Abstract
Background
Migraine is a leading cause of disability worldwide. Several retrospective studies have suggested that the closure of the Patent Foramen Ovale (PFO) may provide relief from migraines. However, three randomized controlled trials did not meet their primary endpoints regarding migraine cessation, reduction in monthly migraine days, and responder rates.
Methods
The SPRING study is a multicenter, prospective, randomized, and open-label trial designed to compare the effectiveness and safety of PFO closure versus medication in the relief of migraines. The primary endpoint is the total cessation of migraines, as recorded in patient headache diaries during the follow-up period. Additional diagnostic tools include echocardiography with agitated saline contrast, transcranial Doppler, and routine laboratory measurements.
Conclusion
The SPRING trial aims to assess the effectiveness and safety of PFO closure versus medication in mitigating migraines in real-world settings. (Clinical Trails ID: NCT04946734).
Similar content being viewed by others
Background
Migraine is recognized the leading cause of disability among individuals under 50, as reported by the World Health Organization's Global Burden of Diseases Study, 2019 [1]. Despite its prevalence, advancements in migraine management have remained relatively stagnant since the 1990s [1]. While effective symptomatic and preventive treatments exist, none can substantially attenuate migraine symptoms. Over the past two decades, numerous studies have indicated an association between migraine risk and the presence of Patent Foramen Ovale (PFO) and its right-left shunt grade, suggesting PFO as a potential causative factor [2,3,4,5]. Proposed mechanisms for this correlation include hypoxia, changes in cerebral autonomic regulation, and venous thromboembolism due to right-left shunting and in situ thrombosis within the PFO [6,7,8,9]. Furthermore, multiple studies have demonstrated that device closure of PFO may result in migraine relief [10,11,12,13]. However, none of the three randomized controlled trials (RCTs) on PFO closure achieved their primary endpoints, including migraine cessation, reduction in monthly migraine days, and responder rates [14,15,16]. Consequently, current guidelines provide minimal endorsement for PFO closure as a treatment for migraine due to inconsistencies between RCTs and retrospective studies. Recognizing the need for a comprehensive evaluation of PFO closure's efficacy in preventing migraines, we have launched a real-world design RCT. This trial aims to compare the effectiveness and safety of PFO closure versus medication in alleviating migraines.
Study design
The Spring study is a multicenter, randomized and open-label trail comparing the effectivity and safety of PFO closure vs medicine in alleviating migraine, with a follow-up period of 12 months (ClinicalTrials.gov ID NCT04946734). The trial is performed in 21 centers in China and has been approved by all local ethics committees. Our hypothesis posits that, compared to medication alone (clopidogrel 75 mg q.d. and aspirin 100 mg q.d.), PFO closure accompanied by one months of clopidogrel (75 mg q.d.) and six months of Aspirin (100 mg q.d.) will be more effective in completely ceasing migraines. Patient recruitment is expected to be completed by the end of 2024, with final results anticipated in 2025.
Patients with migraine and PFO were included in this study. Participant inclusion and exclusion criteria are presented in Table 1.
Study protocol
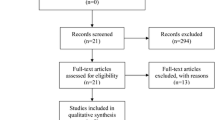
The flow chart of the study design is presented in Fig. 1. Once patients are diagnosed with migraine and PFO, a right-to-left shunt is evaluated using transcranial Doppler (TCD) and transthoracic/transesophageal echocardiography (TTE/TEE) with agitated saline contrast. Upon confirmation of the shunt and PFO, investigators will detail the study protocol to the patients. Following their agreement and the signing of the consent form, we will collect routine laboratory test results, brain imaging (CT/MR), rating scale questionnaires, and other baseline characteristics.
After a comprehensive review of inclusion and exclusion criteria, the investigators will proceed with the randomization and distribute headache diaries. Patients in the control group will be prescribed aspirin (100 mg daily) for 6 months and clopidogrel (75 mg daily) for 1 month. In contrast, patients in the treatment group will undergo percutaneous PFO closure in the catheterization laboratory.
PFO device closure
After local anesthesia, a 6-French MPA 2 catheter was inserted into the right heart system through the right femoral vein by placement of a 6-French vascular sheath. Under fluoroscopy, we measured the pressure in right atrium and pulmonary artery to exclude pulmonary hypertension related PFO. Then, we advanced the catheter through the PFO and anchor in the left inferior pulmonary vein. After accessing the size and location of the PFO, the MPA 2 catheter was exchanged for an 8-French or 9-French delivery sheath and a PFO closure device would be placed across the atrium septum under bedside TTE and fluoroscopy. After successful implement, patients were prescribed same medication of Aspirin 100 mg qd for 6 months and clopidogrel 75 mg qd for 1 month, and would be usually discharged home in the next day.
The success of the percutaneous PFO closure procedure will be defined as implantation of the PFO device without any major complication including death, stroke, or any complication that requires surgical or endovascular treatment.
