Abstract
Background
There is a broad pulse pressure (PP) and a high prevalence of carotid plaques in old adults. Previous studies have indicated that PP is strongly associated with carotid plaque formation. This study aimed to explore this association in old adults with uncontrolled hypertension.
Methods
1371 hypertensive patients aged ≥ 60 years with uncontrolled hypertension were enrolled in a community-based screening in Hangzhou, China. Carotid plaques were assessed using ultrasonography. Logistic regression models were used to estimate the association between PP and carotid plaques by odds ratios (ORs) and 95% confidence intervals (CIs).
Results
Carotid plaques were detected in 639 (46.6%) of subjects. Multiple plaques were found in 408 (63.8%) and soft plaques in 218 (34.1%). Elevated PP was associated with a high prevalence of carotid plaques. After adjusting for traditional risk factors, compared to patients within the lowest tertile of PP, those within the highest tertiles had an increased risk of carotid plaques (OR 2.061, CI 1.547–2.745). For each 1-SD increase, the risk increased by 40.1% (OR 1.401, CI 1.237–1.587). There was a nonlinear association between PP and carotid plaques (P nonlinearity = 0.039). The risk increased rapidly after the predicted PP level reached around 60 mmHg. The associations were stronger among participants with multiple and soft plaques.
Conclusions
Our findings suggested that PP was independently associated with carotid plaques in old adults with uncontrolled hypertension who have an increased risk of atherosclerosis.
Similar content being viewed by others
Introduction
Hypertension is a major public health challenge in China, and its prevalence is rising, but it has not been sufficiently controlled. Nearly 50% of Chinese adults aged 35–75 years have hypertension, while only less than 10% control it [1]. It is well known that hypertension is the leading cause of cardiovascular death. Compared to hypertensive patients with controlled blood pressure (BP), those who fail to control BP have a higher risk of cardiovascular death [2, 3]. This adverse outcome risk increases with increasing age. China follows the global trend of population aging [4], and regulating BP in the senior age group is not ideal [1]. Therefore, the aging group with uncontrolled hypertension deserve more attention in daily healthcare.
Carotid plaque formation is a common risk factor for cardiovascular and cerebrovascular diseases such as stroke, transient ischemic attack (TIA), and acute myocardial infarction [5,6,7]. About 31% of the Chinese population have carotid plaques, and up to 63% of the old adults aged 70–89 years [8]. In 2020, ∼ 200 million people in China were affected by carotid plaques [9]. It was necessary to take timely intervention to prevent carotid atherosclerosis. Pulse pressure (PP) was strongly associated with carotid plaques. Age-related central elastic arteriosclerosis and high systolic blood pressure (SBP) make old adults more likely to have a wide PP [10]. Previous studies in China showed that elevated PP was an independent risk factor for the presence of carotid plaques [11,12,13,14]. However, there is limited information about this correlation in old adults with uncontrolled hypertension, who are more vulnerable to atherosclerosis diseases.
Therefore, this study aimed to find the association between PP and carotid plaques in old adults with uncontrolled hypertension. We also investigated the dose-response relationship between PP levels and carotid plaques, independent of the traditional risk factors.
Methods
Study design and participants
In May 2020, the Centers for Disease Control and Prevention and the medical service community collaborated on a “stroke high-risk population screening and intervention” initiative in Hangzhou, China. This was a large-scale public welfare project that focused on secondary prevention and individualized intervention for high-risk population of stroke. Participants were informed of the screening results by community health workers, and further diagnosis and treatment would be recommended if necessary. Participants aged ≥ 60 years with hypertension or diabetes were selected from the service contractor of the family doctor of their community health service centre, and they were informed of the screening details, including a questionnaire survey, physical examination, and carotid ultrasonography, by their family doctor. Participants meeting the following criteria were excluded from the screening: (1) Patients with mental disorders are unable to complete the questionnaire independently; (2) Long-term disability; (3) Unable to complete the follow-up. This screening included a sample of 15,561 participants according to the above exclusion criteria. For the present study, of the 11,433 participants with hypertension who were included, 3384 participants had incomplete information on biochemical indexes, conventional confounding factors or carotid artery imaging and 6678 participants were excluded due to having SBP < 150 and diastolic blood pressure (DBP) < 90 mmHg. Finally, 1371 patients were included in this analysis (Fig. 1).
