Abstract
Background
Proton pump inhibitors (PPIs) are commonly prescribed for gastroprotection in patients undergoing percutaneous coronary intervention (PCI), who are at increased risk of gastrointestinal bleeding due to antiplatelet therapy. However, emerging evidence suggests that PPIs may adversely impact cardiovascular outcomes. This systematic review and meta-analysis sought to assess the relationship between using PPIs and cardiovascular outcomes in patients following PCI.
Methods
We searched various databases up to March 15, 2024, for observational studies and randomized controlled trials (RCTs) assessing the cardiovascular effects of PPIs in PCI patients. Data were extracted on study characteristics, patient demographics, PPI use, and cardiovascular outcomes. The Newcastle-Ottawa Scale and Cochrane Risk of Bias Tool 2 assessed study quality. Meta-analyses were conducted using a random-effects model using R software version 4.3.
Results
A total of 21 studies involving diverse populations and study designs were included. Observational studies suggested a moderate increase in risk for composite cardiovascular diseases (CVD), myocardial infarction (MI), and major adverse cardiac events (MACE) associated with PPI use, with pooled hazard ratios (HRs) of 1.20 (95% CI: 1.093–1.308) for CVD, 1.186 (95% CI: 1.069–1.303) for MI, and 1.155 (95% CI: 1.001–1.309) for MACE. However, RCTs showed no significant link between PPI therapy and negative cardiovascular events (Relative Risk: 1.016, 95% CI: 0.878–1.175). Substantial heterogeneity was observed among observational studies but not RCTs.
Conclusion
The findings indicate that while observational studies suggest a potential risk of adverse cardiovascular events with post-PCI use of PPI, RCTs do not support this association. Further large-scale, high-quality studies are required to understand the cardiovascular implications of individual PPIs better and optimize patient management post-PCI. This analysis shows the complexity of PPI use in patients with coronary artery diseases and the necessity to balance gastroprotective benefits against potential cardiovascular risks.
Similar content being viewed by others
Introduction
Percutaneous coronary intervention (PCI), commonly referred to as coronary angioplasty, is a minimally invasive technique employed to address obstructive coronary artery disease [1, 2]. It involves the insertion of a catheter into the blocked coronary artery and the subsequent balloon inflation to dilate the artery and improve blood flow. A stent (a small mesh tube) is frequently implanted to keep the artery open. PCI has revolutionized the treatment of coronary artery disease, offering an effective alternative to open-heart surgery for many patients [3].
While PCI has proven to be a life-saving procedure for countless individuals, it is not without potential complications. One significant concern is the increased risk of gastrointestinal (GI) bleeding, particularly among patients who require concomitant antiplatelet therapy, such as aspirin and P2Y12 inhibitors like clopidogrel or prasugrel [3]. While essential for preventing stent thrombosis and reducing the risk of adverse cardiovascular events, these medications can increase the likelihood of GI bleeding by impairing platelet function [4].
To mitigate this risk, proton pump inhibitors (PPIs) are often prescribed prophylactically to patients undergoing PCI. PPIs are a class of medications that potently suppress gastric acid secretion, thereby reducing the risk of peptic ulcers and GI bleeding [5]. Clinical practice guidelines and expert consensus recommendations support their widespread use in this setting. However, studies have raised concerns about potential adverse cardiovascular outcomes linked with PPI use, particularly in individuals with established cardiovascular illnesses [6, 7]. These concerns stem from various proposed mechanisms, including the ability for PPIs to interfere with the antiplatelet effects of clopidogrel, alter endothelial function, promote vascular calcification, and disrupt the gut microbiome, which may have systemic implications for cardiovascular health [8].
