Abstract
Background
Myocardial infarction with non-obstructive coronary arteries (MINOCA) is a rare cause of heart attack, which may not receive sufficient attention from patients during post-discharge treatment, especially among those with normal coronary angiography results.
Case presentation
We present the case of a 65-year-old woman who was readmitted to the hospital with ventricular septal rupture (VSR) complicated by ventricular aneurysm, occurring 2 weeks after myocardial infarction. During the initial admission, coronary angiography revealed normal coronary arteries, leading to a diagnosis of MINOCA. Epicardial coronary vasospasm or coronary embolism was considered as potential causes; however, the patient did not adhere to standardized treatment upon initial discharge. The delayed VSR led to a decline in cardiac function but did not result in severe hemodynamic impairment. Following correction of heart failure with medications, the patient underwent percutaneous VSR repair 19 days after diagnosis and was discharged with a favorable recovery.
Conclusions
The occurrence of delayed VSR complicated with ventricular aneurysm in patients with MINOCA is rare, highlighting the possibility of serious complications in MINOCA cases. Both cardioprotective therapies and cause-targeted therapies are essential in the management of patients with MINOCA.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Background
Myocardial infarction with non-obstructive coronary arteries (MINOCA) is defined as an acute myocardial infarction (AMI) according to the “Fourth Universal.
Definition of Myocardial Infarction” criteria, with no stenosis ≥ 50% in any major epicardial coronary artery and no other specific cause leading to myocardial injury [1]. The reported prevalence of MINOCA ranges from 5 to 6% in patients with AMI [1], and patients with MINOCA generally have a more favorable prognosis compared to patients with AMI due to coronary artery disease (CAD) [1, 2]. Consequently, identifying non-obstructive coronary arteries through coronary angiography in patients with AMI might lead to inappropriate discontinuation of medical therapy [3], particularly in those patients with normal coronary arteries. We present a case of MINOCA in a patient who did not adhere to standardized medications after discharge and was readmitted to the hospital 2 weeks later due to ventricular septal rupture (VSR) complicated with ventricular aneurysm.
Case presentation
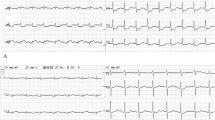
A 65-year-old woman was admitted to our hospital due to recurrent chest pain for 30 days and dyspnea for 7 days. 30 days ago, the patient experienced intermittent chest pain following exposure to cold, with each episode lasting several minutes. She ignored the symptom and did not seek medical attention. 25 days ago, the patient experienced persistent chest pain without relief and went to a local hospital on September 24, 2021. She had no medical history of hypertension, diabetes mellitus, or hyperlipidemia. The electrocardiogram (ECG) revealed sinus rhythm, ST-segment elevation and Q-wave formation in leads II, III, and AVF (Fig. 1).
The patient’s troponin-I level was 9.057ng/ml (reference: < 0.02 ng/ml) upon admission. However, emergency coronary angiography revealed normal coronary arteries (Fig. 2, Supplementary materials), with no evidence of coronary artery stenosis, slow blood flow, or coronary artery spasm.
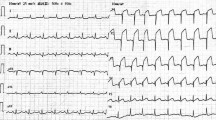
The patient was ruled out for hypercoagulable disorders but declined further provocative testing. Echocardiography demonstrated hypokinesia in the posterior septum, with a normal left ventricular end-diastolic dimension (LVEDD = 42 mm) and a normal left ventricular ejection fraction (LVEF = 68%). During hospitalization, the patient experienced atrial fibrillation, which reverted to sinus rhythm following intravenous administration of amiodarone. The patient was diagnosed with MINOCA and was discharged with rivaroxaban, metoprolol, and perindopril. However, she did not adhere to the prescribed medications after discharge as she no longer experienced chest pain. 7 days ago, the patient was readmitted to the local hospital due to dyspnea. The ECG showed Q-wave formation in leads II, III, and AVF, without evident new ST-T changes (Fig. 3), and troponin I levels did not significantly elevate.
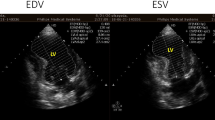
Echocardiography indicated a left ventricular aneurysm complicated with ventricular septal rupture (VSR). After receiving optimized heart failure management, the patient was transferred to our hospital for further management. The echocardiography conducted at our hospital revealed a 10 mm rupture in the posterior interventricular septum near the apex, and a ventricular aneurysm measuring 36 mm × 25 mm in the mid-lower portion of the left ventricular posterior septum, accompanied by moderate pericardial effusion (Fig. 4). The location of the ventricular aneurysm and VSR was further confirmed by the cardiac CT scan (Fig. 5). Considering the stable hemodynamics of the patient and the relatively small area of VSR, we performed a transcatheter closure of the VSR 19 days after its detection (Fig. 6, Supplementary materials). The patient recovered quickly, resumed a normal life, and was prescribed standardized secondary preventive medications. One year after the operation, the patient remained in good condition with a normal level of N-terminal pro-B-type natriuretic peptide (NT-proBNP). The echocardiogram showed normal left ventricular size, preserved ejection fraction, and a residual left ventricular aneurysm.