Data collection
Detailed information on individual demographics, lifestyle, and histories of disease was obtained through standardized self-report questionnaires. Smoking was defined as those who had been continuously or cumulatively smoking for ≥ 6 months. Regular exercise was defined as moderate/vigorous-intensity performed ≥ 30 min 3 or more times per week. Physical examinations include height, weight, SBP, and DBP. Height was measured in a standing position, and weight was measured using a calibrated platform scale. Participants completed the above measurement process in a light dress and without shoes. Body mass index (BMI) was calculated as weight/ height2 (kg/ m2). BP measurements were determined by the participants sitting in a quiet room at their local community health center. Participants were instructed to avoid alcohol consumption, cigarette smoking, coffee/tea, and exercise for at least 30 min before BP measurement. BP was measured using an automatic electronic sphygmomanometer (HEM-741 C; Omron, Tokyo, Japan). For each participant, the BP was measured 3 times by community health workers, and the average of three BP was used for the final analysis and evaluation. For the current participants (age ≥ 60 years), based on the Eighth Joint National Committee [15], uncontrolled hypertension for the present analysis was identified if the SBP was ≥ 150 mmHg or the DBP was ≥ 90 mmHg, regardless of antihypertensive medications use (Supplemental Fig. 1). Biochemical measurements include blood glucose, creatinine and lipid levels, including triglyceride (TG), total cholesterol (TC), low density lipoprotein cholesterol (LDLC), and high density lipoprotein cholesterol (HDLC). Blood samples were drawn from each participant’s antecubital vein in the morning after an overnight fast. Fasting blood glucose, TG, and TC were measured using the glucose oxidase method, glycerol phosphate oxidase-peroxidase, and esterase oxidase-peroxidase, respectively. HDLC was determined after phosphotungstate precipitation. LDLC was calculated using the Friedewald formula [16]. Atherogenic dyslipidemia was defined as TG > 1.69 mmol/L and HDLC < 1.03 mmol/L in males or < 1.29 mmol/L in females [17]. The estimated glomerular filtration rate (eGFR) was calculated as follows: eGFR = 175× Scr− 1.234× age− 0.179 (if female,×0.79), where Scr is serum creatinine concentration (in mg/dL) and age in years. Reduced renal function was defined as eGFR < 60 mL/min per 1.73 m² [18].
Carotid ultrasonography examinations were certified manually by experienced sonographers who received unified training from the local medical service community and were blinded to the characteristics and laboratory results of the participants. All participants were studied lying supine with their head turned 45 degrees from the site being scanned. The colour Doppler ultrasound diagnostic instrument was equipped with a Philips HDI 5000 ultrasound system and a 7.5 MHz probe, which was operated by sonographers. We examined the carotid plaques on the left and right sides, with each side measured at three different locations: common carotid artery, carotid bifurcation, and internal carotid artery. Carotid plaques were defined as focal structures encroaching into the arterial lumen of at least 0.5 mm or 50% of the surrounding intima-media thickness value or demonstrating a thickness > 1.5 mm as measured from the intima-lumen interface to the media-adventitia interface [19].