Numerous observational studies and meta-analyses have explored the relationship between the use of PPIs and cardiovascular outcomes [6, 8, 9]. However, the results have been inconsistent, often marred by methodological heterogeneity and potential confounding factors. While some studies indicate an elevated risk of adverse cardiovascular events such as myocardial infarction (MI), stroke [10], and cardiovascular mortality associated with PPI use, others report no significant connections or even propose potential protective effects [6, 9]. However, systematic reviews focusing specifically on populations that have undergone PCI are sparse. Additionally, previous meta-analyses have not included many recent studies potentially relevant to this topic.
Given the widespread use of PPIs in patients undergoing PCI and the potential implications for cardiovascular outcomes, it is crucial to thoroughly evaluate the available evidence and provide clarity on this contentious issue. This systematic review and meta-analysis aim to address this critical knowledge gap by rigorously evaluating the current literature on the relationship between PPI use and adverse cardiovascular outcomes in patients who have undergone PCI.
Methods
A protocol for this study was registered with the International Prospective Register of Systematic Reviews (PROSPERO). The review was performed adhering to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [11] (Table S1).
Eligibility criteria
We included observational studies cross-sectional, cohort, case-control and randomized controlled trials (RCTs) that assessed the link between PPI use and adverse cardiovascular outcomes in patients who had undergone PCI. Eligibility criteria were established to focus on adult participants (18 years and older) who had undergone PCI, with the intervention of interest being using proton pump inhibitors post-procedure. Comparators included patients not using PPIs post-PCI or those assessed for baseline cardiovascular risk before PPI use. The primary outcomes targeted were myocardial infarction, stroke, cardiovascular mortality, and composite outcomes of these. We limited inclusion to observational studies and RCTS, excluding case reports, editorials, and reviews to ensure the empirical validity of the systematic review.
Information sources and search strategy
A literature search was conducted in databases including PubMed, EMBASE, and Web of Science, from inception to March 15, 2024, with no language restrictions. The search strategy combined terms related to “proton pump inhibitors,” and “cardiovascular outcomes.” An experienced librarian reviewed the search strategy to ensure comprehensiveness. The full search strategy is given in Table S2.
Study selection
Titles and abstracts were initially screened for eligibility by two independent reviewers, followed by a detailed full-text evaluation of studies that potentially met the inclusion criteria. Any disagreements among the reviewers were settled by consulting a third reviewer. We used a semi-automated software (Nested-Knowledge, MN, USA) for screening. The selection process was documented and presented in a PRISMA flow diagram.
Data extraction
A data extraction form was used to collect data from included studies, such as study characteristics, participant demographics, details of PPI use, cardiovascular outcomes, and confounders adjusted for in the analysis. Two reviewers performed data extraction independently, with difference of opinion resolved through discussion or involving a third reviewer. We employed the tagging function of Nested-knowledge for data extraction.
Risk of Bias Assessment
Appropriate methodologies were employed to assess the quality of the studies incorporated in this analysis. The Newcastle-Ottawa Scale was used for observational studies, and the Cochrane Risk of Bias Tool 2 (RoB-2) was applied to RCTs. Two independent evaluators carried out these assessments. In cases of divergent opinions, resolutions were achieved by either reaching a consensus or involving a third reviewer for additional input.
Data synthesis and analysis
Meta-analysis used a random-effects model to account for potential heterogeneity across studies. We pooled hazard ratios (HRs) and confidence intervals for cardiovascular outcomes. Relative risks (RRs) were also calculated from RCTs. Heterogeneity was quantified using the I² statistic, and τ² was estimated to assess the variance between studies [12]. Subgroup analyses were conducted based on the type of PPI and specific cardiovascular outcomes. The evaluation of publication bias was performed using funnel plots and Egger’s test. All statistical analyses were carried out using the R statistical software package (version 4.3), employing the “meta” and “metafor” packages for meta-analysis tasks [13].
