Abstract
Background
While coronary artery calcification (CAC) is recognized as a reliable marker for coronary atherosclerosis, the relationship between the concentration of C-reactive protein (CRP) and the incidence and progression of CAC remains controversial.
Method
PubMed, Embase, Web of Science, and Scopus were systematically searched to identify relevant observational studies until October 2023. The methodological quality of the included studies was evaluated using the Newcastle-Ottawa Scale (NOS). A random-effects meta-analysis was employed to calculate pooled odd ratios (OR) and corresponding 95% confidence intervals, considering heterogeneity among the studies.
Results
Out of the 2545 records, 42 cross-sectional and 9 cohort studies were included in the systematic review. The meta-analysis on 12 eligible cross-sectional studies revealed no significant association between CAC and CRP [pooled OR: 1.03 (1.00, 1.06)]. Additionally, an insignificant association was found between CAC and CRP through meta-analysis on three eligible cohort studies [pooled OR: 1.05 (0.95, 1.15)] with no considerable heterogeneity across studies. Sensitivity analyses indicated that the meta-analysis models were robust. There was no evidence of publication bias.
Conclusion
Based on the meta-analysis findings, elevated levels of CRP did not emerge as a valuable prognostic maker for CAC incidence and progression prediction.
Similar content being viewed by others
Introduction
Approximately 19 million fatalities worldwide in 2020 were linked to cardiovascular disease (CVD), representing a rise of 18.7% compared to the numbers recorded in 2010 [1]. As a result, preventing CVD is crucial for maintaining public health, as CVD substantially affects nations with high medical costs and economic burdens [2, 3]. Atherosclerosis is caused by lipid buildup in large arteries. Inflammatory pathways are activated as a result of endothelial dysfunction, leading to plaque enlargement, necrotic core formation, and plaque calcification [4] The dysfunctional endothelial cells promote lipid infiltration and leukocyte adhesion, further exacerbating the inflammation and contributing to the progression of the condition [5,6,7,8,9]. CRP is an inflammation-associated protein [10] and is primarily synthesized in hepatocytes. It exhibits a brief lifespan of approximately 18–20 h [11, 12]. In non-inflammatory states, CRP release rate is slow but secreted rapidly after an increase in inflammatory cytokines level, most notably Interleukin 6 (IL-6), Interleukin 1 (IL-1), and Tumor necrosis factor- α (TNF-α) [13, 14]. The American Heart Association has recommended the inclusion of high-sensitivity C-reactive protein (hs-CRP) in the overall assessment of cardiovascular risk [15]. Over time, this concept has evolved, and in 2010, the American College of Cardiology Foundation advised that evaluating CRP levels is a sensible approach for individuals at intermediate risk of cardiovascular events [16]. Coronary artery calcification (CAC) is an advanced feature and indicator of atherosclerosis [17]. It performs better than any cardiovascular risk factor and adds predictive value to traditional equations [18]. CAC can happen via the active or passive route; inflammation is critical for both courses [19]. CAC score is usually calculated by Agatston’s method, and it has been widely used to evaluate the risk of an acute coronary event [20, 21] It is generally known that inflammation causes atherosclerotic plaque instability and promotes the expression of osteogenic factors, which leads to the differentiation of local cells into osteoblast-like cells and calcification. Subsequently, anti-inflammatory factors reduce the expression of osteogenic factors [22,23,24]. Although inflammation and calcification may seem to work together, the truth is more complicated. Lee H et al. showed that hs-CRP is significantly associated with CAC progression among clinical parameters. Still, the association disappears after adjusting for traditional risk factors [3]. Oh et al. discovered a significant difference in hs-CRP levels among high-risk subjects with CAC scores > 300 [25]. On the other hand, Zeb et al. reported an insignificant association between hs-CRP concentrations and the CAC progression [26]. This study intends to explore the clinical evidence concerning the prognostic significance of CRP as an inflammatory index in assessing the likelihood of CAC development.
Methods
Registration and protocol
The present systematic review and meta-analysis followed the meta-analysis of observational studies in epidemiology (MOOSE) guidelines (Supplementary Appendix 1). The protocol was registered in the International Prospective Register of Systematic Reviews PROSPERO (CRD42021242397).
