Abstract
Aim
Compared to the conventional cardiopulmonary resuscitation (CCPR), potential benefits of extracorporeal cardiopulmonary resuscitation (ECPR) for patients with cardiac arrest (CA) are still controversial. We aimed to determine whether ECPR can improve the prognosis of CA patients compared with CCPR.
Methods
We systematically searched PubMed, EMBASE, and Cochrane Library from database’s inception to July 2023 to identify randomized controlled trials (RCTs) or cohort studies that compared ECPR with CCPR in adults (aged ≥ 16 years) with out-of-hospital cardiac arrest (OHCA) and in-hospital cardiac arrest (IHCA). This meta-analysis was performed using a random-effects model. Two researchers independently reviewed the relevance of the study, extracted data, and evaluated the quality of the included literature. The primary outcome was short-term (from hospital discharge to one month after cardiac arrest) and long-term (≥ 90 days after cardiac arrest) survival with favorable neurological status (defined as cerebral performance category scores 1 or 2). Secondary outcomes included survival at 1 months, 3–6 months, and 1 year after cardiac arrest.
Results
The meta-analysis included 3 RCTs and 14 cohort studies involving 167,728 patients. We found that ECPR can significantly improve good neurological prognosis (RR 1.82, 95%CI 1.42–2.34, I2 = 41%) and survival rate (RR 1.51, 95%CI 1.20–1.89, I2 = 62%). In addition, the results showed that ECPR had different effects on favorable neurological status in patients with OHCA (short-term: RR 1.50, 95%CI 0.98- 2.29, I2 = 55%; long-term: RR 1.95, 95% CI 1.06–3.59, I2 = 11%). However, ECPR had significantly better effects on neurological status than CCPR in patients with IHCA (short-term: RR 2.18, 95%CI 1.24- 3.81, I2 = 9%; long-term: RR 2.17, 95% CI 1.19–3.94, I2 = 0%).
Conclusions
This meta-analysis indicated that ECPR had significantly better effects on good neurological prognosis and survival rate than CCPR, especially in patients with IHCA. However, more high-quality studies are needed to explore the role of ECPR in patients with OHCA.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Introduction
Cardiac arrest (CA) is one of the leading causes of death in the world due to its high morbidity and mortality, and conventional cardiopulmonary resuscitation (CCPR) is the primary method for treating patients in CA [1]. However, during CCPR, even with optimal manual chest compressions, the cardiac output is approximately 20% to 30% of the normal value [2]. Moreover, the duration of cardiopulmonary resuscitation (CPR) affected the prognosis of CA patients markedly. As the duration of CCPR prolongs, the survival chances of CA patients gradually become remote. In general, when CCPR lasts longer than 35 min, only a very small number of patients achieve the return of spontaneous circulation (ROSC) and good neurological prognosis ultimately [3]. Overall, most CA patients had a low survival rate and unfavorable neurological prognosis, much less refractory CA, which urges us to find an alternative resuscitation method other than CCPR.
Extracorporeal cardiopulmonary resuscitation (ECPR), also known as extracorporeal membrane oxygenation, is an improved cardiopulmonary bypass supporting heart and lung function. Increasingly, ECPR has been used in the past few years due to the improvement and miniaturization of the technology [4]. ECPR can provide sufficient blood perfusion to vital organs such as the brain and heart during CA until the cardiac output is fully restored, thus avoiding organ failure and reducing the risk of repeated CA caused by ischemia-induced myocardial dysfunction [5]. In addition, using ECPR can extend the window for resuscitation, thus providing more treatment time for medical practitioners [6]. Multiple studies have also suggested that ECPR is a feasible alternative to CCPR, it can provide better prognosis for patients with CA in comparison with CCPR [7,8,9,10,11,12,13,14].