Discussion and conclusions
Ventricular septal rupture (VSR) is a highly fatal complication following acute myocardial infarction (AMI) with an incidence of about 0.3% [4]. Advanced age, female gender, and delayed reperfusion are recognized as risk factors for VSR post-AMI [4]. VSR usually occurs within 24 h or 3–5 days post-AMI. The former may be attributed to intramural hematoma dissection or hemorrhage into ischemic myocardium, while the latter could result from ischemic tissue coagulation necrosis accompanied by neutrophil infiltration, leading to thinning and weakening of the interventricular septum [5]. The occurrence of VSR more than 2 weeks post-AMI is relatively rare and may be associated with central perforation of a ventricular aneurysm that develops subsequent to AMI [5]. Reports regarding delayed VSR secondary to ventricular aneurysm formation following MINOCA are scarce in the literature.
VSR is also a rare and serious complication observed during the acute phase of Takotsubo Syndrome [6]. Differential diagnosis between MINOCA and Takotsubo syndrome is crucial. Takotsubo syndrome is a reversible condition where, although some patients may exhibit Q waves during the acute phase, both electrocardiography and echocardiography tend to normalize after several weeks [7]. In this case, the patient developed a left ventricular aneurysm 2 weeks after the onset of chest pain, accompanied by persistent Q waves in the inferior leads on ECG, suggesting myocardial fibrosis resulting from myocardial infarction. Despite the coronary angiography displaying normal results, the progression of the patient’s condition does not align with the expected course of Takotsubo syndrome. Considering these factors, the diagnosis of MINOCA should be considered.
MINOCA can result from plaque disruption, coronary artery spasm, coronary thromboembolism, and coronary dissection [8]. It is essential to identify the underlying cause during the evaluation of patients with MONICA. In this particular case, MINOCA was initially suspected to be associated with coronary artery spasm. The patient experienced recurrent episodes of chest pain preceding an AMI. However, during coronary angiography, the chest pain diminished, and the angiography revealed normal coronary arteries without evidence of coronary artery spasm. Unfortunately, the patient declined provocative testing, preventing a definitive diagnosis. Moreover, the underlying cause of MINOCA in this patient cannot entirely exclude thrombotic occlusion. Although the patient had no history of palpitations, paroxysmal atrial fibrillation occurred during the initial hospitalization, suggesting the possibility of previous asymptomatic atrial fibrillation leading to thrombus formation. However, during subsequent follow-ups, the patient did not experience atrial fibrillation. Considering that the occurrence of atrial fibrillation during the initial hospitalization might be related to AMI and the absence of other risk factors for thrombus formation, the possibility of thrombotic occlusion causing myocardial infarction seems less likely.
In managing VSR post-AMI, delayed repair is often recommended for patients with stable hemodynamics [9]. When encountering hemodynamic instability, the utilization of mechanical circulatory support such as intra-aortic balloon pump (IABP) and extracorporeal membrane oxygenation is advised to prolong the preoperative interval, aiming to enhance the success rate of repair [4]. Currently, there is no precise recommendation regarding the optimal timing for repair. Hobbs et al. suggested performing repairs 7–10 days post-VSR [10]. However, the mortality rate for patients undergoing repair within 1 to 3 weeks after AMI remained at 30% [11]. Histologic studies have also indicated that the proliferation of connective tissue does not occur until the third week post-infarction [12]. Hence, according to the 2019 Chinese Society of Cardiology (CSC) guidelines, repair could be deferred to 3–4 weeks post-AMI through optimized medications and IABP in hemodynamically stable patients [13]. The patient underwent transcatheter repair 19 days after VSR and had a favorable recovery. Delayed VSR accompanied by a ventricular aneurysm is a rare complication post-AMI, especially rarely reported in patients with MINOCA. The conventional treatment for both complications is VSR repair combined with aneurysmectomy surgery [14]. However, surgical aneurysmectomy appears to yield minimal benefits and is only recommended for patients with large aneurysms and uncontrolled heart failure or recurrent ventricular arrhythmias that are not responsive to ablation procedures [15]. Transcatheter closure of VSR alone serves as an alternative treatment for these two complications, as observed in this patient.
MINOCA is a condition often overlooked in clinical practice. While patients with MINOCA generally have a better prognosis compared to those with AMI-CAD [1, 2], this finding is not consistently supported by all reports [1]. Additionally, patients with MINOCA may also be at risk of developing severe complications. After the occurrence of MINOCA, it is crucial to further investigate the underlying causes and not disregard medication therapy solely due to normal findings in coronary angiography. Both cause-targeted therapies and cardioprotective therapies play a vital role in managing patients diagnosed with MINOCA.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Tamis-Holland JE, Jneid H, Reynolds HR, et al. Contemporary diagnosis and management of patients with myocardial infarction in the absence of obstructive coronary artery Disease: A Scientific Statement from the American Heart Association. Circulation. 2019;139(18):e891–908. https://doi.org/10.1161/CIR.0000000000000670.