Databases and search strategy
Until October 2023, four databases, including Pubmed, Scopus, Embase, and Web of Science, were systematically explored to find relevant studies. The population, exposure, comparison, and outcome (PECO) protocol was followed, with participants with symptomatic or asymptomatic CVD as the target population. CRP as the exposure, and CAC as the primary or secondary outcome. Our search was conducted with the MeSH terms of Coronary Artery Calcification, C-Reactive Protein, Coronary Artery Disease, Acute Coronary Syndrome, Ischemic heart disease, ST-elevation Myocardial Infraction, Non-ST Elevation myocardial infraction, Stable Angina, Non-Stable Angina, and Myocardial Infraction. The retrieved studies’ reference lists were examined to find any missing pieces.
Eligibility criteria and study selection
The two investigators assessed the titles and abstracts of the retrieved records before searching the full text of documents for those intended to fulfill our inclusion criteria. In addition, we requested papers by email twice where the full text was not available, and in case we did not receive an answer, the article was excluded. A third author was enlisted to create certain conclusions to settle disagreements. We included observational studies, including case-control, cohort, and cross-sectional studies, without considering language or publication date. Human research was assessed to determine the relationship between CRP and CAC scores in patients with CVD. The following were excluded: technical reports, conference papers, case reports, animal research, and review articles. Studies involving participants with diseases other than CVD were also excluded.
Data abstraction and quality assessment
Two reviewers extracted data independently and sorted by first author, year, country, age, population, sample size, effect size (OR, RR, HR) with confidence intervals (CI), study outcome, and results. NOS was used to assess the study’s methodological quality (Supplementary Appendix 2) [27]. A maximum of 9 points were awarded based on selection (4 points), comparability (2 points), and outcome/exposure (3 points) assessment. Scores under 4 indicate low quality, 4–6 moderate, and more than 6 indicate good study quality. All included studies were assessed by two investigators and verified by a third member. Discrepancies in score allocation were resolved by consensus.
Statistical analysis
We examined the relationship between CRP and CAC using odds ratio (OR) to estimate the effect size. We conducted a random-effects meta-analysis employing the Der-Simonian and Laird method to calculate the combined OR and 95% CIs. Using a random-effects meta-analysis allowed us to consider conceptual and clinical heterogeneity among the studies. We used a forest plot to illustrate the ORs and their respective 95% confidence intervals. To assess the heterogeneity across the studies, we used the I2 statistics, where an I2 value of 50% or higher suggests significant heterogeneity. Additionally, we employed Cochran’s Q statistic with a significance threshold of P < 0.10 to indicate heterogeneity among the studies.
To test the consistency of the results and robustness of the pooled estimates, we conducted sensitivity analysis systematically, removing specific studies or groups of studies (Supplementary Appendix 3). Furthermore, we performed a subgroup meta-analysis to examine the relationship between CAC and CRP based on how CRP was measured, whether in milligrams per deciliter (mg/dl) or Logarithm of milligrams per Liter (log mg/L) and gender. To assess the publication bias, we visually examined funnel plots where we plotted log odds ratios (log ORs) against their standard errors to measure study precision. Also, we conducted Egger’s regression asymmetry test and Begg’s adjusted rank correlation test to evaluate publication bias statistically. We used two-tailed statistical tests, and the significance level was considered less than 0.10. All statistical analyses were conducted using Stata version 14 software developed by STATA Corp. in College Station, TX, USA.
Results
Results of the literature search
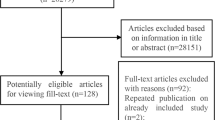
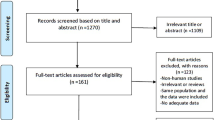
Initially, our systematic review yielded 2545 articles. Among these, 968 were duplicates. After screening the 1577 titles and abstracts, 1446 were excluded, leaving 131 records for full-text examination. Among these 131 articles, 86 reports were discarded as they were reviews, conference reports, or irrelevant to our study; we also collected six articles from looking into references of other papers slightly similar to our work. Finally, 51 studies were chosen for data extraction when they met our inclusion and exclusion criteria. There were 42 cross-sectional studes and 9 cohort studies among the 51 included studes (Fig. 1).
