Abstract
Background
Not all research findings are translated to clinical practice. Reasons for lack of applicability are varied, and multiple frameworks and criteria exist to appraise the general applicability of epidemiological and clinical research. In this two-part study, we identify, map, and synthesize frameworks and criteria; we develop a framework to assist clinicians to appraise applicability specifically from a clinical perspective.
Methods
We conducted a literature search in PubMed and Embase to identify frameworks appraising applicability of study results. Conceptual thematic analysis was used to synthesize frameworks and criteria. We carried out a framework development process integrating contemporary debates in epidemiology, findings from the literature search and synthesis, iterative pilot-testing, and brainstorming and consensus discussions to propose a concise framework to appraise clinical applicability.
Results
Of the 4622 references retrieved, we identified 26 unique frameworks featuring 21 criteria. Frameworks and criteria varied by scope and level of aggregation of the evidence appraised, target user, and specific area of applicability (internal validity, clinical applicability, external validity, and system applicability). Our proposed Framework Appraising the Clinical Applicability of Studies (FrACAS) classifies studies in three domains (research, practice informing, and practice changing) by examining six criteria sequentially: Validity, Indication-informativeness, Clinical relevance, Originality, Risk-benefit comprehensiveness, and Transposability (VICORT checklist).
Conclusions
Existing frameworks to applicability vary by scope, target user, and area of applicability. We introduce FrACAS to specifically assess applicability from a clinical perspective. Our framework can be used as a tool for the design, appraisal, and interpretation of epidemiological and clinical studies.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Introduction
Not all health research findings are translated into clinical or public health interventions [1]. Many reasons for lack of implementation relate to research quality and validity [2,3,4,5]. Excellent frameworks have been developed to assess the quality of epidemiological and clinical research by predominantly assessing the internal validity of research findings (e.g., confounding, selection and measurement biases) [6,7,8,9]. What determines high quality and validity research may not, however, directly determine what is most impactful [10]. The appraisal of applicability, whether study results can impact practice, demands an expanded set of considerations. The cumulative nature of evidence and of the strength of evidence is the focus of many important frameworks, most notably GRADE (Grading of Recommendations, Assessment, Development and Evaluations) [11] used to synthesize evidence and formulate clinical recommendations [12]. The appropriateness and relevance to clinical practice of research questions or findings may need to be considered; not all exposures, interventions, associations, and outcomes are equally informative to practice [13, 14]. External validity is another critical focus when applying study results to specific practice and population contexts (generalizability and transportability) [15,16,17,18].. Implementation science and economic considerations also factor in the practical application of research [19,20,21,22].
Although current frameworks cumulatively cover many important facets of applicability, the specific criteria to assess applicability may vary by the type of research and evidence, and by the stakeholders involved: researchers, clinicians, decision-makers and policymakers. Clinical applicability can be defined as the potential of study findings to inform or directly alter current clinical practice at the individual level. Due to their wide scope, it is unclear whether existing frameworks can concisely assist clinicians in differentiating between studies that change practice, inform practice, or are not clinically applicable. As clinicians must evaluate an ever-expanding research output, there is a need to better identify criteria that may be used to gauge applicability, in particular clinical applicability.
In this two-part study, we conducted a broad literature review to identify, map, and synthesize existing frameworks and criteria pertaining to the applicability of studies. Drawing from this review, current concepts and debates in epidemiology [23,24,25,26] and clinical research [13, 27], and iterative discussions and testing, we developed a concise tool to classify and improve the applicability of studies, with an emphasis on the clinical perspective. FrACAS, our proposed Framework to Appraise the Clinical Applicability of Studies and its checklist (VICORT) are introduced and discussed.
Methods
Search, thematic mapping, and synthesis of available frameworks
We searched PubMed and EMBASE (Ovid) databases since their inception for articles reporting on frameworks appraising the general “applicability” of research findings on November 12, 2020. The eligibility criteria were articles (i) featuring a unique tool, instrument, checklist, or framework (ii) focused on the applicability to practice of (iii) health research evidence, and (iv) published in English. We excluded articles that solely featured a review of frameworks, the application of an existing framework, or were restricted to a specific condition or discipline. Due to the potential multiple understandings of “applicability,” we used combinations of keywords in titles and abstracts to maximize the comprehensiveness of article selection as previously done by others on the topic of applicability [15, 16]; the full search strategy is detailed in the Additional file 1: Methods. Duplicates were removed, titles and abstracts were screened independently by two authors (PD and QDN). We supplemented remaining articles with references in reviews and retrieved articles. Articles were assessed in full to identify unique frameworks. PD and QDN performed conceptual thematic analysis [28] using preliminary themes that were refined iteratively to map the frameworks and to synthesize criteria of applicability by stakeholders. Disagreements were resolved by consensus.