However, ECPR comes with certain limitations. Although ECPR serves as a promising approach for CA patients or refractory CA patients for whom ROSC is hard to achieve by CCPR, there are great difficulties in installing ECPR devices for the short window of resuscitation time under the critical circumstances of CA patients. Moreover, ECPR also requires qualified technical support and may be accompanied by serious complications such as bleeding, infection, and lower limb ischemia [15, 16]. In addition, the criteria for the appropriate population for ECPR treatment are not yet clear. Blindly using ECPR intervention may expose patients for whom ECPR is not necessary to high-risk invasive procedures and may lead to severe complications and causes financial burdens. Conversely, for patients who are suitable for ECPR, postponing ECPR treatment other than using ECPR timely may not only reduce its potential benefits but also increase additional risks. In summary, compared to CCPR, the implementation of ECPR has more uncertainties in population selection, technical operation, and timing. Therefore, there is still controversy over whether ECPR can improve the survival rate and neurological outcome of CA patients ultimately.
A meta-analysis by Kim et al. [17] included 10 cohort studies comparing the impact on the prognosis of ECPR versus CCPR in adult CA patients. The results showed that a higher survival rate and better neurological status appeared in patients who received ECPR. However, this meta-analysis may be seriously biased, for the original study included only involved cohort studies but with no randomized controlled trials (RCTs). Scquizzato et al. [18] conducted a meta-analysis which only included six studies to compare the prognosis of CA patients between ECPR group and CCPR group. The results showed that the survival rate and neurological prognosis of the ECPR group were better than those of the CCPR group, but the overall number of included original studies and sample size were considerably limited, which resulted in less convincing conclusions. In addition, the latest three RCTs assessing the efficacy of ECPR versus CCPR in the treatment of OHCA have yielded inconsistent conclusions. The RCT of Yannopoulos et al. [19] showed that compared with CCPR, ECPR significantly improved the survival rate and good neurological status, but the other two RCTs [20, 21] indicated that ECPR had no effect on survival rate and good neurological status. Based on these conclusions and newly published high-quality cohort studies with propensity-matched data, we believe that this meta-analysis can provide the latest insights into the difference of ECPR and CCPR on the prognosis of CA patients.
Methods
Protocol and registration
The protocol of this study was preregistered on PROSPERO (CRD42022385210). We conducted this meta-analysis based on the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guidelines.
Search strategy
All RCTs or cohort studies which were published by PubMed, EMBASE, and Cochrane Library from the inception to July 2023 were retrieved systematically by two reviewers. The following keywords or medical subject headings (MeSH) terms were used for retrieval: Extracorporeal cardiopulmonary resuscitation, Conventional cardiopulmonary resuscitation, heart arrest, resuscitation, Cardiopulmonary Bypass, and Extracorporeal Circulation. All search records were imported into ENDNOTE and duplicate documents were removed. They independently screened titles and abstracts for initial eligibility, as well as full texts for final eligibility. Any resulting discrepancies were settled by discussion with a third investigator.
Inclusion and exclusion criteria
We included the following studies: (1) The published clinical studies that included patients over 16 years of age with IHCA or OHCA; (2) Studies comparing the treatment of conventional CPR including manual CPR and & or mechanical CPR, with ECPR, including extracorporeal membrane oxygenation or cardiopulmonary bypass in CA patients; (3) Studies reporting the survival rate and neurological outcome after arrest at any time interval; (4) RCTs or observational studies published in English. Studies that include the following criteria are excluded: (1) Patients younger than 16 years old or pregnant; (2) CA Patients who did not receive CCPR or ECPR; (3) CA of non-medical causes such as trauma, drowning and poisoning; (4) letters, conference abstracts, case reports and reviews.
Data extraction
The two reviewers independently used a standard data extraction form to extract data from each study. When any disagreement occurs, it will be resolved through discussions with a third investigator. We collected the following information: (1) authors, publication year, study types, location of arrest, and countries and regions covered in the study; (2) sample size, number of patients with reported outcomes at any time interval after arrest, baseline characteristics of CA patients.
Definition of outcome indicators
The primary outcome was short-term (30 days after cardiac arrest) and long-term (≥ 90 days after cardiac arrest) survival with favorable neurological status. Secondary outcomes included post-arrest survival (measured as survival during follow-up at hospital discharge/1 month, 3–6 months, and 1 year). For long-term data, the longest available follow-up was used. We defined 1 or 2 points of a cerebral performance category (CPC) score [22] or a modified Glasgow Outcome Scale (MGOS > = 4) [23] as good neurological outcome.