Descriptive characteristics of the studies
Four cohort studies were conducted in the USA; two used the Multi-Ethnic Study of Atherosclerosis (MESA) database for their research, one used the Early Identification of Atherosclerosis by Noninvasive Imaging Research (EISNER) data, and one utilized Prospective Army Coronary Calcium (PACC) participants. Two studies were done in Asia and three in Europe. Combined, 13,305 patients were studied. The study also included 42 cross-sectional studies with a total of 124,606 participants. Thirty-six research involved both genders; five included only men [28,29,30,31,32], and one recruited just women [33]. This analysis comprised 42 cross-sectional studies, 14 of which were conducted in the United States, four in South America and Brazil, three in Europe, and 21 in Asia. The data sources of population-based cross-sectional studies included participants from MESA, the Brazilian Longitudinal Study of Adult Health, the ERA-JUMP study, the Study of Inherited Risk of Coronary Atherosclerosis (SIRCA), the Genetic Network of Arteriopathy (GENOA) study, the Framingham Heart Study (FHS), the Dallas Heart Study (DHS), the Shiga Epidemiological Study of Subclinical Atherosclerosis (SESSA), the Mediators of Atherosclerosis in South Asians Living in America study (MASALA), The Swedish CArdioPulmonary bioimage Study (SCAPIS), and the Study of Women’s Health Across the Nation (SWAN).
Association between CRP and CAC in cohort studies
Systematic review findings
Characteristics of cohort studies are presented in Table 1. In a longitudinal study, Zeb et al. investigated the association of inflammatory markers and lipoprotein particle subclasses with the incidence and progression of CAC. Their findings showed no association between hs-CRP and CAC incidents. [RR = 0.97 [0.92, 1.02] p < 0.001] [26]. In 2021, research on subjects with CVD risk factors or atypical chest pain revealed a significant association between hs-CRP ≥ 2.0 mg/L and CAC progression, HR:1.18 (1.01–1.63), but only in the univariate analysis [3]. Intriguingly, Nankeolyar et al. found an inverse association between hs-CRP and CAC measured at baseline but failed to produce an association with CAC when measured continuously or categorically [34]. Diederichsen et al. explored the association between 15 biomarkers and CAC in a population of 1006 particiants and followed up with a second computed tomography scan after five years but couldn’t find an independent association between CAC growth and CRP [35]. In a study by Gauss et al., the association of inflammatory markers with CAC and epicardial fat volume was investigated. Consistent with previous studies, they exhibit no association between hs-CRP and baseline levels or progression of CAC [36]. A study performed on the MESA cohort with low Framingham risk scores pursued whether novel markers that don’t require ionizing radiation can forecast the progression of CAC. In univariate and age-adjusted models, CRP was associated with only increased CAC, not incident CAC. However, this association became non-significant in multivariable models adjusted for age and other traditional CVD risk factors in low-risk participants [37]. In an investigation by Hamer et al. on 466 healthy men and women from the Whitehall II epidemiological cohort, no significant association was found between CAC and CRP after adjusting for follow-up time, pre-stress cortisol level, employment grade, statin use, resting blood pressure, fibrinogen, high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C), body mass index (BMI), and smoking [38]. Lastly, Taylor et al. reported that CRP levels were similar in univariate analysis in those with and without CAC progression [39].
Meta-analysis findings
Seven cohort studies were primarily excluded from the meta-analysis because their data could not be included in the quantitative synthesis. These studies employed various statistical methodologies to report the relationship between CRP and CAC, and they analyzed different variables in their studies. The meta-analysis on three cohorts showed no significant association between CAC and hs-CRP [OR: 1.05(0.95, 1.15)] (Fig. 2). We also presented the results of two studies that measured hs-CRP concentration on a Log mg/L scale [OR: 1.03(0.99, 1.07)]. No considerable heterogeneity was found across studies. Sensitivity analysis demonstrated the consistency of the results, indicating that the meta-analysis model was robust.
Meta-analysis of cohort studies investigating the association between CRP and CAC separated by CRP measurement scale. Diamond represents the summary odds ratio (pooled OR) estimate, and its width shows a corresponding 95% CI with random effects estimate. The square’s size and central point reflect the study-specific statistical weight (inverse of the variance), and the point estimate of the OR and horizontal line reflects the corresponding 95% CI of the study. I2 test and Cochran’s Q statistic were used to assess the statistical heterogeneity (P < 0.10) across studies
Association between CRP and CAC in cross-sectional studies
Systematic review findings
Out of 42 included cross-sectional studies in our paper, five studies [25, 41,42,43,44] reported a positive association between CAC and CRP levels. Another five studies [45,46,47,48,49] also reported a positive association but only in particular populations, for example, in very elderly or diabetic women or women of African-American origins. The remaining thirty-one studies declared no association between CAC and CRP levels (Table 2).