Development of framework for clinical applicability
As illustrated in Fig. 1, we developed our framework by integrating four major inputs: contemporary debates in epidemiology and clinical research, brainstorming and discussion meetings, comparison with existing frameworks for appraisal of clinical applicability, and pilot application testing of our framework. Ten clinicians, researchers, and methodologists with expertise in multiple substantive domains of clinical practice and research (intensive care, pediatrics, internal, emergency, and geriatric medicine), as well as epidemiology, biostatistics, qualitative, and translational research, participated in a total six brainstorming and discussion meetings (in-person and virtual). Each meeting introduced a preliminary version of the framework which was discussed and progressively altered between each subsequent meeting. After the fourth meeting, pilot testing of the preliminary framework was conducted in a mapping review on the clinical applicability of frailty on 10 articles (forthcoming), and feedback was incorporated to the following iteration. Not all participants attended all meetings, and although formal Delphi methodology was not employed, versions of the framework were iteratively refined and circulated by email to reach the final consensus framework.
Results
Analysis, mapping, and synthesis of frameworks for applicability
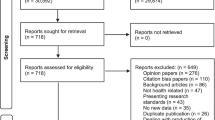
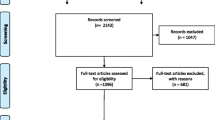
We identified 4622 references, of which 1324 were duplicates and 3265 were excluded following the screening of titles and abstracts, leaving 33 for assessment. Thirty additional references were identified in reviews and references from retrieved articles; we assessed 63 full text articles and included 26 unique frameworks. Additional file 1: Fig. A1 presents the flowchart for article selection.
Description and analysis of frameworks
Table 1 presents the 26 frameworks and their predominant focus [6, 7, 11, 17, 18, 22, 29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55]. Frameworks were published between 1999 and 2021 in epidemiological, clinical, public health, policy, and decision-making journals. Although we only included frameworks related to applicability, the focus varied widely from the quality of clinical practice guidelines (CPG, AGREE I-II [29, 30]), quality and strength of recommendations (GRADE) [11], use of evidence to inform health decisions (GRADE EtD) [18], applicability of prediction model studies (PROBAST) [43], applicability of randomized trials (PRECIS) [41] and health technology assessments (HTA) [47, 55]. Due to distinct purpose and focus in appraising applicability, the complexity of frameworks and the number, nature, and level of criteria detail within frameworks also varied. Some frameworks featured a simple list of key criteria [50, 53] whereas others elaborated on a full system of domains, criteria, and appraisal processes (e.g., RE-AIM [22, 44], GRADE [11], PRECIS [41], RoB2 [7], RoBINS-I [56], Atkins et al. [48]); some adapted to specific concepts and disciplines (GRADE EtD) [18, 34,35,36,37,38]. After comparative analysis of frameworks, we identified three dimensions explaining the variability which we used to map the frameworks and criteria:
-
The primary intended target user or stakeholders (researchers, clinicians, and decision-makers);
-
The evidence type appraised and its level of aggregation, from fundamental research to CPG;
-
The areas of applicability: internal validity, clinical applicability for individual patients, external validity, and applicability at the system level.
Although the categories within these dimensions are not mutually exclusive, they allow the mapping and synthesis of the multiple purposes and understandings of applicability, as illustrated in Figs. 2 and 3.
Mapping of frameworks and synthesis of criteria
Figure 2 maps the 26 frameworks according to the evidence type appraised and the primary intended target user. For most frameworks, the scope of the evidence appraised was directed at a single level of aggregation (e.g. prediction studies [39, 43], trials [7, 17, 41, 48, 50, 51], CPG [29, 31, 54, 57]); a few frameworks bridged evidence types such as the GRADE [11] framework which examines findings from case-control and cohort studies to systematic reviews. Most frameworks were intended for multiple stakeholders (researchers, clinicians, decision-makers), but none encompassed all three. There was a qualitative association between the level of aggregation of evidence and the primary intended users: as the frameworks appraised increasingly aggregated evidence (e.g., HTA or CPG) the target users tended towards decision-makers, whereas frameworks pertaining to prediction and observational studies were more focused on researchers, with in the middle, frameworks on trials focused mostly on clinicians.
Fig. 3 summarizes the criteria extracted from the frameworks. Across all frameworks, 21 criteria were synthesized and qualitatively mapped to evidence type appraised and the applicability areas. Although there was overlap of areas of applicability, 7 criteria fell under internal validity (i.e., risk of bias, confounding, reporting bias, dose-response gradient, precision, directness, consistency of results, and comparison intervention). Clinical applicability at the individual level directly encompassed 5 criteria (i.e., comparison intervention, intervention characteristics, magnitude and trade-offs of harms and benefits, relevance of outcomes, strength/level of evidence); and external validity considered 3 critical criteria (values, beliefs, preferences priority; context and resources for application; representativeness of patients and populations). The latter two criteria along with relevant outcomes were the most frequently featured criteria across frameworks. Finally, six criteria related to applicability at the system level (i.e., acceptability and feasibility, sustainability, cost and cost-effectiveness, scope of practice and actions, equity and ethics, monitoring/audit and support tools). There was a qualitative association between criteria in frameworks about higher level of aggregation of evidence and applicability at the system level. Existing frameworks on clinical applicability span multiple target users, evidence types, and areas of applicability. Applicability holds different meanings whether one is a researcher, clinician, or decision-maker, and is ascertained using different set of criteria depending on the type of evidence and whether internal validity, clinical applicability, external validity, or system applicability is emphasized. Our proposed framework focuses on the clinical perspective and aims to assist clinicians when evaluating all types of primary study results (from fundamental research to RCT and trials) to determine whether and how these apply to clinical practice.