Quality assessment
The quality of the literature included was assessed by two researchers independently. Any unresolved disagreements between reviewers were resolved through discussion. We estimated the quality of RCTs based on the Cochrane Collaboration RCT risk assessment tool, the tool assessed the biases of 7 entries such as random sequence generation, allocation concealment, etc. We used the Newcastle–Ottawa Scale to appraise the quality of cohort studies. The scale assessed risk of bias in the cohort selection, group comparability, and outcomes, with a total score of 9 points.
Statistical analysis
We used the Review Manager version 5.3 for data analysis. The date that belongs to dichotomous outcomes (survival rate, incidence rate of good neurologic status) were summarized according to the Mantel Haenszel method, and we used the relative risk (RR) and 95% confidence interval (95% CI) to compare the results. All analyses were performed using the random-effects models, and heterogeneity was assessed by Q-value tests and I2 tests (I2 > 50% or P < 0.05, indicating substantive heterogeneity).
In this meta-analysis, for the purpose of ensuring the accuracy of comparing the impact of ECPR and CCPR on the prognosis of CA patients in the cohort studies, we performed a statistical analysis based on the propensity score matching method. Propensity score matching is a method that balances observed covariates in the two treatment arms by matching the propensity score representing the probability of receiving ECPR therapy [24]. The Covariates such as age, sex, comorbidities, bystander CPR, witnessed CA, initial rhythms, duration of CPR, and therapeutic hypothermia were used for propensity score matching.
We conducted a subgroup analysis by the location where the arrest occurs (IHCA/OHCA). The results were represented by forest plots. In addition, we evaluated the potential publication bias by using a visual funnel chart, and publication bias was considered to exist when the funnel is asymmetry [25]. The Begg's test and Egger’s test were used to evaluate the plot asymmetry. All significance tests were two-tailed, with P < 0.05 being the statistically significant difference.
Results
Retrieval results
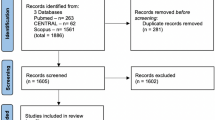
Two independent researchers initially retrieved 6993 articles from three databases (PubMed: 1902, EMBASE: 4960, Cochrane: 131) and excluded 1342duplicate articles. By screening the titles and abstracts, we excluded 5601 articles, while 50 full-text articles were assessed as qualified. Three RCTs including 420 participants [19, 20, 26] and 14 cohort studies including 167,308 participants [7, 8, 27,28,29,30,31,32,33,34,35,36,37,38] were included eventually after reading the full text (Fig. 1).
Study characteristics
The meta-analysis included three RCTs and 14 cohort studies, nine of which had propensity-matched data. Among these 14 cohort studies, five were prospective cohort studies, and the other nine were retrospective cohort studies. The countries of these studies were in Asia (n = 11) and Europe (n = 6). In this meta-analysis, a total of 2308 patients received ECPR treatment and 165,420 patients received CCPR treatment. Among these studies, only three studies included both patients with IHCA and OHCA. Overall, the patients treated with ECPR were significantly younger than those treated with CCPR, and compared with the CCPR group, the ECPR group had more bystander CPR and patients with shockable rhythms, but the CPR duration was significantly prolonged. The basic characteristics of the included studies were summarized in Table 1.
Quality of studies
We used the Cochrane Collaboration's risk assessment tool to assess the quality of the three RCTs, no study was estimated having a “high risk of bias” in each domain. The results were illustrated in Table 2. The Newcastle Ottawa Scale was used to assess the bias risk of 10 cohort studies. The results showed that most studies were considered medium quality (Table 3).
Primary outcome
Short-term favorable neurological status
A total of eleven studies compared the short-term favorable neurological status between the ECPR group and the CCPR group, including 1442 patients receiving ECPR treatment and 51,221 receiving CCPR treatment. We found that ECPR improved the short-term neurological prognosis of CA patients (RR 2.88; 95% CI 1.96–4.23; p < 0.0001; I2 = 76%) (Fig. 2a). In the analysis with matched data, there were three RCTs and seven cohort studies with propensity-matched data. The results showed that a better neurological prognosis occurred in the ECPR group (RR 1.67; 95% CI 1.16–2.40; p = 0.005; I2 = 51%) (Fig. 2b).