A population-based cross-sectional study recruiting 3753 participants revealed no increase in the levels of hs-CRP or GlycA as examined in higher CAC cut-points [53]. An investigation using 1827 individuals with a mean age of 66.96 ± 9.2 from the MESA cohort study demonstrated that in a fully adjusted model, including lipid and glucose measures, the connection between CRP, IL-6, and fibrinogen with CAC persisted as statistically significant. To elaborate, an increase of one standard deviation (SD) in CRP was linked to around a 5% higher prevalence of CAC (95% CI: 1.03, 1.08) [43]. As reported by Okwuosa et al., no statistical association was indicated between CRP levels and CAC [37]. Arps et al., in a study on MESA participants, found a simple association between higher CAC scores and co-morbid atherosclerotic cardiovascular disease (ASCVD) risk factors such as old age, male sex, and white race. Still, CRP was not one of these risk factors [52]. Using 6745 participants of MESA, Kummuri et al. exhibited a strong positive correlation with an increase in CAC score in all anthropometric measurements, except for BMI. However, hs-CRP did not show a significant distribution pattern in this context [59]. Sorensen et al. reported that the likelihood of falling into a higher category of CAC was not associated with increased levels of hs-CRP [62]. Sung et al., in the multivariable fully-adjusted regression analysis, nitoced that individuals with high CRP concentrations and low-level HDL-C are more prone to have CAC score > 0 compared to a group with low levels of CRP and high-level HDL-C [45]. Furthermore, Rhee et al. found no correlation between any groups of increasing CAC categories and hs-CRP [64]. Qasim et al., in a model for diabetic women, adjusted for age, race, medication, metabolic syndrome, Framingham risk score, and BMI, observed a significant association between CAC and CRP. This relationship was attenuated in non-diabetic women, and there was no such association in diabetic or non-diabetic men [47]. Utilizing MESA participants, although in the age, sex, and ethnicity-adjusted model, an increased risk of 13% was reported in those in the highest quartile of CRP for CAC, adding traditional coronary heart disease risk factors demonstrated insignificant association between CAC and CRP [69]. Khera et al., in a multivariable analysis adjusted for traditional CVD risk factors, estrogen, and statin medication use, revealed no significant association between CRP levels and CAC [71]. Recently, a study recruiting 25,408 SCAPIS cohort participants from Sweden demonstrated a significant association between high hs-CRP and CAC by univariate analysis; however, after stepwise adjustments, especially after adjusting for BMI, the association attenuated and wasn’t meaningful anymore [50].
Meta-analysis findings
Overall, twelve cross-sectional studies were included in the meta-analysis, and all of them showed no statistically significant association between CAC and CRP (Fig. 3) [OR: 1.03 (1.00, 1.06)].
We also evaluated these twelve studies based on CAC cut-offs (Fig. 4) and sex (Fig. 5). When stratified according to CAC thresholds, no notable association of CAC > 0 [OR: 1.04(0.99, 1.09)] and CAC > 10 [OR: 1.02(1.00, 1.04)] with CRP was observed. Additionally, upon analysis separated by gender, elevated CRP levels showed an insignificant relationship between CAC and CRP in men [OR: 0.88(0.75, 1.02)] and women [OR: 0.91(0.61, 1.37)]. These preliminary findings warrant further validation through more rigorous research methodologies and longitudinal cohort studies. Additionally, Heterogeneity among cross-sectional studies was not found. Sensitivity analysis demonstrated consistent results, so the meta-analysis model was robust. There was no evidence of publication bias visually and statistically (P = 0.62 for Begg’s adjusted rank correlation test and P = 0.45 for Egger’s regression asymmetry test) (Fig. 6).