Results
Literature search
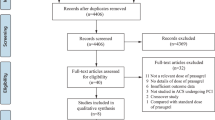
The literature search across various databases yielded a total of 4,975 records. After removing 2,706 duplicate records, 2,269 records were screened. Subsequent evaluation excluded 2,104 records, leaving 165 reports that were sought for retrieval. All 165 full-text reports underwent evaluation to determine their eligibility. Out of these, 144 were excluded due to the following reasons: the outcome of interest was not reported in 73 articles, the exposure was not of interest in 24 articles, and the population was out of scope in 47 articles. Ultimately, 21 studies met the inclusion criteria and were included in qualitative and quantitative analyses [14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34]. Figure 1 depicts the PRISMA flow diagram of the process.
Characteristics of included studies
The included studies in this systematic review exhibited a range of designs, geographic locations, and outcome measures. Table 1 displays the important characteristics of included studies. They predominantly comprised retrospective cohort studies, RCTs, and a few prospective cohort studies. Geographically, the studies were diverse, originating from multiple countries including China, the Netherlands, Italy, Sweden, Japan, the USA, and others, reflecting a global perspective on the subject. The mean age of participants across the studies ranged from approximately 59 to 71 years, indicating a predominantly older adult population. Male predominance was noted in most studies, with the percentage of male participants varying from around 24.7–82.36%. Sample sizes varied widely, from small-scale studies with as few as 86 participants to large cohorts involving up to 99,836 individuals. Regarding medications, the studies focused on patients who had undergone PCI and were prescribed a range of antiplatelet therapies including aspirin, clopidogrel, ticagrelor, and others. The type of population within these studies included those with acute coronary syndromes (ACS), stable angina, and those undergoing elective or emergent PCI. A variety of PPIs were investigated, including omeprazole, pantoprazole, esomeprazole, and lansoprazole. Follow-up periods also showed variability, ranging from 30 days to up to 3 years, allowing for short-term and long-term outcome assessments. The HRs for cardiovascular outcomes, where reported, were provided with 95% CIs, revealing a range of associations from non-significant to moderately increased risks in observational studies. Adjusted variables in the studies were comprehensive, including factors such as sex, age, body mass index (BMI), diabetes, hypertension, previous MI, and others. However, not all studies provided details on adjusted variables. Risk of bias assessment if given in Table S3 and Table S4.
Table 2 presents the clinical characteristics of patients from various studies. Observational studies generally report higher rates of common comorbidities such as hypertension and hyperlipidemia/dyslipidemia, with pooled prevalences of 68.3% and 64.1% respectively. Prevalence of diabetes and smoking was also noted among the patients. A significant observation is the detailed reporting of prior cardiovascular interventions and conditions (such as prior PCI, CABG, MI, PAD, and strokes) in observational studies, unlike in RCTs, where such information may be underrepresented or selectively excluded. There are noticeable differences in the reported rates of conditions, indicating differences in the populations studied.
PPI use and CVD outcomes from observational studies
Observational studies investigating the association between PPI use and CVDs in PCI patients, a diverse array of studies reported on various CVD outcomes. The pooled HR across studies suggested a moderate relationship between PPI use and elevated risk of any CVD outcome, with a pooled HR of 1.20 (95% CI: 1.093–1.308). This indicates that PPI use was associated with an approximately 12% increase in the risk of any cardiovascular event. Individual study HRs varied considerably, with some studies showing a significantly increased risk of specific CVD outcomes, such as coronary revascularization, MI, and stroke, while others reported non-significant associations (Fig. 2). The heterogeneity among studies was moderate, with an I² value of 51%, and the τ² statistic was 0.0206, indicating some variability in the true effect sizes across studies. The p-value for heterogeneity was less than 0.01, confirming the presence of statistically significant heterogeneity. The 95% prediction interval was calculated from 0.859 to 1.542.
