinical case reporting guideline development. BMJ Case Rep. 2013;2013:bcr2013201554. https://doi.org/10.1136/bcr-2013-201554.
Chan A-W, Tetzlaff JM, Altman DG, Laupacis A, Gøtzsche PC, Krleža-Jerić K, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. 2013;158(3):200–7. https://doi.org/10.7326/0003-4819-158-3-201302050-00583.
Patrick B, Johannes R, David B, Constantine G, Paul G, Les L, et al. STARD 2015: an updated list of essential items for reporting diagnositic accuracy studies. BMJ. 2015;351: h5527. https://doi.org/10.1136/bmj.h5527.
Gary C, Johannes R, Douglas A, Karel M. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): the TRIPOD statement. Ann Intern Med. 2015;162(1):55–63. https://doi.org/10.7326/M14-0697.
Melissa Brouwers, Kate Kerkvliet, Karen Spithoff, AGREE Next Steps Consortium. The AGREE Reporting Checklist: a tool to improve reporting of clincal practice guidelines. BMJ. 2016;352:i1152. https://doi.org/10.1136/bmj.i1152.
Chen Y, Yang K, Marušić A, Qaseem A, Meerpohl J, Flottorp S, et al. A reporting tool for practice guidelines in health care: the RIGHT statement. Ann Intern Med. 2017;166(2):128–32. https://doi.org/10.7326/M16-1565.
Wang X, Chen Y, Yang N, Deng W, Wang Q, Li N, et al. Methodology and reporting quality of reporting guidelines: systematic review. BMC Med Res Methodol. 2015;15:74. https://doi.org/10.1186/s12874-015-0069-z.
Yuan Tian-wei, Li Ping-ping, Li Su-ning, Yin Jun-xiang, Fan Yue-lei, Xiong Yan, et al. Current status and future perspective of clinical medical research in China. Chinese Journal of Clinical Medicine. 2019;26(5):673–678. https://doi.org/10.12025/j.isssn.1008-6358.2019.20191792.
Viergever RF, Li K. Trends in global clinical trial registration: an analysis of numbers of registered clinical trials in different parts of the world from 2004 to 2013. BMJ Open. 2015;5(9): e008932. https://doi.org/10.1136/bmjopen-2015-008932.
National Science Board. Science and engineering indicators 2018. NSB-2018–1. National Science Foundation. Published 2018. https://www.nsf.gov/statistics/indicators/. Accessed at 29 Nov 2021.
Bian Z, Li Y, Moher D. Further improve the reporting quality of clinical research in China (in Chinese). Chin J Evid Based Med. 2020;20(12):1365–6. https://doi.org/10.7507/1672-2531.202009123 in Chinese.
Jia YX, Huang DD, Wen JJ, Qureshi R, Wang YH, et al. Assessment of duplicate publication of chinese-sponsored randomized clinical trials. JAMA Netw Open. 2020;3(12): e2027104. https://doi.org/10.1001/jamanetworkopen.2020.27104.
Song TJ, Leng HF, Zhong LLD, Wu TX, Bian ZX. CONSORT in China: past development and future direction. Trials. 2015;16:243. https://doi.org/10.1186/s13063-015-0769-z.
http://www.sinomed.ac.cn/zh/journalSearch.jsp?type=qk. Accessed from Dec 2020 to Jan 2021.
https://navi.cnki.net/knavi/journals/index. Accessed from Dec 2020 to Jan 2021.
https://c.wanfangdata.com.cn/periodical?class_code=R. Accessed from Dec 2020 to Jan 2021.
http://www.cqvip.com/qikan/search.aspx?cid=30. Accessed from Dec 2020 to Jan 2021.
http://hkmpnpub.lib.cuhk.edu.hk/. Accessed from Dec 2020 to Jan 2021.
http://hkinchippub.lib.cuhk.edu.hk/. Accessed from Dec 2020 to Jan 2021.
http://libdigital.umac.mo/macau_periodical/year. Accessed from Dec 2020 to Jan 2021.
https://www.airitilibrary.com/Search/alJnlbrowse. Accessed from Dec 2020 to Jan 2021.
https://jcr.clarivate.com/jcr/home. Accessed at Aug 2021.
https://csci.istic.ac.cn/cjcr/Citation?httproute=True. Accessed from Jan to Aug 2021.
Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ, et al. CONSORT 2010 Explanation and Elaboration: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340: c869. https://doi.org/10.1136/bmj.c869.
Schulz KF, Altman DG, Moher D, for the CONSORT Group. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. Ann Int Med. 2010;152(11):726–32. https://doi.org/10.7326/0003-4819-152-11-201006010-00232.
https://www.equator-network.org/. Assessed at 8 Oct 2021.
http://www.consort-statement.org/about-consort/endorsers1. Accessed at 6 Oct 2021.
Liu Y, Yao C, Chen F, Yang Y. The CONSORT statement: uniform requirements for reporting randomized controlled trials (in Chinese). Natls J Androl. 2001;(5):288–91. https://doi.org/10.13699/j.cnki.1001-6821.2004.01.020
Li XQ, Tao KM, Zhou QH, Moher D, Chen HY, Wang FZ, et al. Endorsement of the CONSORT Statement by High-Impact Medical Journals in China: A Survey of Instructions for Authors and Published Papers. PLoS ONE. 2012;7(2):e30683. https://doi.org/10.1371/journal.pone.0030683.
Xiao L, Hu J, Zhang L, Shang HC. Endorsement of CONSORT by Chinese medical journals: a survey of “instruction to authors.” Chin J Integr Med. 2014;20(7):510–5.
Li XQ, Tao KM, Zhou QH, Moher D, Chen HY, Wang FZ, Ling CQ. Endorsement of the CONSORT statement by high-impact medical journals in China: a survey of instructions for authors and published papers. PLoS ONE. 2012;7(2): e30683. https://doi.org/10.1371/journal.pone.0030683.
Li X, Yu X, Xie Y, Zhao H, Zhang H, Li J. Evaluation and Thinking on the Report Quality of N-of-1 Randomized Controlled Trials of Traditional Chinese Medicine Published in Journals in Mainland China (in Chinese). J Tradit Chin Med. 2020;61(15):1325–9. https://doi.org/10.13288/j.11-2166/r.2020.15.010.
Xin Y, Chen Z, Wu Y, Liu Y, Chen H, Tang C. Reporting quality of randomized controlled trials on acupuncture treatment of dry eye (in Chinese). Chin J Evid Based Med. 2020;20(10):1199–207. https://doi.org/10.7507/1672-2531.202003044.
Hu J, Zhao C, Tian R, Li J, Chen Y, Zhang X, et al. Study on the reporting standard system of clinical research of traditional Chinese medicine. Chin J Evid-based Med. 2018;18(11):1151–7. https://doi.org/10.7507/1672-2531.201807070 in Chinese.
Ma B, Ke FY, Zheng EL, Yang ZX, Tang QN, Qi GQ. Endorsement of the CONSORT statement by Chinese journals of traditional Chinese medicine: a survey of journal editors and review of journals’ instructions for authors. Acup Med. 2016;34:178–83. https://doi.org/10.1136/acupmed-2015-010870.
Ma B, Chen ZM, Xu JK, Wang YN, Chen KY, Ke FY, Niu JQ, Li L, Huang CB, Zheng JX, Yang JH, Zhu QG, Wang YP. Do the CONSORT and STRICTA checklists improve the reporting quality of acupuncture and Moxibustion randomized controlled trials published in Chinese journals? A systematic review and analysis of trends. PLoS ONE. 2016;11(1): e0147244.
Central Propaganda Department of the Communist Party of China, Ministry of Education, Ministry of Science and Technology (2021) Opinions on promoting the prosperity and development of academic journals. http://www.nppa.gov.cn/nppa/contents/279/76206.shtml. Accessed at 30 Nov 2021.
Nosek BA, Errington TM. What is replication? PLoS Biol. 2020;18(3): e3000691. https://doi.org/10.1371/journal.pbio.3000691.