Statistical analysis
Demographic and clinical characteristics were expressed as mean ± standard deviation (SD) for continuous variables and proportions with corresponding percentages (%) for categorical and binary variables. The preliminary analysis explored differences in characteristics of participants by uncontrolled hypertension according to PP levels, using ANOVA or Kruskal-Wallis tests for continuous variables, while for nominal variables, chi-square tests were performed, as appropriate. Participants were grouped into tertiles according to the PP levels: T3, ≥ 79 mmHg; T2, 65–79 mmHg; T1, < 65 mmHg. Binary logistic regressions were conducted to assess the association between PP levels and carotid plaques and to calculate the odds ratios (ORs) and 95% confidence intervals (CIs). A test for multicollinearity would be conducted using an ordinary least squares (OLS) model before analysis. No violation was found as all the variables displayed a variance inflation factor (VIF) < 5. Two models were used. Model 1 was unadjusted. In model 2, we adjusted for a propensity score. The propensity score was calculated with a linear regression model entering PP as the dependent variable. The independent variables included sex, age, smoking, regular exercise, history of stroke, history of transient ischemic attacks (TIA), history of atrial fibrillation/valvular heart disease, diabetes, family history of stroke, BMI, blood glucose, TC, TG, LDLC, HDLC, eGFR, antihypertensive medications and lipid-lowing medications. In the above models, PP was entered as a categorical variable with T1 as the reference group or as a continuous variable with per 1-SD increment. Restricted cubic splines were performed with four knots at the 5th, 33.3th, 50th, 66.6th, and 95th centiles to flexibly model the association between PP and the risk of carotid plaques with adjusted model 2. We also assessed the relationship between PP and the risk of multiple plaques (plaques ≥ 2) and plaque type, including soft, hard and mixed plaques, by using multinomial Logistic regression. In addition, the risk of carotid plaques by categories of age and PP (grouped by the median) would be evaluated. Subgroup analyses were carried out using multivariable Logistic models stratified by traditional clinical risk factors, including sex, smoking, BMI, BP, atherogenic dyslipidemia and reduced renal function, and were summarized via forest plots. An interaction term within each subgroup was also included using the likelihood ratio test. Finally, for sensitivity analysis, we excluded patients with a history of stroke, TIA, taking lipid-lowering or not taking antihypertensive medications and used Logistic models to repeat the analysis to verify the robustness of the results.
Data analysis was performed using the SPSS 25.0 software (SPSS Inc., Chicago, IL, USA) and SAS 9.4 software (SAS Institute Inc., Cary, NC, USA). All statistical tests were two-tailed, with P < 0.05 considered statistically significant.
Results
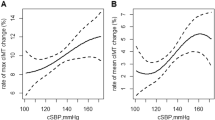
The characteristics of the study population according to tertiles of PP were presented in Table 1. Among the studied 1371 participants, the mean age was 70.21 ± 5.99 years, and 776 (56.60%) were female. Participants in the highest tertiles of PP were more likely to be female, relatively older and have a higher SBP. The proportions of family history of stroke and smoking were more frequent in participants with the lowest tertiles of PP. The median PP was 71 mmHg (interquartile range, 60–81 mmHg). As shown in Fig. 2, the prevalence of carotid plaques increased with the increase in PP levels, regardless of sex. Higher prevalence was more often observed in older groups. A statistical difference in the distribution of carotid plaques prevalence on PP tertiles was found only among participants with SBP ≥ 150 mmHg and DBP < 90 mmHg (Supplemental Fig. 2).
In Tables 2, 639 (46.6%) participants were found to have carotid plaques. Logistic regression analyses showed significant associations between PP and carotid plaques. In the crude model (model 1), compared with the participant with PP < 65 mmHg (T1), 65–79 mmHg (T2) and ≥ 79 mmHg (T3) both had a higher risk of carotid plaques [unadjusted odds ratio (OR): 1.440; 95% confidence interval (CI): 1.010–1.884 and OR: 2.510; 95% CI: 1.926–3.270, respectively.] After fully adjusted (model 2), compared with the T1 group, T3 remained a positive association with the risk of carotid plaques (OR: 2.061; 95% CI: 1.547–2.745), no significant differences were observed in the T2 group (OR: 1.269; 95% CI: 0.960–1.677). When considering PP as per 1-SD mmHg, there was also a positive association in both models. As shown in Tables 3 and 408 (63.8%) of participants with carotid plaques have multiple plaques. Compared with the T1 group, T3 increased by 136.6% risk of having multiple plaques than no plaques (OR: 2.366; 95% CI: 1.700–3.293). In addition, 218 (34.1%) have soft plaques. Compared with the T1 group, T3 increased by 208.5% risk of having soft plaques than no plaques (OR: 3.085; 95% CI: 1.423–6.687).
As shown in Fig. 3, Logistic regression with restricted cubic splines was conducted to model and visualize the association between predicted PP and carotid plaques. When considering the PP at 60 mmHg as the reference point [20], the risk of carotid plaques increased slowly until 60 mmHg of predicted PP and then started to grow rapidly (P nonlinearity = 0.039). There was a substantial increase in the associated risk after 60 mmHg.