The pooled HR for MACE was calculated at 1.155 (95% CI: 1.001–1.309), signifying a statistically significant increase in the risk of MACE associated with PPI use. This indicates that the use of PPIs was associated with a 15.5% increased risk of major cardiac events, including myocardial infarction, stroke, or cardiovascular death. There was moderate heterogeneity observed across the included studies, with an I² of 60% and a τ² of 0.0201, which suggests variability in the effect estimates that the individual studies reported. The p-value for heterogeneity was 0.02, indicating the presence of statistically significant variability among the study outcomes (Fig. 3). The 95% prediction interval was calculated from 0.738 to 1.571.
The overall pooled HR for MI was determined to be 1.186 (95% CI: 1.069–1.303), indicating an approximately 18.6% increased risk of myocardial infarction associated with the use of PPIs. This finding suggests a significant association between PPI use and the occurrence of MI in the studied populations. Heterogeneity amongst the included studies was minimal, with an I² value of 3% and τ² of 0.0039, suggesting a high level of consistency in the effects reported across studies. The p-value for heterogeneity was 0.41, further indicating that there was not significant variation between the studies’ results (Fig. 4). The 95% prediction interval was calculated from 0.982 to 1.389.
In the analysis of studies examining the risk of stroke linked with PPI use, the pooled HR was found to be 1.129 (95% CI: 0.720–1.539). This suggests a potential 12.9% increase in the risk of stroke among PPI users compared to non-users, although the confidence interval indicates that this increase may not be statistically significant. The heterogeneity among the included studies was moderate with an I² value of 52% (Fig. 5).
PPI use and CVD outcomes from RCTs
Six RCTs reported CVD outcome with PPI use in patients undergoing PCI. In this meta-analysis of RCTs assessing the relationship between PPI use and CVD outcomes, the pooled RR was found to be 1.016 (95% CI: 0.878–1.175). This analysis 3,740 individuals in the PPI group and 3,606 individuals in the control group, with a total of 665 and 638 cardiovascular events, respectively. The overall heterogeneity among the studies was low, with an I² of 30%, and a tau-squared (τ²) of 0.0050, indicating relatively little variation between the study outcomes. The p-value for heterogeneity was 0.19, suggesting that there is no statistically significant inconsistency across the included studies (Fig. 6).
Publication bias
We used funnel plot and Egger’s test to evaluate the publications bias (Figures S1 and S2). The analysis revealed no significant publication bias for either observational studies (Egger’s test p-value = 0.335) or RCTs (Egger’s test p-value = 0.907).
Discussion
This meta-analysis critically evaluates the relationship between PPI use and adverse cardiovascular outcomes following PCI. Amid conflicting evidence from observational studies and RCTs, our analysis offers a contemporary synthesis, casting light on a controversial topic in cardiovascular pharmacotherapy. While PPI treatment was associated with an elevated risk of adverse cardiovascular outcomes in meta-analyses of observational studies, no such association was observed in patients with PCI in the RCT meta-analysis.
There are several proposed mechanisms by which PPIs may elevate the CVD risk. co-administration of PPIs and dual antiplatelet therapy (DAPT) may elevate cardiovascular risk. Prior research suggests that PPI treatment could reduce aspirin’s absorption and oral bioavailability by inhibiting gastric acid secretion. Some PPIs, including omeprazole and esomeprazole, share a metabolic pathway with clopidogrel, a prodrug, potentially affecting its antiplatelet function through competitive enzymatic interaction. Additional hypotheses suggest that PPIs may elevate plasma levels of asymmetric dimethylarginine, which inhibits nitric oxide synthase, thereby disrupting nitric oxide synthesis. PPIs may also undermine the effectiveness of clopidogrel due to shared cytochrome P450 metabolism, leading to concerns about their concurrent use and potential for cardiovascular harm.