Shamseer L, Hopewell S, Altman DG, Moher D, Scuilz KF. Update on the endorsement of CONSORT by high impact factor journals: a survey of journal “Instructions to Authors” in 2014. Trials. 2016;17:301. https://doi.org/10.1186/s13063-016-1408-z.
Li N, Li J, Sun F, Luan J, Liu Y, Li YL, et al. Cognition degree of guidelines on medical research reports for medical journals editors in China. Chin J Sci Tech Period. 2019;30(4):6 in Chinese.
Guo SN, Qi SL, Yang LL, Wang XH, Zhu Q, Meng X, et al. Recognition status of quality assessment and standards for reporting randomized controlled trials of traditional Chinese medicine researchers. Chin J Tradit Chin Med Pharm. 2018;33(3):1077–81.
Agha RA, Barai I, Rajmohan S, Lee S, Anwar MO, Fowler AJ. Support for reporting guidelines in surgical journals needs improvement: A systematic review. Int J Surg. 2017;45:14–7. https://doi.org/10.1016/j.ijsu.2017.06.084.
https://www.equator-network.org/about-us/chinese-equator-centre/. Accessed at 18 Oct 2021.
Duan YT, Zhang X, Chen JX, Chen Z, Bian ZX. The Application of Reporting Guidelines in Chinese Core Medical Journals. Berlin, Germany: REWARD |EQUATOR Conference 2020, Feb 19–22; 2020.
Sharp MK, Tokalic R, Gomez G, Wager E, Altman DG, Hren D. A cross-sectional bibliometric study showed suboptimal journal endorsement rates of STROBE and its extensions. J Clin Epidemiol. 2019;107:42–50. https://doi.org/10.1016/j.jclinepi.2018.11.006.
Xu L, Li J, Zhang M, Ai C, Wang L. Chinese authors do need CONSORT: Reporting quality assessment for five leading Chinese medical journals. Contemp Clin Trials. 2008;29:727–31. https://doi.org/10.1016/j.cct.2008.05.003.
Ghosn L, Boutron I, Ravaud P. Consolidated Standards of Reporting Trials (CONSORT) extensions covered most types of randomized controlled trials, but the potential workload for authors was high. J Clin Epidemiol. 2019;113:168e175.
Acknowledgements
The authors thank Dr. Martha Dahlen for her critical English editing of the manuscript. We also thank Ms. Yan Zheng, Ms. Zhirui Xu, Mr. Xin He, Mr. Huijie Guo, Mr. Cheng Zheng, and Ms. Lee Hiu Ching for their generous help in screening and visualizing the data.
Funding
This work was supported by the National Key R&D Program of China (2019YFC1710400; 2019YFC1710403). None of the sponsors had a role in any aspect of this study, including design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, approval of the manuscript, or decision to publish. All authors had full access to all the data in the study and accept the responsibility to submit it for publication.
Author information
Authors and Affiliations
Contributions
ZB, DM, and YD conceptualized the study. ZB and DM supervised the study. ZB and YD developed the search strategies and made the assessment standards. YD, LZ, YM, and JC screened, extracted, and analysed data. YD, YM, and LZ drafted the manuscript. ZB and DM revised the manuscript. JL, JM, and XZ provided critical comments and substantially improved the quality of the manuscript. All authors provided detailed comments on earlier drafts and approved the final manuscript. The corresponding authors attest that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent to publish
Not applicable.
Competing interests
All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: DM is chair of EQUATOR Network and led the initial development of CONSORT reporting guideline. ZB is the Director of the Chinese EQUATOR Centre. YTD, YFM, LYZ, JYL, and XZ are the academic staff in the Chinese EQUATOR Centre. All authors consider incomplete research reporting to be a common and important problem and believe RGs and checklists to be useful tools.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Appendix1. Included Journals.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Duan, Y., Zhao, L., Ma, Y. et al. A cross-sectional study of the endorsement proportion of reporting guidelines in 1039 Chinese medical journals. BMC Med Res Methodol 23, 20 (2023). https://doi.org/10.1186/s12874-022-01789-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12874-022-01789-1