Proposed framework: the framework to appraise the clinical applicability of studies (FrACAS) and VICORT checklist
Operational definition and classification of “clinical applicability:” the FrACAS framework
FrACAS uses an operational definition of clinical applicability that classifies a study according to the following questions: “are these research results valid?”, “can these results inform [my] practice?”, or “do these results change [my] current practice?”. As shown in Fig. 4, studies are classified in one of three evidence domains: research, practice-informing, or practice-changing domains, based on six criteria that examine study design elements and related data sources.
Criteria for appraisal and classification in FrACAS: the VICORT checklist
The six criteria that determine study classification in FrACAS are: Validity, Indication-informativeness, Clinical relevance, Originality, Risk-benefit comprehensiveness, and Transposability (VICORT checklist). Study findings are considered progressively more informative and practice changing as they sequentially meet these criteria. Table 2 presents each criterion’s definition and comparisons with criteria synthesized in the review.
Validity
Validity is the criterion most discussed, established, and assessed by researchers and clinicians [2, 3]. Internal validity is a necessary criterion for study findings to be considered research evidence. As our review shows, most quality assessment tools, including the Cochrane Risk-of-Bias tool (RoB 2) [7] and the Risk Of Bias In Non-randomised Studies of Intervention (ROBINS-I) [6], focus on the validity of methods (randomization, blinding, and missing data; confounding, information, and endogenous selection bias). The importance of validity in general applicability of study results is highlighted by the 7 validity-related criteria shown in Table 2. When considered outside of the traditional epidemiology and medical research contexts, the scope of validity may vary by scientific disciplines. As a general term, validity may encompass other criteria such as clinical relevance and elements related to transposability (e.g. in psychology and medical education when referring to test validity and psychometrics; see below) [68,69,70]. Although internal validity is a prerequisite, it is not sufficient for clinical applicability.
Indication-informativeness
Validity ensures that estimates are unbiased. Indication-informativeness ensures that these estimates are applicable in clinical practice. Study findings produce estimates, but not all estimates can lead to action in clinical practice. To do so, the study should produce results that inform a clinical indication, i.e., an intervention in a specific population. An indication entails the identification of what clinicians should do and which population would benefit from this being done. To inform a clinical indication, a study must include a well-defined intervention whose effect is identifiable in the results (i.e., identifiability). The ability to identify and to promise the future effects of an intervention under consideration is the key criterion to achieve indication-informativeness and move from the research domain to the clinical practice domain.
Only some study designs fulfill this criterion. Firstly, randomized control trials (RCT) where an intervention is evaluated in an eligible/target population. Secondly, observational studies of an exposure for which there exists an intervention (or where one is envisioned) to remove or modify the exposure of interest [71]. If validity is ensured, the effect of the intervention can be identified and generally assumed to approximate the effect of the exposure (e.g., smoking cessation and smoking). The existence (or lack thereof) of an exposure-removing intervention is the core of the indication-informativeness criterion. HIV, smoking, atherosclerosis, frailty, and age are exposures with decreasing levels indication-informativeness since eliminating each is increasingly challenging. Third, observational studies can also inform a clinical indication by descriptively reporting absolute outcomes of an already/otherwise-indicated intervention in a specific population of interest. For example, reporting the absolute mortality following heart surgery indicated for coronary artery disease, in patients with frailty, informs this indication by allowing the counterfactual contrast between undergoing an intervention and the natural history when forgoing the intervention, in those with frailty. Of note in this scenario, the well-defined intervention is not indicated on the basis of frailty. Following these three study designs, exposures can form the basis of an indication (i.e., inform an intervention or specific population) only when they are used in a study as a selection criterion, predictor, mediator, or effect modifier, not when used as a confounder or outcome.
Indication-informativeness does not currently feature explicitly in any identified frameworks. However, it is strongly related to the widely debated requirement of well-defined interventions in epidemiology [23, 72,73,74]. Our framework contextualizes the presence of the well-defined intervention/consistency assumption [26, 61] as a requirement for evidence that is clinically informative and applicable, not for epidemiological evidence itself [75].