Our meta-analysis of RCTs revealed no significant link between PPI use and cardiovascular outcomes in PCI patients, with minimal study heterogeneity. Conversely, the observational studies meta-analysis showed a significant increase in composite CVD, MACE, and MI risks, but not for stroke. The variability in outcomes might be ascribed to differences in study designs, populations, and confounder adjustments. Inherent biases and unmeasured confounding factors, though mitigated, remained a concern. The discrepancy between observational studies and RCTs may stem from the former’s inherent biases and the latter’s controlled environment, better capturing the intervention’s true effect. Moderate heterogeneity was detected among RCTs included in this meta-analysis, but unmeasured confounders appeared more impactful in the observational studies meta-analysis. Inconsistencies suggest that the increased risk observed in patients on PPI therapy could reflect their higher inherent risk due to poor prognosis, regardless of PPI use. In real-world practice, patients with high bleeding risk are more likely to receive proton pump inhibitors PPIs than those without high bleeding risk, and these patients also possess high ischemic risk. Therefore, patients who are prescribed PPIs may have high ischemic risks. This could be a reason for the discrepancy between the results of observational studies and RCTs.
Assessing the combined PPI effect should consider individual drug profiles rather than amalgamating data from multiple drugs in a class. Although we aimed to lessen the impact due to discrepancies in baseline characteristics among patients using PPIs and non-users through adjusted estimates from each study, some provided only unadjusted data.
Several prior meta-analyses have examined the link between PPI use and the risk of CVD. For instance, Jeridi et al.‘s analysis indicated that PPIs, as a category of drugs, did not correlate with a heightened risk of cardiovascular events [6]. Nonetheless, mixed outcomes emerged for concurrent use of PPIs with clopidogrel. Specifically, examining omeprazole’s impact on cardiovascular well-being suggested no adverse effects. Another meta-analysis from prospective observational studies implied that short-term PPI use for treating gastroesophageal conditions did not elevate the risk of initial cardiovascular incidents [9]. The reported increase in cardiovascular mortality has been largely attributed to publication bias and the inherent biases of observational studies, such as unmeasured confounding variables and indication bias. Considering these findings alongside RCT outcomes, it appears dubious to consider PPI consumption as an independent CVD risk factor, separate from any potential interaction with clopidogrel.
The clinical implications of this study are significant for the management of patients PCI. The divergent findings from observational studies and RCTs underscore the necessity for clinicians to critically evaluate the use of proton PPIs in the context of DAPT. Given that PPIs are widely prescribed to mitigate gastrointestinal bleeding risks in patients on antiplatelet therapy, understanding their potential cardiovascular impact is paramount. While observational studies suggest an increased risk of adverse cardiovascular events with PPI use, RCTs do not corroborate this finding. Therefore, it may not be necessary to universally avoid PPIs in patients post-PCI if they have a clear indication for their use, particularly in those with a high risk of gastrointestinal complications. Clinicians should consider the individual patient’s risk profile, the specific PPI being prescribed, and the potential for drug-drug interactions, particularly with clopidogrel.
Future research should focus on large-scale, prospective studies that can control for the wide array of confounding factors not accounted for in observational studies. Investigations should delineate the cardiovascular risks associated with individual PPIs, as there may be variability within this drug class. There is also a need for further pharmacodynamic studies to understand the interactions between PPIs and DAPT, especially with newer antiplatelet agents. Additionally, genomic studies may provide insights into the variability of patients’ responses to combined PPI and antiplatelet therapy.
The present study has a few limitations. The inclusion of studies was restricted to those published exclusively in English, potentially introducing language bias and omitting relevant data available in other languages. Most included studies adjusted for multiple confounders, but the adjustment variables varied significantly between studies, potentially leading to residual confounding. Also, the analysis depended heavily on the quality of the included studies, where the presence of unreported biases within the original studies could skew the meta-analysis results. The analysis of observational studies may be particularly susceptible to indication bias, as patients prescribed PPIs might have had higher baseline risks for cardiovascular events. The scope of PPIs reviewed was broad, and individual PPI effects, which may vary significantly in terms of cardiovascular risk, were not extensively differentiated, which could mask specific risks associated with particular PPIs. These limitations indicate the requirement for cautious interpretation of the findings and suggest a pressing need for more targeted research that can provide clearer insights into the cardiovascular safety of specific PPIs in the post-PCI setting. While PPIs are essential for gastrointestinal protection, especially post-PCI, their potential cardiovascular risks warrant a tailored approach. Further investigations should focus on individual PPIs and consider patient-specific factors to refine our understanding of the cardiovascular implications of PPI use. Furthermore, studies should focus on individual PPIs rather than combines effect of PPI class.