Pasupathy S, Air T, Dreyer RP, Tavella R, Beltrame JF. Systematic review of patients presenting with suspected myocardial infarction and nonobstructive coronary arteries. Circulation. 2015;131(10):861–70. https://doi.org/10.1161/CIRCULATIONAHA.114.011201.
Kunadian V, Chieffo A, Camici PG, et al. An EAPCI Expert Consensus Document on Ischaemia with non-obstructive coronary arteries in collaboration with European Society of Cardiology Working Group on Coronary Pathophysiology & Microcirculation endorsed by Coronary Vasomotor Disorders International Study Group. Eur Heart J. 2020;41(37):3504–20. https://doi.org/10.1093/eurheartj/ehaa503.
Damluji AA, van Diepen S, Katz JN, et al. Mechanical complications of Acute Myocardial Infarction: A Scientific Statement from the American Heart Association. Circulation. 2021;144(2):e16–35. https://doi.org/10.1161/CIR.0000000000000985.
Birnbaum Y, Fishbein MC, Blanche C, Siegel RJ. Ventricular septal rupture after acute myocardial infarction. N Engl J Med. 2002;347(18):1426–32. https://doi.org/10.1056/NEJMra020228.
Zalewska-Adamiec M, Bachorzewska-Gajewska H, Dobrzycki S. Cardiac rupture-the most serious complication of Takotsubo Syndrome: a Series of five cases and a systematic review. J Clin Med. 2021;10(5):1066. https://doi.org/10.3390/jcm10051066.
Ghadri JR, Wittstein IS, Prasad A, et al. International Expert Consensus Document on Takotsubo Syndrome (Part II): diagnostic workup, outcome, and management. Eur Heart J. 2018;39(22):2047–62. https://doi.org/10.1093/eurheartj/ehy077.
Agewall S, Beltrame JF, Reynolds HR, et al. ESC working group position paper on myocardial infarction with non-obstructive coronary arteries. Eur Heart J. 2017;38(3):143–53. https://doi.org/10.1093/eurheartj/ehw149.
Rashid H, Kumar K, Ullah A, et al. Delayed ventricular septal rupture repair on patient outcomes after myocardial infarction: a systematic review. Curr Probl Cardiol. 2023;48(3):101521. https://doi.org/10.1016/j.cpcardiol.2022.101521.
Ronco D, Matteucci M, Ravaux JM, et al. Mechanical circulatory support as a bridge to definitive treatment in Post-infarction Ventricular Septal rupture. JACC Cardiovasc Interv. 2021;14(10):1053–66. https://doi.org/10.1016/j.jcin.2021.02.046.
Arnaoutakis GJ, Zhao Y, George TJ, Sciortino CM, McCarthy PM, Conte JV. Surgical repair of ventricular septal defect after myocardial infarction: outcomes from the Society of thoracic surgeons National Database. Ann Thorac Surg. 2012;94(2):436–43. https://doi.org/10.1016/j.athoracsur.2012.04.020. discussion 43 – 4.
Fishbein MC, Maclean D, Maroko PR. The histopathologic evolution of myocardial infarction. Chest. 1978;73(6):843–9. https://doi.org/10.1378/chest.73.6.843.
Chinese Society of Cardiology of Chinese Medical Association; Editorial Board of Chinese Journal of Cardiology. [2019 Chinese Society of Cardiology (CSC) guidelines for the diagnosis and management of patients with ST-segment elevation myocardial infarction]. Zhonghua Xin Xue Guan Bing Za Zhi. 2019 Oct 24;47(10):766–83. Chinese. https://doi.org/10.3760/cma.j.issn.0253-3758.2019.10.003. PMID: 31648459.
Kawamura M, Monta O, Shibata K, Tsutsumi Y. Unrecognized concomitant ventricular septal rupture and left ventricular aneurysm 10 months after myocardial infarction in a patient presenting with chronic heart failure. BMC Cardiovasc Disord. 2021;21(1):544. https://doi.org/10.1186/s12872-021-02360-4.
Ibanez B, James S, Agewall S, et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39(2):119–77. https://doi.org/10.1093/eurheartj/ehx393.
Acknowledgements
Not applicable.
Funding
This work was supported by the Sichuan Science and Technology Program (Grant numbers: 2023NSFSC0581, Sichuan, China).
Author information
Authors and Affiliations
Contributions
Si Wang and Xu Huang collected the data and wrote the main manuscript. Qianfeng Xiao prepared the figures. Ying Xu and Xin Wei revised the initial manuscript. All authors reviewed and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The ethics committee approval from the authors’ institution and patients’ informed consent have been obtained for this study.
Consent for publication
Written informed consent was obtained from the patients for publication of this Case report and any accompanying images. A copy of the written consent is available for review by the Editor of this journal.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, S., Huang, X., Xiao, Q. et al. Delayed ventricular septal rupture complicated with ventricular aneurysm in a case of myocardial infarction with non-obstructive coronary arteries. BMC Cardiovasc Disord 24, 419 (2024). https://doi.org/10.1186/s12872-024-04100-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-024-04100-w