Long-term favorable neurological status
Eleven studies that included 896 cases in the ECPR group and 1977 cases in the CCPR group reported the long-term favorable neurological status. We found that the long-term favorable neurological status of the ECPR group was markedly higher than the CCPR group (RR 2.11; 95% CI 1.40–3.19; p = 0.0004; I2 = 69%) (Fig. 3a). By analyzing the matched data, there were three RCTs and six cohort studies with propensity-matched data. As with the above results, the long-term favorable neurological status of the ECPR group tended to be higher than that of the CCPR group (RR 1.83; 95% CI 1.32–2.53; p = 0.0003; I2 = 14%) (Fig. 3b).
Secondary outcomes
Survival rate at various times of follow-up
Propensity-matched data from cohort studies were used to analyze secondary outcomes. There were three RCTs and nine cohort studies with propensity-matched data. The results indicated that the overall survival rate of the ECPR group was higher than that of the CCPR group (RR 1.51; 95% CI 1.20–1.89; p = 0.0004; I2 = 62%), especially the 3–6-month survival rate. (at discharge: RR 1.25, 95% CI 1.00–1.56, p = 0.05, I2 = 57%; at 3-6 months: RR 2.73, 95% CI 1.67–4.48, p < 0.0001, I2 = 0%; at one year: RR 1.92, 95% CI 1.14–3.25, p = 0.01, I2 = 0%) (Fig. 4).
Subgroup analysis
Short-term and long-term favorable neurological status stratified by IHCA and OHCA
Ten studies reported the short-term favorable neurological status and eight studies reported the long-term favorable neurological status. The results showed that ECPR had different effects on favorable neurological status in patients with OHCA (short-term: RR 1.50, 95%CI 0.98- 2.29, I2 = 55%; long-term: RR 1.95, 95% CI 1.06–3.59, I2 = 11%). However, ECPR had significantly better effects than CCPR in patients with IHCA. (short-term: RR 2.18, 95%CI 1.24- 3.81, I2 = 9%; long-term: RR 2.17, 95% CI 1.19–3.94, I2 = 0%) (Table 4).
Short-term and long-term survival rates stratified by IHCA and OHCA
Twelve studies reported short-term survival rate and six studies reported long-term survival rate. We found that the short-term and long-term survival rates of the ECPR group tended to be higher than that of the CCPR group in patients with IHCA (short-term: RR 2.03, 95%CI 1.30- 3.18, I2 = 0%; long-term: RR 1.92, 95% CI 1.14–3.25, I2 = 0%). However, ECPR had different effects on survival rates in patients with OHCA (short-term: RR 1.10, 95%CI 0.91- 1.34, I2 = 44%; long-term: RR 3.16, 95% CI 1.36–7.38, I2 = 0%) (Table 4).
Publication bias
The Begg's test and Egger's test were used to evaluate the publication bias of survival rate and good neurological prognosis at discharge. As shown in the following results, no publication bias was found (P > 0.05). [survival rate: Begg's test value (P = 1.000) and Egger's test value (P = 0.284), good neurological prognosis: Begg's test value (P = 0.436) and Egger's test value (P = 0.626)].
Discussion
Based on the RCTs and large sample size cohort studies latest published, we re-performed the meta-analysis to investigate whether ECPR improved the prognosis of CA patients compared to CCPR. In this meta-analysis, we found that ECPR significantly improved the short-term and long-term neurological outcomes and survival rate in CA patients. Although this result did accord with the meta-analysis by Scquizzato et al. [18] in 2022, our current meta-analysis included more original studies with larger sample sizes with more convincing findings.