Conclusion
The current evidence is insufficient to establish a definitive relationship between PPI use and adverse cardiovascular events in individuals undergoing PCI due to conflicting results between RCTs and observational studies. More comprehensive, high-quality studies are needed to clarify this relationship, particularly studies that can better control for confounding factors and provide a more nuanced analysis of the effects of individual PPIs.
Data availability
All data are presented within the manuscript and are available by contacting the corresponding author.
References
Stergiopoulos K, Boden WE, Hartigan P, Möbius-Winkler S, Hambrecht R, Hueb W, et al. Percutaneous coronary intervention outcomes in patients with stable obstructive coronary artery disease and myocardial ischemia: a collaborative meta-analysis of contemporary randomized clinical trials. JAMA Intern Med. 2014;174(2):232–40.
Al-Lamee RK, Nowbar AN, Francis DP. Percutaneous coronary intervention for stable coronary artery disease. Heart. 2019;105(1):11–9.
Khera S, Kolte D, Bhatt DL. Percutaneous coronary intervention. Translational research in coronary artery disease. Elsevier; 2016. pp. 179–94.
You SC, Rho Y, Bikdeli B, Kim J, Siapos A, Weaver J, et al. Association of Ticagrelor vs clopidogrel with net adverse clinical events in patients with acute coronary syndrome undergoing percutaneous coronary intervention. JAMA. 2020;324(16):1640–50.
Han Y-Y, Li Z-X, Duan R. Efficacy and safety of proton pump inhibitors combined with clopidogrel in patients undergoing percutaneous coronary intervention: a meta-analysis. Rev Cardiovasc Med. 2021;22(1):167–74.
Jeridi D, Pellat A, Ginestet C, Assaf A, Hallit R, Corre F, Coriat R. The safety of long-term proton pump inhibitor use on cardiovascular health: a meta-analysis. J Clin Med. 2022;11(14):4096.
Bell EJ, Bielinski SJ, Sauver JLS, Chen LY, Rooney MR, Larson NB, et al. editors. Association of proton pump inhibitors with higher risk of cardiovascular disease and heart failure. Mayo Clinic Proceedings; 2021: Elsevier.
Shang Y-S, Zhong P-Y, Ma Y, Bai N, Niu Y, Wang Z-L. Efficacy and safety of Proton pump inhibitors in patients with coronary artery diseases receiving oral antiplatelet agents and/or anticoagulants: a systematic review and meta-analysis. J Cardiovasc Pharmacol. 2022;80(1):1–12.
Nolde M, Ahn N, Dreischulte T, Krause E, Güntner F, Günter A, et al. Proton pump inhibitors and the risk of cardiovascular events and cardiovascular mortality: a systematic review and meta-analysis of observational studies. Eur J Intern Med. 2022;106:80–9.
Yang M, He Q, Gao F, Nirantharakumar K, Veenith T, Qin X, et al. Regular use of proton-pump inhibitors and risk of stroke: a population-based cohort study and meta-analysis of randomized-controlled trials. BMC Med. 2021;19:1–12.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372.
Gandhi AP, Satapathy P, Rustagi S, Hermis AH, Sah R, Padhi BK. Comments on shigellosis in Southeast Asia: a systematic review and meta-analysis. Travel Med Infect Dis. 2023:102593-.
Shamim MA, Gandhi AP, Dwivedi P, Padhi BK. How to perform meta-analysis in R: a simple yet comprehensive guide. Evid. 2023;1(1):60–80.