Clinical relevance
Epidemiological research spans a broad range of outcome types including basic science mechanisms, intermediate outcomes, and patient-centered outcomes [13]. Clinical relevance requires that study outcomes be directly relevant and informative to practice. The precise delimitation of what outcomes are informative to practice varies [13]. It may be easy to restrict measures of heart stem cell transplantation survival to being clinically non-informative, but cholesterol levels, coronary calcium scores, atherosclerotic cardiovascular disease hospitalization, mortality, and health-related quality of life (HRQoL) all have some clinically relevant information. Achieving full clinical relevance benefits from incorporating patient-centered outcomes, of which mortality and HRQoL are examples. Ignoring outcomes that are patient-centered has led to increased numbers of studies using surrogate outcomes with unclear patient benefit and potential overdiagnoses [27, 62]. Clinical relevance in FrACAS is related to the directness [11, 14] and relevance of outcomes criteria identified in our review.
Originality: clinical significance and novelty
The originality criterion comprises significance and novelty. Under our framework, significance centers on demonstrating a clinically meaningful magnitude of effect (effect size), not only statistical significance [64]. Even if results are clinically meaningful, they can only alter current practice if they are novel compared to the current evidence base and standard practice, as shown in Fig. 4. Appraising novelty requires contrasting study results with a careful examination of the cumulative substantive evidence (e.g., reviews, practice guidelines) and current practices. Appraisal is thus practice-setting dependent. Under an evidence-based research approach, the broader context of study question and results should be systematically considered in the planning and interpretation of the study itself [12, 76]. The novelty of a study involves changing an intervention-population coupling: this requires altering (i.e., adding or removing) an intervention in a specific population or, conversely, modifying a specific population as eligible for an intervention. For example, finding that exercise benefits older adults with frailty may not be novel since exercise is already recommended to older adults in general. The difference between statistical and clinical significance (magnitude of benefits) has been highlighted in frameworks [11, 17, 18, 31, 40, 46, 47], but the importance of the novelty of findings to alter practice has not. The lack of novelty may explain why some prediction studies do not alter practice: if all modifiable predictive exposures are already addressed in standard care, then no new indication can be identified.
Risk-benefit comprehensiveness
Will altering an indication in current practice prove comprehensively beneficial to patients? Two sides must be examined: first, the intervention and displaced alternatives and, secondly, their summary net effect on overall outcomes [77]. Comparing a drug to placebo will not displace the same alternatives as comparing a drug with another active agent; if the study outcome is condition-specific at the expense of remaining patient-centered, important complications or outcomes may be overlooked that would outweigh the observed benefit. The withdrawal of the nonsteroidal anti-inflammatory drug rofecoxib due to unanticipated cardiovascular events is one example of the importance of comprehensively considering risks and benefits [78]. The risk-benefit comprehensiveness criteria emphasizes the necessity of examining explicitly and comprehensively the magnitude and trade-offs of harms and benefits criterion identified in available frameworks [11, 17, 18, 31, 40, 46, 47]. The correct calculation of comprehensive health outcomes to estimate net-benefit requires that outcomes be integrated on the absolute scale rather than on the relative scale [66].
Transposability
Appraising transposability involves taking all elements of study design, including the broader context of the study, and applying them to a specific practice setting. Epidemiologists and clinicians readily consider the external validity rubrics of generalizability and transportability [25, 79, 80]. Our transposability criterion has a wider scope. In addition to considering the population and effect modifiers (effectiveness) [25], transposability includes all other facets of implementing the intervention in a given practice setting, e.g., acceptability and feasibility, cost-effectiveness, ethics, and sustainability [18, 22, 46, 48, 53]. These will vary by practice context: resource settings, income levels, healthcare systems and payers, preferences priority, etc. [18, 21, 46, 52, 81]. As these additional questions enter into the realm of implementation science and economic evaluation, they may be beyond the direct purview of epidemiological research and are not exhaustively detailed in FrACAS.
Discussion
We identified 26 unique frameworks that appraise applicability of studies varying according to the evidence type assessed and the intended target user. Within these frameworks we synthesized 21 criteria focused on four facets of applicability (internal validity, clinical applicability at the individual level, external validity, and applicability at the population or system level). Our mapping of frameworks can help researchers, clinicians, and decision-makers select the most suitable framework depending on the appraisal question and context; selected framework may be further customized by including other synthesized criteria.
We propose a framework aiming to assist clinicians in the appraisal of clinical applicability. FrACAS shares many criteria with existing more structured and widely adopted frameworks. We believe that FrACAS is complementary to established frameworks. First, our framework creates three practical and operational domains of clinical applicability that are meaningful from a clinical practice standpoint: research evidence (i.e., does not inform clinical practice directly), practice informing, and practice changing. Rather than having the full body of existing evidence on a topic as the primary area of focus, FrACAS takes each individual study and characterizes its clinical applicability and impact, which is typically how new findings are examined and consumed in daily practice.