Since this meta-analysis included more cohort studies, confounding factors may not be excluded completely. These confounders also made the basic characteristics of patients in the ECPR and CCPR groups differ significantly: patients treated with ECPR were younger and had a higher proportion of bystander CPR and shockable rhythms than those treated with CCPR, and these basic characteristics play a role in whether CA patients have a good prognosis [39, 40]. After adjusting the baseline characteristics of the cohort studies by the “propensity score matching method”, the results still suggested that patients treated with ECPR had a better neurological outcome than those treated with CCPR. The possible reasons are as follows:
Firstly, technically speaking, ECPR provides more oxygenated blood flow to CA patients, and this volume of oxygenated blood does not decrease over time. Due to the fact that CA patients can only tolerate short-term blood circulation disorders, the chance of survival will decline rapidly if CPR continues for more than 15–30 min [41]. Even high-quality chest compressions can only produce up to 25% of normal cardiac output, and the blood flow reduces with the prolongation of CPR duration [2]. Clearly, we should be committed to shortening the duration of cardiopulmonary resuscitation to minimize the risk of extensive hypoxic brain injury. Although the general information table in this meta-analysis showed that in most studies, the duration of cardiopulmonary resuscitation in the ECPR group was longer than that in the CCPR group, the prognosis of patients in the ECPR group was better than that in the CCPR group. This may be because ECPR can maintain blood and oxygen supply for CA patients through blood pumps and oxygenators, ensuring sufficient blood supply to important organs such as the brain and myocardium, thereby improving the survival rate and neurological prognosis [17, 42].
Secondly, ECPR may act as a bridge to subsequent invasive or appropriate therapies (e.g., percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG)) for reversible causes of CA such as myocardial infarction and coronary ischemia, which is virtually infeasible in CCPR [43]. In addition, ECPR makes it easier to perform early therapeutic hypothermia (TH) that alleviates ischemia–reperfusion injury [29]. In our meta-analysis, we found that patients in the ECPR group were more likely to receive TH treatment than the CCPR group, which mainly because ECPR provided a basis for subsequent TH for CA patients. For another, hemodynamic instability in patients occurs more often in the CCPR group, and TH may aggravate the patient's condition [7]. Therefore, TH is less performed on the patients in the CCPR group.
In the secondary outcome, we found that the long-term survival rate of patients in the ECPR group was significantly higher than that of patients in the CCPR group. This result indicated that the benefits of ECPR for patients with CA may be long-term and related to survival with good neurological outcome. Furthermore, the improvement of long-term survival rate in CA patients may be attributed to the recovery of some brain functions.
We conducted a subgroup analysis on the survival rate and favorable neurological status of IHCA and OHCA. Firstly, we found that patients receiving ECPR treatment had better long-term neurological outcomes than those receiving CCPR treatment in both IHCA and OHCA. ECPR provides stable systemic perfusion and rapid pathways for PCI or CABG, enabling it to treat potential causes of cardiac arrest [42, 44]. In addition, ECPR makes it easier to perform TH in CA patients, thereby reducing oxygen consumption and alleviating brain edema, which improves the recovery of nervous system function [45].
Secondly, the results suggested that the patients with IHCA had higher short-term and long-term survival rate in the ECPR group, which was similar to the meta-analysis results of Gravesteijn et al. [46]. However, in terms of short-term survival rate of OHCA patients, we found that there was no obvious difference between the ECPR group and the CCPR group, the cause of which may be that OHCA patients suffer from a longer period of no-flow or low-flow circulation status, let alone the varied and complex potential causes of CA.
Limitations
The limitations of our meta-analysis are as follows. Firstly, there are only three RCTs but 14 cohort studies included in this meta-analysis. Although we try to use high-quality research with propensity score matching method to minimize the impact of confounding factors, the influence of unknown confounding factors in cohort studies and the limitation of insufficient RCTs should also be put into consideration. Secondly, among the indications for ECPR, the inclusion criteria vary across study populations in terms of the location of the arrest, duration of no-flow and CPR. Moreover, most studies on ECPR are observational, and ECMO usage cannot be blinded. Consequently, the subjective selection of ECPR participants may introduce bias into the estimation of survival and neurological outcomes. Finally, since the included studies come from different countries and regions, it inevitably leads to varying heterogeneity between studies for differences in the quality of ECPR teams, the characteristics of CA patients, medical facilities, emergency medical service systems, the systematic accessibility to ECPR.