Aihara H, Sato A, Takeyasu N, Nishina H, Hoshi T, Akiyama D, et al. Effect of individual proton pump inhibitors on cardiovascular events in patients treated with clopidogrel following coronary stenting: results from the Ibaraki Cardiac Assessment Study Registry. Catheter Cardiovasc Interv. 2012;80(4):556–63.
Burkard T, Kaiser C, Brunner-La Rocca H, Osswald S, Pfisterer M, Jeger R, Investigators B. Combined clopidogrel and proton pump inhibitor therapy is associated with higher cardiovascular event rates after percutaneous coronary intervention: a report from the BASKET trial. J Intern Med. 2012;271(3):257–63.
Chandrasekhar J, Bansilal S, Baber U, Sartori S, Aquino M, Farhan S, et al. Impact of proton pump inhibitors and dual antiplatelet therapy cessation on outcomes following percutaneous coronary intervention: results from the PARIS Registry. Catheter Cardiovasc Interv. 2017;89(7):E217–25.
Dunn SP, Steinhubl SR, Bauer D, Charnigo RJ, Berger PB, Topol EJ. Impact of proton pump inhibitor therapy on the efficacy of clopidogrel in the CAPRIE and CREDO trials. J Am Heart Association. 2013;2(1):e004564.
Gargiulo G, Costa F, Ariotti S, Biscaglia S, Campo G, Esposito G, et al. Impact of proton pump inhibitors on clinical outcomes in patients treated with a 6-or 24-month dual-antiplatelet therapy duration: insights from the PROlonging dual-antiplatelet treatment after Grading stent-induced intimal hyperplasia studY trial. Am Heart J. 2016;174:95–102.
Harjai KJ, Shenoy C, Orshaw P, Usmani S, Boura J, Mehta RH. Clinical outcomes in patients with the concomitant use of clopidogrel and proton pump inhibitors after percutaneous coronary intervention: an analysis from the Guthrie Health off-label stent (GHOST) investigators. Circulation: Cardiovasc Interventions. 2011;4(2):162–70.
Jensen BE, Hansen JM, Larsen KS, Junker AB, Lassen JF, Jensen SE, De Muckadell OBS. Randomized clinical trial: the impact of gastrointestinal risk factor screening and prophylactic proton pump inhibitor therapy in patients receiving dual antiplatelet therapy. Eur J Gastroenterol Hepatol. 2017;29(10):1118–25.
Liu Y-H, Cao Z-Y, Dai Y-N, Zeng L-H, Zhang Y-S, Fan H-L, et al. Association of Proton Pump Inhibitor and Infection and major adverse clinical events in patients with ST-Elevation myocardial infarction: a propensity score matching analysis. Front Med. 2022;9:882341.
Macaione F, Montaina C, Evola S, Novo G, Novo S. Impact of dual antiplatelet therapy with proton pump inhibitors on the outcome of patients with acute coronary syndrome undergoing drug-eluting stent implantation. International Scholarly Research Notices. 2012;2012.
Maret-Ouda J, Santoni G, Xie S, Rosengren A, Lagergren J. Proton pump inhibitor and clopidogrel use after percutaneous coronary intervention and risk of major cardiovascular events. Cardiovasc Drugs Ther. 2022:1–8.
Nicolau JC, Bhatt DL, Hohnloser SH, Kimura T, Lip GY, Miede C, et al. Dabigatran dual therapy vs warfarin triple therapy post-percutaneous coronary intervention in patients with atrial fibrillation with/without a proton pump inhibitor: a pre-specified analysis of the RE-DUAL PCI trial. Drugs. 2020;80:995–1005.
Ono M, Onuma Y, Kawashima H, Hara H, Gao C, Wang R, et al. Impact of proton pump inhibitors on efficacy of antiplatelet strategies with ticagrelor or aspirin after percutaneous coronary intervention: insights from the GLOBAL LEADERS trial. Catheter Cardiovasc Interv. 2022;100(1):72–82.