Next, to distinguish between level of evidence domains, FrACAS proposes two additional criteria not explicitly featured in other frameworks: indication-informativeness and originality. Many frameworks emphasize study design to determine clinical applicability and give more weight to RCT and meta-analyses than to cohort and case-control designs. The indication-informativeness criterion makes clear that it is not the study design per se that allows a study to inform and alter practice but its ability to validly inform an indication. Many health-improving interventions did not originate from experimental evidence (e.g., smoking cessation). RCT evidence has an easier claim to validity, indication-informativeness, and thus clinical applicability. However, one cannot invalidate causal inference from observational studies, only require more caution [71]. The criterion of originality is important to differentiate studies between being practice-informing or practice-changing. Determining originality (novelty and significance) is clinically consequential: practice-informing studies can go unnoticed by clinicians without major detriment since they do not alter any indication, but practice-changing studies cannot. The novelty of study results is often the prime answer to the “so what?” question of clinical applicability, following the “is it credible?” question of internal validity.
Our framework and criteria span multiple evidence types and target users, from fundamental research up to trials and, though focused on clinicians, can be relevant to researchers and decision-makers. FrACAS proposes six relatively orthogonal criteria and does not reduce them to one or two dimensions to summarize the strength or certainty of evidence [82]. FrACAS can be used as a checklist to diagnose which study design elements should be addressed for a study to change practice. Clinical translation can and does occur in the absence of one or many criteria, but we believe that careful analysis would reveal that missing criteria are assumed. We believe that the conciseness of our framework and checklist will help clinicians and trainees appraise and discuss study findings in daily practice.
Finally, our framework emphasizes the highly contextual and potentially subjective nature of appraising clinical applicability. By explicitly describing study design elements and data sources to be examined for each criterion, we show that determining practice-changing status requires the consideration of an increasing number of features. Whereas classifying articles as practice informing can be based on the appraisal of the individual study in question, a practice changing classification requires consideration of the cumulative evidence base, current standard and specific practice setting. Changing practice is an interdisciplinary and concerted effort requiring both methodological and substantive expertise.
Limitations
Although we carried out a robust literature search, extraction, and synthesis process, we did not conduct a formal systematic review. Even if we used a very wide search strategy, we may have omitted applicability frameworks. Our review serves primarily as a map to compare frameworks and criteria rather than to examine their relative strengths and weaknesses [15, 16, 83,84,85]. The process of developing a conceptual framework entails some subjectivity and variability; although a formal Delphi method was not employed, we included a wide range of inputs to iterate versions of our framework (current frameworks, debates in epidemiology, multiple stakeholders, and pilot testing). This representativity and the relative overlap with existing frameworks provide face and content validity. Ultimately, the most proper test of validity and usefulness of our framework will be determined in its usage and application in the real world; further refinements may benefit from wider inclusion of patient and institutional stakeholders.
Conclusion
Frameworks appraising applicability can be classified according to the types of evidence assessed, target users, and areas of applicability (internal validity, clinical applicability, external validity, applicability at population/system level). We proposed a concise framework focusing on clinical applicability which uses six criteria to classify studies into three evidence domains: research, practice informing, or practice changing. Our framework can be used as a tool for the design, appraisal, and interpretation of epidemiological and clinical studies to improve their clinical applicability.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Morris ZS, Wooding S, Grant J. The answer is 17 years, what is the question: understanding time lags in translational research. J R Soc Med. 2011;104:510–20.
Maclure M, Schneeweiss S. Causation of bias: the episcope. Epidemiology. 2001;12:114–22.
Greenland S. Validity and bias in epidemiological research. Oxford: Oxford University Press; 2011. https://doi.org/10.1093/med/9780199218707.003.0037.
Campbell DT. Factors relevant to the validity of experiments in social settings. Psychol Bull. 1957;54:297–312.
Jüni P, Altman DG, Egger M. Systematic reviews in health care: assessing the quality of controlled clinical trials. Br Med J. 2001;323:42–6.
Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. https://doi.org/10.1136/bmj.i4919.
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. https://doi.org/10.1136/bmj.l4898.
Greenland S, Morgenstern H. Confounding in health research. Annu Rev Public Health. 2001;22:189–212.
Hernán MA, Hernández-Díaz S, Robins JM. A structural approach to selection bias. Epidemiology. 2004;15:615–25.
Galea S. An argument for a consequentialist epidemiology. Am J Epidemiol. 2013;178:1185–91.
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924 LP–926. https://doi.org/10.1136/bmj.39489.470347.AD.
Lund H, Juhl CB, Nørgaard B, Draborg E, Henriksen M, Andreasen J, et al. Evidence-based research series-paper 3: using an evidence-based research approach to place your results into context after the study is performed to ensure usefulness of the conclusion. J Clin Epidemiol. 2021;129:167–71. https://doi.org/10.1016/j.jclinepi.2020.07.021.
Methodology Committee of the Patient-Centered Outcomes Research Institute. Methodological standards and patient-centeredness in comparative effectiveness research: the PCORI perspective. JAMA. 2012;307:1636–40.
Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 8. Rating the quality of evidence--indirectness. J Clin Epidemiol. 2011;64:1303–10.
Burchett H, Umoquit M, Dobrow M. How do we know when research from one setting can be useful in another? A review of external validity, applicability and transferability frameworks. J Heal Serv Res Policy. 2011;16:238–44.