Begg’s funnel plot for assessing the presence of publication bias. The logarithm of the odds ratio was plotted against the precision of the study (P = 0.61 for Egger’s regression asymmetry test). There was no evidence of publication bias statistically (P = 0.15 for Egger’s regression asymmetry test)
Discussion
Our systematic review and meta-analysis examined 15 observational studies, including 12 cross-sectional and three cohort studies, to describe the relationship between CRP and CAC. The majority of the studies have stated no association between CRP and CAC. Nevertheless, several studies indicated a statistically significant correlation between CRP and CAC, whether in univariate or multivariable analysis. Moreover, our results showed no significant role of CRP in risk stratification for CAC score, supporting a different pathophysiology approach of CRP and CAC development. Therefore, additional prospective cohort studies must be conducted, including well-designed methodologies and recruiting healthy individuals. To validate the association, these studies should examine the relationship between CRP and CAC at baseline and follow-up stages.
Measuring CAC can be costly and involve radiation exposure. Recent studies showed the potential role of novel biomarkers as a prognostic value in predicting CAC score [75,76,77,78,79], suggesting novel pharmacological targets in reducing CAC burden.
The recent guidelines from the American Heart Association emphasize the importance of performing CAC testing for predicting cardiovascular events and stratifying CVD risk. This underscores the significance of CAC as a diagnostic marker in CVD [80,81,82,83,84].
CRP is a useful marker in assessing inflammation because it rapidly increases in concentration following a stimulus, reaching its peak around two days later, and takes several days to return to baseline levels, indicating the duration and intensity of the inflammatory response [85,86,87,88,89,90].
Local vascular inflammation is linked to calcification. It’s widely recognized that inflammation within atherosclerotic lesions plays a significant role in triggering the rupture of these plaques [23].
Local factors, including inflammatory cytokines stimulate the transformation of vascular smooth muscle cells (VSMCs) into osteogenic cells. On the other hand, unsaturated fatty acids like eicosapentaenoic acid can counteract this process by reducing the expression of factors related to bone formation [24]. Clinical evidence has established a direct connection between vascular inflammation and the development of calcification. Interestingly, positron emission tomography (PET) reveals that vascular inflammation tends to occur before calcification becomes visible through standard tomography [91]. Furthermore, research findings suggest that inflammation in the vascular system can fluctuate over time, and each episode of acute inflammation might contribute to the progression of calcification. Importantly, from a clinical standpoint, it’s unclear whether calcification can trigger inflammation [19].
Our nine included cohort studies found no significant association between CAC and CRP except for one, Lee H et al. In a retrospective observational cohort study, they investigated 1015 Korean subjects who underwent CAC scoring by computed tomography. During 39 months of follow-up, they found a significant association between CAC progression and CRP in men and not women. However, the association was not adjusted for additional risk factors. Also, male subjects were the predominant study population (80.6%), a big difference from the general composition of society [3].
In thirty-two of our included cross-sectional studies, researchers found no association between CRP and CAC. In the remaining ten studies, however, Oh et al. investigated 456 participants from South Korea; subjects with a CAC score of ≥ 300 agatston units, in univariate regression analysis, found log hs-CRP to be significantly associated with the high CAC group [OR: 2.812 (1.600,4.942)]. Nevertheless, the study with cross-sectional design and no adjustment for conventional CVD risk factors, failed to verify the association between CAC and CRP [25]. In another study, Fu et al. found an association between CAC and hs-CRP in multiple linear regression analysis. However, they only included subjects with complaints of chest pain in a cross-sectional manner, making it challenging to establish a clear causal link [42]. In the research involving individuals without any evident cardiovascular issues, Wang et al. observed a connection between CRP levels and the presence of CAC in both male and female participants. This association remained significant even after considering age, individual conventional risk factors, and Framingham risk score. Nonetheelss, CRP was measured 4 to 8 years before conducting electron beam computed tomography; it’s possible that the relationship between CRP and the presence of CAC was influenced by the progression of atherosclerosis in individuals who had elevated CRP levels [44]. The remaining cross-sectional studies found an association between CAC and CRP, but the association can not be generalized to the community. In this regard, Qasim et al. found a significant link between CAC and CRP levels in type 2 diabetic women. Conversely, there was no evident relationship between CRP and CAC in diabetic or nondiabetic men [47]. Sung et al. found CAC score > 0 to be significantly associated with high CRP concentration levels in individuals with low HDL-C [45]. Additionally, Quagli et al. discovered a distinct and independent relationship between CRP levels and the presence of CAC in older adults (aged 80 years or older) without underlying health issues [46].
Based on the pooled estimation in our study, sub-group analysis by gender and CAC threshold revealed no significant relationship between CAC and CRP. To confidently address this issue, however, more research is needed, specifically focusing on gender-specific analysis.