Ren Y-h, Zhao M, Chen Y-d, Chen L, Liu H-b, Wang Y, et al. Omeprazole affects clopidogrel efficacy but not ischemic events in patients with acute coronary syndrome undergoing elective percutaneous coronary intervention. Chin Med J. 2011;124(06):856–61.
Sarafoff N, Sibbing D, Sonntag U, Ellert J, Schulz S, Byrne RA, et al. Risk of drug-eluting stent thrombosis in patients receiving proton pump inhibitors. Thromb Haemost. 2010;104(09):626–32.
Tentzeris I, Jarai R, Farhan S, Brozovic I, Smetana P, Geppert A, et al. Impact of concomitant treatment with proton pump inhibitors and clopidogrel on clinical outcome in patients after coronary stent implantation. Thromb Haemost. 2010;104(12):1211–8.
Wei P, Zhang Y-G, Ling L, Tao Z-Q, Ji L-Y, Bai J, et al. Effects of the short-term application of pantoprazole combined with aspirin and clopidogrel in the treatment of acute STEMI. Experimental Therapeutic Med. 2016;12(5):2861–4.
Weisz G, Smilowitz NR, Kirtane AJ, Rinaldi MJ, Parvataneni R, Xu K, et al. Proton pump inhibitors, platelet reactivity, and cardiovascular outcomes after drug-eluting stents in clopidogrel-treated patients: the ADAPT-DES study. Circulation: Cardiovasc Interventions. 2015;8(10):e001952.
Yano H, Tsukahara K, Morita S, Endo T, Sugano T, Hibi K, et al. Influence of Omeprazole and Famotidine on the Antiplatelet effects of Clopidogrel in Addition to aspirin in patients with Acute Coronary Syndromes–A prospective, randomized, Multicenter Study–. Circ J. 2012;76(11):2673–80.
Zhang F, Su S, Hou Y, Zhao L, Wang Z, Liu F, et al. Effects (MACE and bleeding events) of ticagrelor combined with omeprazole on patients with acute myocardial infarction undergoing primary PCI. Hellenic J Cardiol. 2020;61(5):306–10.
Zhu P, Gao Z, Tang X-F, Xu J-J, Zhang Y, Gao L-J, et al. Impact of proton-pump inhibitors on the pharmacodynamic effect and clinical outcomes in patients receiving dual antiplatelet therapy after percutaneous coronary intervention: a propensity score analysis. Chin Med J. 2017;130(24):2899–905.
Zou J-J, Chen S-L, Tan J, Lin L, Zhao Y-Y, Xu H-M, et al. Increased risk for developing major adverse cardiovascular events in stented Chinese patients treated with dual antiplatelet therapy after concomitant use of the proton pump inhibitor. PLoS ONE. 2014;9(1):e84985.
Acknowledgements
The authors acknowledge the Nested-Knowledge, MN, USA for providing the access to the software.
Funding
None.
Author information
Authors and Affiliations
Contributions
Bijaya K Padhi: Conceptualization (lead); writing – original draft (lead), review and editing (equal); Quazi Syed Zahiruddin, Mahalaqua Nazli Khatib: formal analysis (lead); writing – review and editing (equal). Sarvesh Rustagi: Software (lead); writing – review and editing (equal). Rakesh Kumar Sharma, Ranjit Sah, Prakasini Satapathy: Methodology (lead); writing – review and editing (equal). Arathi P Rao : Data analysis (equal).
Corresponding authors
Ethics declarations
Ethic approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Padhi, B.K., Khatib, M.N., Zahiruddin, Q.S. et al. Adverse cardiovascular outcomes associated with proton pump inhibitor use after percutaneous coronary intervention: a systematic review and meta-analysis. BMC Cardiovasc Disord 24, 372 (2024). https://doi.org/10.1186/s12872-024-04029-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-024-04029-0