Follow up
Follow-up assessments will be conducted at 1, 3, 6, and 12 months post-intervention. These will include the recording of migraine diaries, completion of questionnaires assessing migraine impact, quality of life, medication history, and any serious adverse events. At the 6 and 12-month marks, or upon reaching the primary endpoint, additional follow-up assessments will be conducted. These will consist of TTE/TEE with right heart contrast and TCD with a bubble test to re-evaluate the right-to-left shunt. When adverse events are identified during follow-up, investigators will assess the condition and severity to determine whether to terminate the trial.
Study outcomes
Primary and secondary endpoints are presents in Table 2. The outcomes of the migraine changes are adjudicated based on the headache diary data. The total days of migraine is defined as migraine frequency per month plus migraine average duration, divided by 24, round up to nearest integral. The definition of responder rate is the percentage of patients who have at least 50% reduction of monthly migraine attacks from their baseline. Other efficacy measures include migraine severity, as assessed by the HIT-6 scale [17], and quality of life alterations, determined by the SF-36v2 questionnaire [18]. These changes would be also analyzed the relationship with a successful PFO closure and residual shunt.
Data management and monitoring
All clinical data will be de-identified and stored in an electronic data capture system. The Headache Diary Review Committee, blinded to the randomization, will oversee the calculation and recording of migraine severity and frequency. The Independent Event Committee and the Data Safety Monitoring Board will ensure the integrity of the data and monitor the safety of the participants.
Sample size and statistical considerations
This was a randomized controlled study with PFO blocking group as the intervention group and drug treatment group as the control group. Migraine frequency (monthly migraine days), improvement in migraine frequency, severity (migraine scale score) and quality of life score were the outcome indicators. According to previous reports, the frequency of migraines treated with medication was 6.5 ± 2.4 days/month. The migraine HIT-6 score was 66.2 ± 5.1; After treatment, the number of migraine attacks per month can be reduced by 25%; SF12 quality of life score increased by 1.2 ± 6.9 points after treatment. The frequency of migraine was expected to decrease by 0.6 days in PFO occlusion group. Migraine scale HIT-6 score decreased by 1.5 points; After treatment, the number of migraine attacks per month can be reduced by 50%; SF12 quality of life score improved by 4.2 points after treatment. The test level was set as bilateral α = 0.05, and the assurance was 80%. PASS 15.0 software was used to calculate the required sample size of 113, 183, 60 and 156 cases in each group based on the above four observation indicators. Taking the maximum sample size and considering 20% of the cases of loss and refusal to visit, the final requirement was 220 cases in the PFO blocking group and 220 cases in the drug treatment group.
The primary endpoints will be analyzed across different analysis sets: Intention-to-Treat, per-protocol, and as-treated, with the primary analysis set being Intention-to-Treat. Categorical variables will be described using rates and composition ratios. Group differences in variables such as migraine termination, sociodemographic factors, migraine incidence rate, changes in migraine characteristics (with or without aura and their changes), effective closure rates, or residual leakage will be compared using the chi-square test. Continuous variables will be characterized based on their data distribution, utilizing means, standard deviations, medians, and interquartile ranges. Group differences in variables such as sociodemographic factors, severity and frequency of migraine attacks, and quality of life scores derived from the SF-36v2 questionnaire will be compared using t-tests or the Wilcoxon test.
Additional analysis will involve covariance analysis to account for factors significantly influencing the observed indicators. Different centers will be treated as random effects, and generalized linear models will be employed to further control for the potential impact of center effects. Stratified analysis will be performed based on factors such as gender and age. Predefined subgroups will include migraine with or without aura. Survival function estimation will be conducted using the Kaplan–Meier method. Relevant covariates will be controlled for using the Cox proportional hazards model, and truncated data will be analyzed accordingly.
For all analyses, a two-tailed P value < 0.05 was used as the criterion for statistical significance. Analyses were performed using SPSS 22 software (IBM Corp., Armonk, NY, USA).
Discussion
This study is a multicenter, randomized trail evaluating the effectivity of PFO closure in alleviating migraine. Previously, the three RCT set their primary endpoints as complete migraine cessation (MIST, 2008) [16], monthly migraine days reduction (PRIMA, 2016) [15] and responder rate (PREMIUM, 2017) [14]. Although some non-randomized studies suggest the efficacy of PFO closure in migraine relief, none of the aforementioned RCTs achieved their set endpoints. In the 2008 MIST study, patients in the closure group were implanted with the STARFlex Technology device, which has been reported with a higher complication. In addition, a relatively shorter follow up time might also underpower the PFO closure effect. While in the PRIMA and PREMIUM study, both use Amplatzer PFO Occluder and had a 12 months follow up time, but still had an in-neglectable frequency of residual shunt (12% & 11% V.S. 6%). Additionally, small sample sizes might have contributed to these studies not meeting their endpoints. Notably, a pooled analysis of PRIMA and PREMIUM demonstrated a significant mean reduction in monthly migraine days, attacks, and an increase in complete cessation rates in the PFO closure groups [19]. A significant discrepancy between RCTs and retrospective studies might stem from the meticulous screening of patients in RCTs for refractoriness to migraine prevention medication, which may not align with real-world clinical practice. To solve these problems, our SPRING study employs a more recent generation of PFO closure devices and strategy, along with a larger sample size. Unlike previous approaches, we are not mandating preventive medication before closure, aiming to assess if patients can achieve migraine relief post-intervention, thus aligning more closely with the scenario when patients first seek medical advice for migraines.