Research conducted in animal models has yielded mixed findings. In a study by Paul et al., they observed a correlation between the presence of CRP in atherosclerotic plaques and enlargement in their size [92]. On the other hand, Tennent et al., who worked with mice expressing transgenic human CRP, found no indications of heightened atherosclerotic buildup, increased complexity of these lesions, or occurrences of spontaneous thrombosis and plaque-related fatalities [93].
The current systematic review comes with certain limitations. CRP concentrations were measured by traditional and highly sensitive methods, which can detect low levels of chronic inflammation suited for detecting CVD. This might lead to the heterogenicity in results.
The variability in recorded CAC cutoff values has led to heterogenicity in findings among different studies.
The studies exhibited differences in several aspects, including the ratio of males to females, the existence of other cardiovascular risk factors among participants, the presence of documented CVD, the duration of the follow-up period, and the average age of the subjects.
Therefore, conducting additional primary studies, particularly prospective cohort studies with larger sample sizes, while considering the shortcomings of previous research could help confirm the link between CRP and CAC score.
Conclusion
The present meta-analysis findings indicate no significant association between high CRP levels and CAC in cohort and cross-sectional studies. Therefore, it appears that CRP concentration cannot be used as a predictor to determine the necessity of CAC measurement and screening. Hence, it seems imperative to carry out large-scale prospective cohort studies. These investigations ought to employ robust methodologies and entail recruiting individuals in good health. To validate the observed association, the focus should be on investigating the correlation between CRP and CAC during both the initial assessment and follow-up phases.
Availability of data and materials
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, et al. Heart disease and stroke statistics—2022 update: a report from the American Heart Association. Circulation. 2022;145(8):e153–639.
Eun Y, Lee SN, Song SW, Kim HN, Kim SH, Lee YA, et al. Fat-to-muscle ratio: a New Indicator for Coronary Artery Disease in healthy adults. Int J Med Sci. 2021;18(16):3738–43.
Lee H, Park HE, Yoon JW, Choi SY. Clinical significance of body fat distribution in coronary artery calcification progression in Korean population. Diabetes Metabolism J. 2021;45(2):219–30.
Jebari-Benslaiman S, Galicia-García U, Larrea-Sebal A, Olaetxea JR, Alloza I, Vandenbroeck K, et al. Pathophysiology of atherosclerosis. Int J Mol Sci. 2022;23(6):3346.
Verma S, Buchanan MR, Anderson TJ. Endothelial function testing as a biomarker of vascular disease. Circulation. 2003;108(17):2054–9.
Verma S, Anderson TJ. Fundamentals of endothelial function for the clinical cardiologist. Circulation. 2002;105(5):546–9.
Teh YC, Ding JL, Ng LG, Chong SZ. Capturing the fantastic voyage of monocytes through time and space. Front Immunol. 2019;10:834.
Gerhardt T, Ley K. Monocyte trafficking across the vessel wall. Cardiovascular Res. 2015;107(3):321–30.
Gerrity RG. The role of the monocyte in atherogenesis: I. Transition of blood-borne monocytes into foam cells in fatty lesions. Am J Pathol. 1981;103(2):181.
Tillett WS, Francis T Jr. Serological reactions in pneumonia with a non-protein somatic fraction of pneumococcus. J Exp Med. 1930;52(4):561.
Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation. 2003;107(3):363–9.
Calabró P, Willerson JT, Yeh ET. Inflammatory cytokines stimulated C-reactive protein production by human coronary artery smooth muscle cells. Circulation. 2003;108(16):1930–2.
Du Clos TW, Mold C. C-reactive protein: an activator of innate immunity and a modulator of adaptive immunity. Immunol Res. 2004;30:261–77.
Zhang D, Sun M, Samols D, Kushner I. STAT3 participates in Transcriptional activation of the C-reactive protein gene by Interleukin-6 (∗). J Biol Chem. 1996;271(16):9503–9.
Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO III, Criqui M, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107(3):499–511.
Greenland P, Alpert JS, Beller GA, Benjamin EJ, Budoff MJ, Fayad ZA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines developed in collaboration with the American Society of Echocardiography, American Society of Nuclear Cardiology, Society of Atherosclerosis Imaging and Prevention, Society for Cardiovascular Angiography and interventions, Society of Cardiovascular Computed Tomography, and Society for Cardiovascular Magnetic Resonance. J Am Coll Cardiol. 2010;56(25):e50–103.