Dose-response relationship between pulse pressure levels and the risk of carotid plaque. Red lines indicated hazard ratios, the green line indicated the reference line (the median of pulse pressure as a reference point), and the dashed lines indicated 95% CI from restricted cubic spline regression. ORs were calculated using Logistic regression analysis after adjustments for a propensity score. OR odds ratio; CI confidence interval
In Fig. 4, we further examined the association of carotid plaques based on categories of age and PP. The increase in age and PP were associated with the risk of carotid plaques. Compared to the participants with aged < 65 years and PP < 71 mmHg, those aged 80 ≥ years and PP ≥ 71 mmHg had the highest risk of carotid plaques (OR: 4.804; 95% CI: 2.367–9.749).
Figure 5 presented the carotid plaques for the PP (per 1-SD mmHg) in various subgroups. The association between PP levels and carotid plaques risk was stronger in atherogenic dyslipidemia and participants without reduced renal function. There were no significant interactions of sex, smoking, BMI, BP groups and diabetes.
Associations between pulse pressure and the risk of carotid plaque in patients stratified by the sex, BMI, smoking, blood pressure, diabetes, atherogenic dyslipidemia and reduced renal function. Data were adjusted for a propensity score other than variables for stratification. ORs per 1-SD mmHg increase in pulse pressure. The P-value for interaction was calculated. OR odds ratio; CI confidence interval. Other abbreviations as in Table 1
In the sensitivity analysis, participants with a history of stroke, TIA, taking lipid-lowering or not taking antihypertensive medications were excluded. PP (per 1-SD mmHg or grouped by tertiles) was still related to a higher risk of carotid plaques after adjusting for multiple covariates (Supplemental Table 1).
Discussion
In this cross-sectional study, we used 1371 community-dwelling old adults with uncontrolled hypertension aged ≥ 60 years to evaluate the association between PP and carotid plaques. The results revealed a positive association between PP and the risk of carotid plaques. We found that the risk of carotid plaques significantly increased with PP levels after the inflection point (60 mmHg). The association between the PP and carotid plaques was closer among patients with multiple and soft carotid plaques. Our results shed light on the association between PP and the risk of carotid plaques and tried to provide a potential threshold for intervention to reduce the arteriosclerosis burden in old adults with uncontrolled hypertension.
Previous results of studies on the relationship between PP and carotid plaques risk in old adults or hypertensive patients have been limited and challenging to replicate. Consistent with our results, Dong et al. found that elevated PP was an independent risk factor for carotid plaques in participants aged ≥ 60 years without histories of stroke or cardiovascular disease in rural China [11]. PP was also associated with stenosis of the asymptomatic carotid artery and angiographic ulceration of the symptomatic carotid artery [21], as well as promoting an increase in plaque number [12], independently of antihypertensive medications use. Among old adults with uncontrolled hypertension, PP was strongly associated with multiple plaques and soft plaques. Soft plaques, also called vulnerable plaques, are more likely to be unstable and dislodged. In 323 hypertensive patients with a mean age of 61.7 ± 14 years, high SBP and low DBP were associated with uncontrolled hypertension. High PP was associated with increased common artery intima-media thickness but not with carotid or iliofemoral plaques [22]. However, the present study only evaluated the association between PP and carotid plaques, its number and type, but not with plaque in other sites, such as coronary, iliofemoral intracranial or extracranial arteries, and common artery intima-media thickness size. These inevitably limited the comparison of our findings with others. In addition, participants in the above study were recruited from the Department of Cardiology, and at least 30% were diagnosed with cardiovascular disease. In contrast, 1355 (98.83%) of the community residents with uncontrolled hypertension taking at least one antihypertensive medication were enrolled, and the positive association remained significant in participants without a history of stroke or TIA. A longitudinal study from Norway found that PP could not effectively predict plaque burden, echolucent plaques, and carotid intima-media thickness. A single time-point measurement of PP at age 40 was not associated with atherosclerosis two decades later [23]. The effects of PP and its dynamic changes on atherosclerosis need to be further studied in essential/ secondary hypertensive patients. Therefore, generalizability or potential selection bias should be considered when interpreting our results.