Conclusion
This multicenter, randomized trial aims to compare the efficacy and safety of PFO closure versus medication in alleviating migraines. Given the inconclusive evidence from previous RCTs, which failed to reach their primary endpoints, our study is designed to provide a robust evaluation of the effectiveness of these treatments in a real-world setting.
Availability of data and materials
No datasets were generated or analysed during the current study.
References
Diseases GBD, Injuries C. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1204–22.
Takagi H, Umemoto T, Group A. A meta-analysis of case-control studies of the association of migraine and patent foramen ovale. J Cardiol. 2016;67(6):493–503.
Tang Y, Peng A, Peng B, He S, Zhao X, Zhu Y, Lai W, Song T, Chen L. Association between patent foramen ovale and migraine without aura: a community-based cross-sectional study in China. BMJ Open. 2022;12(3):e056937.
De Giuli V, Grassi M, Locatelli M, Gamba M, Morotti A, Bonacina S, Mazzoleni V, Pezzini D, Magoni M, Monastero R, et al. Cardiac sources of cerebral embolism in people with migraine. Eur J Neurol. 2021;28(2):516–24.
Martinez-Majander N, Artto V, Ylikotila P, von Sarnowski B, Waje-Andreassen U, Yesilot N, Zedde M, Huhtakangas J, Numminen H, Jakala P, et al. Association between migraine and cryptogenic ischemic stroke in young adults. Ann Neurol. 2021;89(2):242–53.
Yan C, Li H, Wang C, Yu H, Guo T, Wan L, Yundan P, Wang L, Fang W. Frequency and size of in situ thrombus within patent foramen ovale. Stroke. 2023;54(5):1205–13.
Dong B, Lu Y, He S, Li B, Li Y, Lai Q, Li W, Ji S, Chen Y, Dai L, Chen L. Multisite and multitimepoint proteomics reveal that patent foramen ovale closure improves migraine and epilepsy by reducing right‐to‐left shunt‐induced hypoxia. MedComm (2020). 2023;4(4):e334.
Guo ZN, Qu Y, Gao Y, Xing Y, Ma H, Liu J, Guo YZ, Chang J, Zhang P, Jin H, et al. Changes in cerebral autoregulation, stroke-related blood biomarkers, and autonomic regulation after patent foramen ovale closure in severe migraine patients. CNS Neurosci Ther. 2023;29(10):3031–42.
Khasiyev F, Arsava EM, Topcuoglu MA. Cerebral vasomotor reactivity in migraine: effect of patent foramen ovale and aerogenic microembolism. Neurol Res. 2020;42(9):795–804.
Zhao E, Xie H, Zhang Y. A nomogram for the prediction of cessation of migraine among patients with patent foramen ovale after percutaneous closure. Front Neurol. 2020;11:593074.
Ben-Assa E, Rengifo-Moreno P, Al-Bawardy R, Kolte D, Cigarroa R, Cruz-Gonzalez I, Sakhuja R, Elmariah S, Pomerantsev E, Vaina LM, et al. Effect of residual interatrial shunt on migraine burden after transcatheter closure of patent foramen ovale. JACC Cardiovasc Interv. 2020;13(3):293–302.
Qi Y, Zhang Y, Luo X, Cheng G, Du Y, Liu R, Xie H, Cheng Y, Guo Y, Luo G. Efficacy of patent foramen ovale closure for treating migraine: a prospective follow-up study. J Investig Med. 2021;69(1):7–12.
He YD, Yan XL, Qin C, Zhang P, Guo ZN, Yang Y. Transcatheter patent foramen ovale closure is effective in alleviating migraine in a 5-year follow-up. Front Neurol. 2019;10:1224.
Tobis JM, Charles A, Silberstein SD, Sorensen S, Maini B, Horwitz PA, Gurley JC. Percutaneous closure of patent foramen ovale in patients with migraine: the PREMIUM Trial. J Am Coll Cardiol. 2017;70(22):2766–74.