Demer LL, Tintut Y. Vascular calcification: pathobiology of a multifaceted disease. Circulation. 2008;117(22):2938–48.
Greenland P, Blaha MJ, Budoff MJ, Erbel R, Watson KE. Coronary calcium score and cardiovascular risk. J Am Coll Cardiol. 2018;72(4):434–47.
Panh L, Lairez O, Ruidavets J-B, Galinier M, Carrié D, Ferrières J. Coronary artery calcification: from crystal to plaque rupture. Arch Cardiovasc Dis. 2017;110(10):550–61.
Mark DB, Berman DS, Budoff MJ, Carr JJ, Gerber TC, Hecht HS, ACCF/ACR/AHA/NASCI/SAIP/SCAI, et al. SCCT 2010 Expert Consensus Document on Coronary computed Tomographic Angiography: a report of the American College of Cardiology Foundation Task Force on Expert Consensus documents. J Am Coll Cardiol. 2010;55(23):2663–99.
Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15(4):827–32.
New SE, Goettsch C, Aikawa M, Marchini JF, Shibasaki M, Yabusaki K, et al. Macrophage-derived matrix vesicles: an alternative novel mechanism for microcalcification in atherosclerotic plaques. Circul Res. 2013;113(1):72–7.
Libby P. Mechanisms of acute coronary syndromes and their implications for therapy. N Engl J Med. 2013;368:2004–13.
Kageyama A, Matsui H, Ohta M, Sambuichi K, Kawano H, Notsu T, et al. Palmitic acid induces osteoblastic differentiation in vascular smooth muscle cells through ACSL3 and NF-κB, novel targets of Eicosapentaenoic Acid. PLoS ONE. 2013;8(6):e68197.
Oh J, Park S, Yu HT, Chang HJ, Lee SH, Kang SM, Choi D. Lack of superiority for Soluble ST2 over high sensitive C-Reactive protein in Predicting High Risk Coronary Artery Calcium score in a community cohort. Yonsei Med J. 2016;57(6):1347–53.
Zeb I, Jorgensen NW, Blumenthal RS, Burke GL, Lloyd-Jones D, Blaha MJ, et al. Association of inflammatory markers and lipoprotein particle subclasses with progression of coronary artery calcium: the multi-ethnic study of atherosclerosis. Atherosclerosis. 2021;339:27–34.
Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. In: The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2000.
Lukito AA, Bakri S, Kabo P, Wijaya A. The mechanism of coronary artery calcification in centrally obese non-diabetic men: study on the interaction of leptin, free leptin index, adiponectin, hs-C reactive protein, bone morphogenetic protein-2 and matrix Gla Protein. Mol Cell Biomedical Sci. 2020;4(2):88–93.
Kimani C, Kadota A, Miura K, Fujiyoshi A, Zaid M, Kadowaki S, et al. Differences between coronary artery calcification and aortic artery calcification in relation to cardiovascular disease risk factors in Japanese men. J Atheroscler Thromb. 2019;26(5):452–64.
Nagasawa SY, Ohkubo T, Masaki K, Barinas-Mitchell E, Miura K, Seto TB, et al. Associations between inflammatory markers and subclinical atherosclerosis in middle-aged white, Japanese-American and Japanese men: the ERA-JUMP study. J Atheroscler Thromb. 2015;22(6):590–8.
Zhang ZY, Bian LQ, Kim SJ, Zhou CC, Choi YH. Inverse Relation of Total Serum Bilirubin to coronary artery calcification score detected by Multidetector computed tomography in males. Clin Cardiol. 2012;35(5):301–6.
Zhang Z, Bian L, Choi Y. Serum uric acid: a marker of metabolic syndrome and subclinical atherosclerosis in Korean men. Angiology. 2012;63(6):420–8.
Wang NC, Matthews KA, Barinas-Mitchell EJM, Chang CCH, El Khoudary SR. Inflammatory/hemostatic biomarkers and coronary artery calcification in midlife women of African-American and white race/ethnicity: the study of women’s Health across the Nation (SWAN) heart study. Menopause-the J North Am Menopause Soc. 2016;23(6):653–61.