This study found a cut-off value for the association between PP and carotid plaques. Previous studies showed that both cardiac target organ damage and cardiovascular prognosis were related to increased PP, suggesting a specific cut-off (> 60 mmHg) for participants aged ≥ 60 years [24, 25]. Another large longitudinal cohort study of patients with a high risk of atherosclerosis indicated a PP ≥ 70 mmHg increased risk of cardiovascular events [26]. The results of the study on high-risk participants were consistent with the results we reported. Previous studies indicated that PP and SBP were superior to DBP in predicting intima-media thickening and early plaques [27]. High PP levels could induce endothelial dysfunction to promote carotid plaques [28]. However, PP may be a maker of widespread atherosclerosis rather than a cause, whereas stiffness of the arterial tree leads to an increase in SBP and a decrease in DBP [29]. In addition, aging was closely related to high PP, mainly due to age-related alterations in collagen to elastin ratio and disruption of elastic fibres [30]. Increased PP was associated with the markers of subclinical cardiovascular disease, and this association was stronger with aging in a cohort study [31]. Our results found that advanced age and high PP were closely related to the risk of carotid plaques. After the age of 60 years, DBP decreased with age, while SBP increased [32]. Isolated systolic hypertension was the primary type of hypertension in old adults [33]. However, the absence of a significant interaction between hypertension type and PP with carotid plaques may reflect a lack of statistical power to detect effect in this not large sample, despite the association between PP and carotid plaques being larger among those with SDP ≥ 150 and DBP < 90 mmHg than those with SDP < 150 and DBP ≥ 90 mmHg. In cross-sectional studies of patients with kidney disease [34, 35], PP was weakly associated with large vessel calcification and atherosclerosis. The authors indicated that the influence of hypervolemia may overwhelm the impact of atherosclerosis and arterial calcification on PP. Patients with chronic kidney disease tend to have retention of water and sodium, which led to blood volume expansion [36]. Consistently, our results found that the association between PP and carotid plaques risk was weaker in participants with reduced renal function.
The main strength of this study was that we presented a general description of carotid plaques prevalence in old adults with uncontrolled hypertension and comprehensively investigated the associations between PP and the risk of carotid plaques from a community screening. To our knowledge, this association has not previously been reported in detail in such a high-risk group. However, several limitations should be noted here. First, this study was a cross-sectional study and was not randomized. The ability to clarify a cause-effect of PP and carotid plaques in old adults with uncontrolled hypertension was limited. Therefore, it is necessary to continue to record the BP and the occurrence of atherosclerosis in these old adults. Second, this study was restricted to a not large urban sample in southeast China, which is a potential limiting factor to the generalization of our results under the social background of aging and poor blood pressure control. Third, carotid artery imaging results were measured by one certified sonographer. Apart from the fact that we could not assess inter-rater variability for the measurements, residual bias from diagnostic suspicion was required to be considered. Finally, we did not collect some potential confounders, such as inflammatory biomarkers, dietary habits, and herb use. The absence of these data may compromise the validity of the present results.
Conclusions
In conclusion, in a community screening of old adults with uncontrolled hypertension, an elevated PP was associated with the risk of carotid plaques as well as promoting multiple plaques and unstable plaques, independently of lifestyle characteristics and other cardiovascular risk factors. Our findings may guide community health workers to help old adults control the PP at an optimal level to reduce the risk of carotid plaques. Future prospective studies with a large sample are necessary to verify our findings and to provide a more precise intervention threshold of PP in old adults with uncontrolled hypertension.
Data availability
The data sets used during the current study are available from the corresponding author on reasonable request.
Abbreviations
- TIA:
-
Transient ischemic attack
- PP:
-
Pulse pressure
- SBP:
-
Systolic blood pressure
- DBP:
-
Diastolic blood pressure
- BMI:
-
Body mass index
- TC:
-
Total cholesterol
- LDLC:
-
Low density lipoprotein cholesterol
- HDLC:
-
High density lipoprotein cholesterol
- SD:
-
Standard deviation
- OR:
-
Odds ratio
- CI:
-
Confidence interval
- OLS:
-
Ordinary least squares
- VIF:
-
Variance inflation factor
References
Lu J, Lu Y, Wang X, et al. Prevalence, awareness, treatment, and control of hypertension in China: data from 1.7 million adults in a population-based screening study (China PEACE million persons project). Lancet. 2017;390(10112):2549–58.