Conclusions
Compared with CCPR, ECPR had significantly better effects on good neurological prognosis and survival rate. In addition, ECPR has the potential to improve neurological status and survival rate of IHCA patients, whereas its effect on the short-term outcome of patients with OHCA was not significant. More high-quality studies are still needed to investigate the potential benefits of ECPR in CA patients.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- CA:
-
Cardiac arrest
- CPR:
-
Cardiopulmonary resuscitation
- CCPR:
-
Conventional cardiopulmonary resuscitation
- ECPR:
-
Extracorporeal cardiopulmonary resuscitation
- ROSC:
-
Return of spontaneous circulation
- RCTs:
-
Randomized controlled trials
- IHCA:
-
In-hospital cardiac arrest
- OHCA:
-
Out-of-hospital cardiac arrest
- VT:
-
Ventricular tachycardia
- VF:
-
Ventricular fibrillation
References
Panchal AR, Bartos JA, Cabañas JG, et al. Part 3: Adult basic and advanced life support: 2020 american heart association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2020;142(16_suppl_2):S366-s468.
Jiang L, Zhang JS. Mechanical cardiopulmonary resuscitation for patients with cardiac arrest. World J Emerg Med. 2011;2(3):165–8.
Perkins GD, Callaway CW, Haywood K, et al. Brain injury after cardiac arrest. Lancet. 2021;398(10307):1269–78.
Conrad SA, Broman LM, Taccone FS, et al. The Extracorporeal life support organization maastricht treaty for nomenclature in extracorporeal life support. a position paper of the extracorporeal life support organizatio. Am J Respir Crit Care Med. 2018;198(4):447–51.
Bol ME, Suverein MM, Lorusso R, et al. Early initiation of extracorporeal life support in refractory out-of-hospital cardiac arrest: Design and rationale of the INCEPTION trial. Am Heart J. 2019;210:58–68.
Angelos MG, Gaddis M, Gaddis G, et al. Cardiopulmonary bypass in a model of acute myocardial infarction and cardiac arrest. Ann Emerg Med. 1990;19(8):874–80.
Sakamoto T, Morimura N, Nagao K, et al. Extracorporeal cardiopulmonary resuscitation versus conventional cardiopulmonary resuscitation in adults with out-of-hospital cardiac arrest: a prospective observational study. Resuscitation. 2014;85(6):762–8.
Chen YS, Lin JW, Yu HY, et al. Cardiopulmonary resuscitation with assisted extracorporeal life-support versus conventional cardiopulmonary resuscitation in adults with in-hospital cardiac arrest: an observational study and propensity analysis. The Lancet. 2008;372(9638):554–61.
Kagawa E, Inoue I, Kawagoe T, et al. Assessment of outcomes and differences between in- and out-of-hospital cardiac arrest patients treated with cardiopulmonary resuscitation using extracorporeal life support. Resuscitation. 2010;81(8):968–73.
Bellezzo JM, Shinar Z, Davis DP, et al. Emergency physician-initiated extracorporeal cardiopulmonary resuscitation. Resuscitation. 2012;83(8):966–70.
Haneya A, Philipp A, Diez C, et al. A 5-year experience with cardiopulmonary resuscitation using extracorporeal life support in non-postcardiotomy patients with cardiac arrest. Resuscitation. 2012;83(11):1331–7.
Lamhaut L, Jouffroy R, Soldan M, et al. Safety and feasibility of prehospital extra corporeal life support implementation by non-surgeons for out-of-hospital refractory cardiac arrest. Resuscitation. 2013;84(11):1525–9.
Le Guen M, Nicolas-Robin A, Carreira S, et al. Extracorporeal life support following out-of-hospital refractory cardiac arrest. Critical Care. 2011;15(1):R29.
Wang CH, Chou NK, Becker LB, et al. Improved outcome of extracorporeal cardiopulmonary resuscitation for out-of-hospital cardiac arrest–a comparison with that for extracorporeal rescue for in-hospital cardiac arrest. Resuscitation. 2014;85(9):1219–24.
Otani T, Sawano H, Natsukawa T, et al. D-dimer predicts bleeding complication in out-of-hospital cardiac arrest resuscitated with ECMO. Am J Emerg Med. 2018;36(6):1003–8.