Burchett HED, Blanchard L, Kneale D, Thomas J. Assessing the applicability of public health intervention evaluations from one setting to another: a methodological study of the usability and usefulness of assessment tools and frameworks. Heal Res Policy Syst. 2018;16:15–7.
Green LW, Glasgow RE. Evaluating the relevance, generalization, and applicability of research: issues in external validation and translation methodology. Eval Heal Prof. 2006;29:126–53.
Alonso-Coello P, Schünemann HJ, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, et al. GRADE Evidence to Decision (EtD) frameworks: A systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ. 2016;353:i2016.
Eccles MP, Mittman BS. Welcome to implementation science. Implement Sci. 2006;1:1–3.
Bauer MS, Damschroder L, Hagedorn H, Smith J, Kilbourne AM. An introduction to implementation science for the non-specialist. BMC Psychol. 2015;3:1–12. https://doi.org/10.1186/S40359-015-0089-9.
Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the economic evaluation of health care programmes. Oxford: Oxford university press; 2015.
Glasgow RE, Vogt TM, Boles SM. Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am J Public Health. 1999;89:1322–7.
Hernán MA, Taubman SL. Does obesity shorten life? The importance of well-defined interventions to answer causal questions. Int J Obes. 2008;32:S8–14.
Daniel RM, De Stavola BL, Vansteelandt S. Commentary: the formal approach to quantitative causal inference in epidemiology: misguided or misrepresented? Int J Epidemiol. 2016;45:1817–29.
Cole SR, Stuart EA. Generalizing evidence from randomized clinical trials to target populations: the ACTG 320 trial. Am J Epidemiol. 2010;172:107–15.
Hernán MA, Robins JM. Causal Inference : what if. Boca Raton: Chapman & Hall/CRC; 2020.
Fleming TR. Surrogate endpoints and FDA’s accelerated approval process. Health Aff (Millwood). 2005;24:67–78. https://doi.org/10.1377/hlthaff.24.1.67.
Creswell JW, Creswell JD. Research design: qualitative, quantitative, and mixed methods approaches. Thousand Oaks: Sage Publications; 2017.
Cluzeau F, Burgers J, Brouwers M, Grol R, Mäkelä M, Littlejohns P, et al. Development and validation of an international appraisal instrument for assessing the quality of clinical practice guidelines: the AGREE project. Qual Saf Heal Care. 2003;12:18–23.
Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, et al. AGREE II: advancing guideline development, reporting and evaluation in health care. Cmaj. 2010;182:839–42.
Brouwers MC, Spithoff K, Kerkvliet K, Alonso-Coello P, Burgers J, Cluzeau F, et al. Development and validation of a tool to assess the quality of clinical practice guideline recommendations. JAMA Netw Open. 2020;3:e205535.
Cambon L, Minary L, Ridde V, Alla F. A tool to analyze the transferability of health promotion interventions. BMC Public Health. 2013;13:1184. https://doi.org/10.1186/1471-2458-13-1184.
Khorsan R, Crawford C. External validity and model validity: a conceptual approach for systematic review methodology. Evid Based Complement Alternat Med. 2014;2014:1–12.
Alonso-Coello P, Oxman AD, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, et al. GRADE Evidence to Decision (EtD) frameworks: A systematic and transparent approach to making well informed healthcare choices. 2: Clinical practice guidelines. BMJ. 2016;353:i2089.
Parmelli E, Amato L, Oxman AD, Alonso-Coello P, Brunetti M, Moberg J, et al. GRADE evidence to decision (EtD) framework for coverage decisions. Int J Technol Assess Health Care. 2017;33:176–82.
Schünemann HJ, Wiercioch W, Brozek J, Etxeandia-Ikobaltzeta I, Mustafa RA, Manja V, et al. GRADE evidence to decision (EtD) frameworks for adoption, adaptation, and de novo development of trustworthy recommendations: GRADE-ADOLOPMENT. J Clin Epidemiol. 2017;81:101–10.
Moberg J, Oxman AD, Rosenbaum S, Schünemann HJ, Guyatt G, Flottorp S, et al. The GRADE evidence to decision (EtD) framework for health system and public health decisions. Heal Res Policy Syst. 2018;16:1–15.
Piggott T, Brozek J, Nowak A, Dietl H, Dietl B, Saz-Parkinson Z, et al. Using GRADE evidence to decision frameworks to choose from multiple interventions. J Clin Epidemiol. 2021;130:117–24. https://doi.org/10.1016/j.jclinepi.2020.10.016.
Khalifa M, Magrabi F, Gallego B. Developing a framework for evidence-based grading and assessment of predictive tools for clinical decision support. BMC Med Inform Decis Mak. 2019;19:207. https://doi.org/10.1186/s12911-019-0940-7.
Milat A, Lee K, Conte K, Grunseit A, Wolfenden L, Van Nassau F, et al. Intervention scalability assessment tool: a decision support tool for health policy makers and implementers. Heal Res Policy Syst. 2020;18:1–17.