Lewington S, Lacey B, Clarke R, et al. The Burden of Hypertension and Associated Risk for Cardiovascular Mortality in China. JAMA Intern Med. 2016;176(4):524–32.
Armas Rojas N, Dobell E, Lacey B, et al. Burden of hypertension and associated risks for cardiovascular mortality in Cuba: a prospective cohort study. Lancet Public Health. 2019;4(2):e107–15.
Fang EF, Xie C, Schenkel JA et al. A research agenda for ageing in China in the 21st century (2nd edition): Focusing on basic and translational research, long-term care, policy and social networks. Ageing Res Rev, 2020. 64: p. 101174.
Naqvi TZ, Lee MS. Carotid intima-media thickness and plaque in cardiovascular risk assessment. JACC Cardiovasc Imaging. 2014;7(10):1025–38.
Hollander M, Bots ML, Del Sol AI, et al. Carotid plaques increase the risk of stroke and subtypes of cerebral infarction in asymptomatic elderly: the Rotterdam study. Circulation. 2002;105(24):2872–7.
Jashari F, Ibrahimi P, Bajraktari G, et al. Carotid plaque echogenicity predicts cerebrovascular symptoms: a systematic review and meta-analysis. Eur J Neurol. 2016;23(7):1241–7.
Clarke R, Du H, Kurmi O, et al. Burden of carotid artery atherosclerosis in Chinese adults: implications for future risk of cardiovascular diseases. Eur J Prev Cardiol. 2017;24(6):647–56.
Song P, Fang Z, Wang H, et al. Global and regional prevalence, burden, and risk factors for carotid atherosclerosis: a systematic review, meta-analysis, and modelling study. Lancet Glob Health. 2020;8(5):e721–9.
Swaminathan RV, Alexander KP. Pulse pressure and vascular risk in the elderly: associations and clinical implications. Am J Geriatr Cardiol, 2006. 15(4): p. 226 – 32; quiz 133-4.
Dong S, Gao J, Wang C, et al. Association between blood pressure components and the presence of carotid plaque among adults aged 45 years and older: a population-based cross-sectional study in rural China. Blood Press Monit. 2019;24(5):234–40.
Song X, Zhao X, Liebeskind DS, et al. Associations between systemic blood pressure parameters and intraplaque hemorrhage in symptomatic intracranial atherosclerosis: a high-resolution MRI-based study. Hypertens Res. 2020;43(7):688–95.
Cheng G, Fan F, Zhang Y, et al. Different associations between blood pressure indices and carotid artery damages in a community-based population of China. J Hum Hypertens. 2016;30(12):750–4.
Cai A, Mo Y, Zhang Y, et al. Relationship of pulse pressure index and carotid intima-media thickness in hypertensive adults. Clin Exp Hypertens. 2015;37(4):267–70.
James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507–20.
Friedewald WT, Levy RI, Fredrickson DS, Fredrickson. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502.
Castañer O, Pintó X, Subirana I, et al. Remnant cholesterol, not LDL cholesterol, is Associated With Incident Cardiovascular Disease. J Am Coll Cardiol. 2020;76(23):2712–24.
Zhang L, Wang F, Wang L, et al. Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet. 2012;379(9818):815–22.
Touboul PJ, Hennerici MG, Meairs S et al. Mannheim carotid intima-media thickness and plaque consensus (2004-2006-2011). An update on behalf of the advisory board of the 3rd, 4th and 5th watching the risk symposia, at the 13th, 15th and 20th European Stroke Conferences, Mannheim, Germany, 2004, Brussels, Belgium, 2006, and Hamburg, Germany, 2011. Cerebrovasc Dis, 2012. 34(4): pp. 290-6.
Liu M, Wang JH, Wang SS, He Y. Blood pressure level, hypertension prevalence and control status in oldest old in China. Chin J Endemiology. 2019;40(3):290–5.
Lovett JK, Howard SC, Rothwell PM, Howard, Rothwell PM. Pulse pressure is independently associated with carotid plaque ulceration. J Hypertens. 2003;21(9):1669–76.