Ha TS, Yang JH, Cho YH, et al. Clinical outcomes after rescue extracorporeal cardiopulmonary resuscitation for out-of-hospital cardiac arrest. Emerg Med J. 2017;34(2):107–11.
Kim SJ, Kim HJ, Lee HY, et al. Comparing extracorporeal cardiopulmonary resuscitation with conventional cardiopulmonary resuscitation: A meta-analysis. Resuscitation. 2016;103:106–16.
Scquizzato T, Bonaccorso A, Consonni M, et al. Extracorporeal cardiopulmonary resuscitation for out-of-hospital cardiac arrest: a systematic review and meta-analysis of randomized and propensity score-matched studies. Artif Organs. 2022;46(5):755–62.
Yannopoulos D, Bartos J, Raveendran G, et al. Advanced reperfusion strategies for patients with out-of-hospital cardiac arrest and refractory ventricular fibrillation (ARREST): a phase 2, single centre, open-label, randomised controlled trial. Lancet (london, england). 2020;396(10265):1807–16.
Belohlavek J, Smalcova J, Rob D, et al. Effect of intra-arrest transport, extracorporeal cardiopulmonary resuscitation, and immediate invasive assessment and treatment on functional neurologic outcome in refractory out-of-hospital cardiac arrest: a randomized clinical trial. JAMA. 2022;327(8):737–47.
Low CJW, Ramanathan K, Ling RR, et al. Extracorporeal cardiopulmonary resuscitation versus conventional cardiopulmonary resuscitation in adults with cardiac arrest: a comparative meta-analysis and trial sequential analysis. Lancet Respir Med. 2023;11(10):883–93.
Sauneuf B, Dupeyrat J, Souloy X, et al. The CAHP (cardiac arrest hospital prognosis) score: a tool for risk stratification after out-of-hospital cardiac arrest in elderly patients. Resuscitation. 2020;148:200–6.
Rana OR, Schröder JW, Kühnen JS, et al. The modified glasgow outcome score for the prediction of outcome in patients after cardiac arrest: a prospective clinical proof of concept study. Clin Res Cardiol. 2012;101(7):533–43.
Haviland A, Nagin DS, Rosenbaum PR. Combining propensity score matching and group-based trajectory analysis in an observational study. Psychol Methods. 2007;12(3):247–67.
Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54(10):1046–55.
Suverein MM, Delnoij TSR, Lorusso R, et al. Early extracorporeal cpr for refractory out-of-hospital cardiac arrest. N Engl J Med. 2023;388(4):299–309.
Maekawa K, Tanno K, Hase M, et al. Extracorporeal cardiopulmonary resuscitation for patients with out-of-hospital cardiac arrest of cardiac origin: a propensity-matched study and predictor analysis. Crit Care Med. 2013;41(5):1186–96.
Blumenstein J, Leick J, Liebetrau C, et al. Extracorporeal life support in cardiovascular patients with observed refractory in-hospital cardiac arrest is associated with favourable short and long-term outcomes: a propensity-matched analysis. Eur Heart J Acute Cardiovasc Care. 2016;5(7):13–22.
Choi DS, Kim T, Ro YS, et al. Extracorporeal life support and survival after out-of-hospital cardiac arrest in a nationwide registry: a propensity score-matched analysis. Resuscitation. 2016;99:26–32.
Fukushima K, Aoki M, Nakajima J, et al. Favorable prognosis by extracorporeal cardiopulmonary resuscitation for subsequent shockable rhythm patients. Am J Emerg Med. 2022;53:144–9.
Jeong D, Lee GT, Park JE, et al. Extracorporeal Life-support for Out-of-hospital Cardiac Arrest: A Nationwide Multicenter Study. Shock (Augusta, Ga). 2022;57(5):680–6.
Kim SJ, Jung JS, Park JH, et al. An optimal transition time to extracorporeal cardiopulmonary resuscitation for predicting good neurological outcome in patients with out-of-hospital cardiac arrest: A propensity-matched study. Crit Care. 2014;18(5):1–15.
Patricio D, Peluso L, Brasseur A, et al. Comparison of extracorporeal and conventional cardiopulmonary resuscitation: a retrospective propensity score matched study. Critical Care. 2019;23(1):27.