Mattle HP, Evers S, Hildick-Smith D, Becker WJ, Baumgartner H, Chataway J, Gawel M, Gobel H, Heinze A, Horlick E, et al. Percutaneous closure of patent foramen ovale in migraine with aura, a randomized controlled trial. Eur Heart J. 2016;37(26):2029–36.
Dowson A, Mullen MJ, Peatfield R, Muir K, Khan AA, Wells C, Lipscombe SL, Rees T, De Giovanni JV, Morrison WL, et al. Migraine intervention with STARFlex Technology (MIST) trial: a prospective, multicenter, double-blind, sham-controlled trial to evaluate the effectiveness of patent foramen ovale closure with STARFlex septal repair implant to resolve refractory migraine headache. Circulation. 2008;117(11):1397–404.
Rendas-Baum R, Yang M, Varon SF, Bloudek LM, DeGryse RE, Kosinski M. Validation of the Headache Impact Test (HIT-6) in patients with chronic migraine. Health Qual Life Outcomes. 2014;12:117.
Ware JE Jr., Sherbourne CD: The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483.
Mojadidi MK, Kumar P, Mahmoud AN, Elgendy IY, Shapiro H, West B, Charles AC, Mattle HP, Sorensen S, Meier B, et al. Pooled analysis of PFO occluder device trials in patients with PFO and migraine. J Am Coll Cardiol. 2021;77(6):667–76.
Acknowledgments
SPRING Investigators
Zhang Caojin, M.D. 1, Chen Weibin, M.D.4, Guo Tao, M.D. 5, Cui Tongtao, M.D.5, Wang Zhanhang, M.D. 6, Xiong Zhaojun, M.D. 7, Gao Hanhua, M.D.8, Lai Junxing, M.D.9, Yuan Jie, M.D.10, Chen Jianying, M.D.11, Wang Xiaodong, M.D.12, Liu Wei, M.D.13, Zhang Hongwei, M.D.14, Zhang Gangcheng, M.D.15, Zheng Xuan, M.D., PhD 15, Shen Qunshan, M.D.16, Chen Xiaobin, M.D.17, Xie Dujiang, M.D.18, Zhang Wenqi, M.D.19, Wang Zhongchao, M.D.20, Wei Wenbin, M.D.21, Zhou Yang, M.D.22, Zhang Wei, M.D.23 (in no particular order)
1Guangdong Provincial People's Hospital, Guangdong Academy of Medical Sciences, Guangzhou, Guangdong, China
4Xiamen Cardiovascular Hospital Xiamen University, Xiamen, Fujian, China
5The First Affiliated Hospital of Guangzhou Medical University, Guangzhou, Guangdong, China
6Guangdong Sanjiu Brain Hospital, Guangzhou, Guangdong, China
7The Third Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong, China
8Huizhou First Hospital, Huizhou, Guangdong, China
9Jiangmen Central Hospital, Jiangmen, Guangdong, China
10Shenzhen People's Hospital, Shenzhen, Guangdong, China
11Affiliated Hospital of Guangdong Medical University, Zhanjiang, Guangdong, China
12The First People's Hospital of Nanning, Nanning, Guangxi, China
13Shangqiu First People's Hospital, Shangqiu, Henan, China
14Hubei Huiyi Medical Group Cardiovascular Center, Enshi, Hubei, China
15Zhongnan Hospital of Wuhan University, Wuhan, Hubei, China
16Wuhan Asia Heart Hospital, Wuhan, Hubei, China
17Xiangya Hospital of Central South University, Changsha, Hunan, China
18Nanjing First Hospital, Nanjing, Jiangsu, China
19China-Japan Union Hospital of Jilin University, Changchun, Jilin, China
20Shanxi Cardiovascular Hospital, Taiyuan, Shanxi, China
21The Eighth Affiliated Hospital, Sun Yat-sen University, Shenzhen, Guangdong, China
22The people's hospital of Leshan, Leshan, Sichuan, China
23Tianjin Chest Hospital, Tianjin, China
Funding
This trail was supported by the Chinese Society of Cardiology [CSCF2020B02].
Author information
Authors and Affiliations
Consortia
Contributions
YZY wrote the main manuscript. LHZ provided expertise on the echocardiography details, while LDL and WXM offered statistical guidance. ZCJ is the principle investigator of this trail and design the protocol. All authors reviewed and contributed to the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Research Ethics Committee of Guangdong General Hospital, Guangdong, China on April 20, 2021 (No. KY-H-2020–454-04). Written informed consent was obtained from all participants before any study procedures were initiated.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zi-yang, Y., Hezhi, L., Dongling, L. et al. Rationale and design of the SPRING trail: effectivity and safety of Pfo closuRe vs medIcine in alleviatiNg migraine, a multicenter, randomized and open-label trail. BMC Cardiovasc Disord 24, 198 (2024). https://doi.org/10.1186/s12872-024-03866-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-024-03866-3