Thorpe KE, Zwarenstein M, Oxman AD, Treweek S, Furberg CD, Altman DG, et al. A pragmatic-explanatory continuum indicator summary (PRECIS): a tool to help trial designers. C Can Med Assoc J. 2009;180(10):E47-57.
Koppenaal T, Linmans J, Knottnerus JA, Spigt M. Pragmatic vs. explanatory: an adaptation of the PRECIS tool helps to judge the applicability of systematic reviews for daily practice. J Clin Epidemiol. 2011;64:1095–101.
Moons KGM, Wolff RF, Riley RD, Whiting PF, Westwood M, Collins GS, et al. PROBAST: a tool to assess risk of bias and applicability of prediction model studies: explanation and elaboration. Ann Intern Med. 2019;170:W1–33.
Glasgow RE, Klesges LM, Dzewaltowski DA, Estabrooks PA, Vogt TM. Evaluating the impact of health promotion programs: using the RE-AIM framework to form summary measures for decision making involving complex issues. Health Educ Res. 2006;21:688–94.
Lavis JN, Permanand G, Oxman AD, Lewin S, Fretheim A. SUPPORT Tools for evidence-informed health Policymaking (STP) 13: Preparing and using policy briefs to support evidence-informed policymaking. Heal Res Policy Syst. 2009;7(Suppl 1):S13.
Stratil JM, Baltussen R, Scheel I, Nacken A, Rehfuess EA. Development of the WHO-INTEGRATE evidence-to-decision framework: an overview of systematic reviews of decision criteria for health decision-making. Cost Eff Resour Alloc. 2020;18:1–15. https://doi.org/10.1186/s12962-020-0203-6.
Almeida ND, Mines L, Nicolau I, Sinclair A, Forero DF, Brophy JM, et al. A framework for aiding the translation of scientific evidence into policy: the experience of a hospital-based technology assessment unit. Int J Technol Assess Health Care. 2019;35:204–11.
Atkins D, Chang SM, Gartlehner G, Buckley DI, Whitlock EP, Berliner E, et al. Assessing applicability when comparing medical interventions: AHRQ and the effective health care program. J Clin Epidemiol. 2011;64:1198–207. https://doi.org/10.1016/j.jclinepi.2010.11.021.
Berger ML, Martin BC, Husereau D, Worley K, Allen JD, Yang W, et al. A questionnaire to assess the relevance and credibility of observational studies to inform health care decision making: an ISPOR-AMCP-NPC good practice task force report. Value Health. 2014;17:143–56. https://doi.org/10.1016/j.jval.2013.12.011.
Bonell C, Oakley A, Hargreaves J, Strange V, Rees R. Assessment of generalisability in trials of health interventions: suggested framework and systematic review. BMJ. 2006;333:346 LP–349. https://doi.org/10.1136/bmj.333.7563.346.
Bornhöft G, Maxion-Bergemann S, Wolf U, Kienle GS, Michalsen A, Vollmar HC, et al. Checklist for the qualitative evaluation of clinical studies with particular focus on external validity and model validity. BMC Med Res Methodol. 2006;6:1–13.
Burford B, Lewin S, Welch V, Rehfuess E, Waters E. Assessing the applicability of findings in systematic reviews of complex interventions can enhance the utility of reviews for decision making. J Clin Epidemiol. 2013;66:1251–61. https://doi.org/10.1016/j.jclinepi.2013.06.017.
Gruen RL, Morris PS, McDonald EL, Bailie RS. Making systematic reviews more useful for policy-makers. Bull World Health Organ. 2005;83:480.
Linan Z, Qiusha Y, Chuan Z, Chao H, Hailong L, Chunsong Y, et al. An instrument for evaluating the clinical applicability of guidelines. J Evid Based Med. 2020;14(1):75–81.
Polus S, Pfadenhauer L, Brereton L, Leppert W, Wahlster P, Gerhardus A, et al. A consultation guide for assessing the applicability of health technologies: a case study. Int J Technol Assess Health Care. 2017;33:577–85.
Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:1–7.
Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, et al. AGREE II: advancing guideline development, reporting, and evaluation in health care. Prev Med (Baltim). 2010;51:421–4. https://doi.org/10.1016/j.ypmed.2010.08.005.
Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses 2000.
Lewis D. Counterfactuals. Hoboken: Wiley; 2013.
Höfler M. Causal inference based on counterfactuals. BMC Med Res Methodol. 2005;5:1–12.
Cole SR, Frangakis CE. The consistency statement in causal inference: a definition or an assumption? Epidemiology. 2009;20:3–5.
Fleming TR, Powers JH. Biomarkers and surrogate endpoints in clinical trials. Stat Med. 2012;31:2973–84. https://doi.org/10.1002/sim.5403.
Brodersen J, Schwartz LM, Heneghan C, O’Sullivan JW, Aronson JK, Woloshin S. Overdiagnosis: what it is and what it isn’t. BMJ Evid Based Med. 2018;23:1–3. https://doi.org/10.1136/ebmed-2017-110886.