Shin TG, Jo IJ, Sim MS, et al. Two-year survival and neurological outcome of in-hospital cardiac arrest patients rescued by extracorporeal cardiopulmonary resuscitation. Int J Cardiol. 2013;168(4):3424–30.
Siao FY, Chiu CC, Chiu CW, et al. Managing cardiac arrest with refractory ventricular fibrillation in the emergency department: Conventional cardiopulmonary resuscitation versus extracorporeal cardiopulmonary resuscitation. Resuscitation. 2015;92:70–6.
Chou TH, Fang CC, Yen ZS, et al. An observational study of extracorporeal CPR for in-hospital cardiac arrest secondary to myocardial infarction. Emerg Med J. 2014;31(6):441–7.
Bougouin W, Dumas F, Lamhaut L, et al. Extracorporeal cardiopulmonary resuscitation in out-of-hospital cardiac arrest: a registry study. Eur Heart J. 2020;41(21):1961–71.
Lee SH, Jung JS, Lee KH, et al. Comparison of extracorporeal cardiopulmonary resuscitation with conventional cardiopulmonary resuscitation: is extracorporeal cardiopulmonary resuscitation beneficial? Korean J Thorac Cardiovasc Surg. 2015;48(5):318–27.
Paul M, Bougouin W, Geri G, et al. Delayed awakening after cardiac arrest: prevalence and risk factors in the Parisian registry. Intensive Care Med. 2016;42(7):1128–36.
Schmidt M, Burrell A, Roberts L, et al. Predicting survival after ECMO for refractory cardiogenic shock: the survival after veno-arterial-ECMO (SAVE)-score. Eur Heart J. 2015;36(33):2246–56.
Reynolds JC, Frisch A, Rittenberger JC, et al. Duration of resuscitation efforts and functional outcome after out-of-hospital cardiac arrest: when should we change to novel therapies? Circulation. 2013;128(23):2488–94.
Inoue A, Hifumi T, Sakamoto T, et al. Extracorporeal Cardiopulmonary Resuscitation for Out-of-Hospital Cardiac Arrest in Adult Patients. J Am Heart Assoc. 2020;9(7):e015291.
Kuroki N, Abe D, Iwama T, et al. Association between delay to coronary reperfusion and outcome in patients with acute coronary syndrome undergoing extracorporeal cardiopulmonary resuscitation. Resuscitation. 2017;114:1–6.
Bian Y, Pan Y, Zheng J, et al. Extracorporeal Versus Conventional Cardiopulmonary Resuscitation for In-Hospital Cardiac Arrest: A Propensity Score Matching Cohort Study. Crit Care Med. 2024;52(6):e268–78.
Duan J, Ma Q, Zhu C, et al. ECPR combined with therapeutic hypothermia could improve survival and neurologic outcomes for patients with cardiac arrest: A meta-analysis. Front Cardiovasc Med. 2021;8:703567.
Gravesteijn BY, Schluep M, Disli M, et al. Neurological outcome after extracorporeal cardiopulmonary resuscitation for in-hospital cardiac arrest: a systematic review and meta-analysis. Critical Care. 2020;24(1):505.
Acknowledgements
Not applicable.
Funding
Financial support and sponsorship: this work was supported by the National Natural Science Foundation of China (82060346).
Author information
Authors and Affiliations
Contributions
MHZ chaired the group, conceived and designed the study, performed statistical analysis, and contributed to data collection, data interpretation, and critical revision of the manuscript. HZ, ZHY, YZW, PS, and GLH conducted a literature search. SMH, JHW, SH, LD and ZWL performed statistical analysis. MHZ, HZ, PS, and RLD wrote the manuscript and performed a critical review of the manuscript. All authors contributed to subsequent drafts and examined the paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhong, H., Yin, Z., Wang, Y. et al. Comparison of prognosis between extracorporeal CPR and conventional CPR for patients in cardiac arrest: a systematic review and meta-analysis. BMC Emerg Med 24, 128 (2024). https://doi.org/10.1186/s12873-024-01058-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12873-024-01058-y