Tartière JM, Kesri L, Safar H, et al. Association between pulse pressure, carotid intima-media thickness and carotid and/or iliofemoral plaque in hypertensive patients. J Hum Hypertens. 2004;18(5):325–31.
Vigen T, Ihle-Hansen H, Lyngbakken MN, et al. Blood pressure at age 40 predicts carotid atherosclerosis two decades later: data from the Akershus Cardiac Examination 1950 study. J Hypertens. 2019;37(10):1982–90.
Franklin SS, Larson MG, Khan SA, et al. Does the relation of blood pressure to coronary heart disease risk change with aging? The Framingham Heart Study. Circulation. 2001;103(9):1245–9.
Anderson RJ, Bahn GD, Emanuele NV, et al. Blood pressure and pulse pressure effects on renal outcomes in the Veterans affairs Diabetes Trial (VADT). Diabetes Care. 2014;37(10):2782–8.
Selvaraj S, Steg PG, Elbez Y, et al. Pulse pressure and risk for Cardiovascular events in patients with atherothrombosis: from the REACH Registry. J Am Coll Cardiol. 2016;67(4):392–403.
Högberg D, Kragsterman B, Björck M, et al. Carotid artery atherosclerosis among 65-year-old Swedish men-a population-based screening study. Eur J Vasc Endovasc Surg. 2014;48(1):5–10.
Ryan SM, Waack BJ, Weno BL, et al. Increases in pulse pressure impair acetylcholine-induced vascular relaxation. Am J Physiol. 1995;268(1 Pt 2):H359–63.
Safar ME, Blacher J, Mourad JJ, et al. Stiffness of carotid artery wall material and blood pressure in humans: application to antihypertensive therapy and stroke prevention. Stroke. 2000;31(3):782–90.
Franklin SS, Gustin W 4th, Wong ND, et al. Hemodynamic patterns of age-related changes in blood pressure. Framingham Heart Study Circulation. 1997;96(1):308–15.
Winston GJ, Palmas W, Lima J, et al. Pulse pressure and subclinical cardiovascular disease in the multi-ethnic study of atherosclerosis. Am J Hypertens. 2013;26(5):636–42.
Duprez DA. Systolic hypertension in the elderly: addressing an unmet need. Am J Med. 2008;121(3):179–e1843.
Liu X, Rodriguez CJ, Wang K. Prevalence and trends of isolated systolic hypertension among untreated adults in the United States. J Am Soc Hypertens. 2015;9(3):197–205.
Kim JK, Song YR, Kim MG, et al. Clinical significance of subclinical carotid atherosclerosis and its relationship with echocardiographic parameters in non-diabetic chronic kidney disease patients. BMC Cardiovasc Disord. 2013;13:96.
Yazici H, Oflaz H, Pusuroglu H, et al. Hypervolemia rather than arterial calcification and extracoronary atherosclerosis is the main determinant of pulse pressure in hemodialysis patients. Int Urol Nephrol. 2012;44(4):1203–10.
Andersen MJ, Agarwal R. Etiology and management of hypertension in chronic kidney disease. Med Clin North Am. 2005;89(3):525–47.
Acknowledgements
All of the investigators and staff members were gratefully acknowledged. Thanks to all the enthusiastic participants.
Funding
This work was supported by the Hangzhou General Research Project on Agriculture and Social Development [Grant # 20201203B207] and the Hangzhou Health Science and Technology Projects [Grant # A20230326].
Author information
Authors and Affiliations
Contributions
Jue Xu and Caixia Jiang foresaw this work and designed the overall approach. Zhecong Yu wrote the paper. Haifeng Yang, Biqi Shou, and Zongxue Cheng contributed to the data analysis. All authors joined in the critical discussion and edited the paper.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki and approved by the Hangzhou Center for Disease Control and Prevention Research Ethics Committee (Ethics approval number: 2023-5). Informed consent was obtained from all individual participants included in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yu, Z., Yang, H., Shou, B. et al. Association between pulse pressure and carotid plaques in old adults with uncontrolled hypertension: results from a community-based screening in Hangzhou, China. BMC Cardiovasc Disord 24, 249 (2024). https://doi.org/10.1186/s12872-024-03914-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-024-03914-y