Wasserstein RL, Lazar NA. The ASA’s statement on p-values: context, process, and purpose. Am Stat. 2016;70:129–33. https://doi.org/10.1080/00031305.2016.1154108.
Ioannidis JPA. The Proposal to Lower P Value Thresholds to .005. JAMA. 2018;319:1429–30. https://doi.org/10.1001/jama.2018.1536.
Poole C. On the origin of risk relativism. Epidemiology. 2010;21:3–9.
Noordzij M, Van Diepen M, Caskey FC, Jager KJ. Relative risk versus absolute risk: One cannot be interpreted without the other. Nephrol Dial Transplant. 2017;32:ii13–8.
de Vet HCW, Terwee CB, Mokkink LB, Knol DL. Measurement in medicine: a practical guide. Cambridge: Cambridge University Press; 2011. https://doi.org/10.1017/CBO9780511996214.
Cook DA, Brydges R, Ginsburg S, Hatala R. A contemporary approach to validity arguments: a practical guide to Kane’s framework. Med Educ. 2015;49:560–75. https://doi.org/10.1111/medu.12678.
Kelley TL. Interpretation of educational measurements; 1927.
Hernán MA. The C-word: scientific euphemisms do not improve causal inference from observational data. Am J Public Health. 2018;108:616–9.
Vandenbroucke JP, Broadbent A, Pearce N. Causality and causal inference in epidemiology: the need for a pluralistic approach. Int J Epidemiol. 2016;45:1776–86.
Krieger N, Smith GD. The tale wagged by the DAG: broadening the scope of causal inference and explanation for epidemiology. Int J Epidemiol. 2016;45:1787–808.
VanderWeele TJ, Hernán MA, Tchetgen Tchetgen EJ, Robins JM. Re: causality and causal inference in epidemiology: the need for a pluralistic approach. Int J Epidemiol. 2016;45:2199–200.
Glymour C, Glymour MR. Commentary: race and sex are causes. Epidemiology. 2014;25:488–90. https://doi.org/10.1097/EDE.0000000000000122.
Lund H, Juhl CB, Nørgaard B, Draborg E, Henriksen M, Andreasen J, et al. Evidence-Based Research Series-Paper 2 : Using an Evidence-Based Research approach before a new study is conducted to ensure value. J Clin Epidemiol. 2021;129:158–66. https://doi.org/10.1016/j.jclinepi.2020.07.019.
Hunink MGM, Weinstein MC, Wittenberg E, Drummond MF, Pliskin JS, Wong JB, et al. Decision making in health and medicine: integrating evidence and values. 2nd ed. Cambridge: Cambridge University Press; 2014. https://doi.org/10.1017/CBO9781139506779.
Singh D. Merck withdraws arthritis drug worldwide. BMJ. 2004;329:816. https://doi.org/10.1136/bmj.329.7470.816-a.
Hernán MA, Vanderweele TJ. Compound treatments and transportability of causal inference. Epidemiology. 2011;22:368–77.
Bareinboim E, Pearl J. Causal inference and the data-fusion problem. Proc Natl Acad Sci. 2016;113:7345–52. https://doi.org/10.1073/pnas.1510507113.
Cambon L, Minary L, Ridde V, Alla F. A tool to facilitate transferability of health promotion interventions: ASTAIRE. Sante Publique (Paris). 2014;26:783–6.
Viswanathan M, Ansari MT, Berkman ND, Chang S, Hartling L, McPheeters M, et al. Assessing the Risk of Bias of Individual Studies in Systematic Reviews of Health Care Interventions. In: Methods Guide for Effectiveness and Comparative Effectiveness Reviews. Rockville; 2012.
Baker A, Potter J, Young K, Madan I. The applicability of grading systems for guidelines. J Eval Clin Pract. 2011;17:758–62.
Siering U, Eikermann M, Hausner E, Hoffmann-Eßer W, Neugebauer EA. Appraisal tools for clinical practice guidelines: a systematic review. PLOS ONE. 2013;8(12):e82915.
Graham ID, Calder LA, Hébert PC, Carter AO, Tetroe JM. A comparison of clinical practice guideline appraisal instruments. Int J Technol Assess Health Care. 2000;16:1024–38.
Acknowledgements
Not applicable.
Funding
QDN reports doctoral training funding from the Canadian Institutes of Health Research and the Fonds de recherche Québec-Santé.
Author information
Authors and Affiliations
Contributions
QDN, PD, EMM, CW, and MRK designed the study. QDN and PD were responsible for data extraction and analysis. QDN, PD, RG, MFF, EP and SS contributed to the qualitative analysis and interpretation of data. All authors contributed significantly to the content, critically reviewed, and provided input during manuscript revision. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Methods. Figure A.1. Flowchart for selection of articles.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Nguyen, Q.D., Moodie, E.M., Desmarais, P. et al. Appraising clinical applicability of studies: mapping and synthesis of current frameworks, and proposal of the FrACAS framework and VICORT checklist. BMC Med Res Methodol 21, 248 (2021). https://doi.org/10.1186/s12874-021-01445-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12874-021-01445-0