Nandkeolyar S, Naqvi A, Fan W, Sharma A, Rana JS, Rozanski A, et al. Utility of novel serum biomarkers to predict subclinical atherosclerosis: a sub-analysis of the EISNER study. Atherosclerosis. 2019;282:80–4.
Diederichsen SZ, Grønhøj MH, Mickley H, Gerke O, Steffensen FH, Lambrechtsen J, et al. CT-Detected growth of coronary artery calcification in asymptomatic middle-aged subjects and Association with 15 biomarkers. JACC Cardiovasc Imaging. 2017;10(8):858–66.
Gauss S, Klinghammer L, Steinhoff A, Raaz-Schrauder D, Marwan M, Achenbach S, Garlichs CD. Association of systemic inflammation with epicardial fat and coronary artery calcification. Inflamm Res. 2015;64(5):313–9.
Okwuosa TM, Greenland P, Burke GL, Eng J, Cushman M, Michos ED, et al. Prediction of coronary artery calcium progression in individuals with low Framingham risk score: the multi-ethnic study of atherosclerosis. JACC: Cardiovasc Imaging. 2012;5(2):144–53.
Hamer M, Endrighi R, Venuraju SM, Lahiri A, Steptoe A. Cortisol responses to mental stress and the progression of coronary artery calcification in healthy men and women. PLoS One. 2012;7(2):e31356.
Taylor AJ, Bindeman J, Le TP, Bauer K, Byrd C, Feuerstein IM, et al. Progression of calcified coronary atherosclerosis: relationship to coronary risk factors and carotid intima-media thickness. Atherosclerosis. 2008;197(1):339–45.
Wu YJ, Mar GY, Wu MT, Wu FZ. A LASSO-Derived risk model for subclinical CAC progression in Asian Population with an initial score of Zero. Front Cardiovasc Med. 2021;7:619798.
Sajjadieh A, Arefpour A. Evaluation of the relation between serum level of high-sensitivity reactive protein (Hs-CRP) and coronary calcification with the presence of coronary artery disease in computed tomography angiography. J Isfahan Med School. 2019;37(351):673–9.
Fu K, Liu H-D, MaMuTi K, Hu D-N, Hao P. Relationship between carbohydrate antigen 125 and coronary artery calcification in patients without known coronary artery disease. Med Sci Monitor: Int Med J Experimental Clin Res. 2018;24:2873.
Hughes-Austin JM, Wassel CL, Jiménez J, Criqui MH, Ix JH, Rasmussen-Torvik LJ, et al. The relationship between adiposity-associated inflammation and coronary artery and abdominal aortic calcium differs by strata of central adiposity: the multi-ethnic study of atherosclerosis (MESA). Vasc Med. 2014;19(4):264–71.
Wang TJ, Larson MG, Levy D, Benjamin EJ, Kupka MJ, Manning WJ, et al. C-reactive protein is associated with subclinical epicardial coronary calcification in men and women - the Framingham heart study. Circulation. 2002;106(10):1189–91.
Sung KC, Cho EJ, Lim YH, Shin J, Pyun WB, Kang SM, Rosenson RS. HDL-C levels modify the association between C-reactive protein and coronary artery calcium score. Nutr Metab Cardiovasc Dis. 2014;24(11):1240–5.
Quaglia LA, Freitas WM, Soares AA, Santos RD, Nadruz W, Blaha M, et al. C-reactive protein is independently associated with coronary atherosclerosis burden among octogenarians. Aging Clin Exp Res. 2014;26:19–23.
Qasim AN, Budharaju V, Mehta NN, St Clair C, Farouk S, Braunstein S, et al. Gender differences in the association of C-reactive protein with coronary artery calcium in type-2 diabetes. Clin Endocrinol (Oxf). 2011;74(1):44–50.
Okwuosa TM, Greenland P, Lakoski SG, Ning H, Kang J, Blumenthal RS, et al. Factors associated with presence and extent of coronary calcium in those predicted to be at low risk according to Framingham risk score (from the multi-ethnic study of atherosclerosis). Am J Cardiol. 2011;107(6):879–85.
Freitas WM, Quaglia LA, Santos SN, Soares AAS, Japiassu AVT, Boaventura V, et al. Association of systemic inflammatory activity with coronary and carotid atherosclerosis in the very elderly. Atherosclerosis. 2011;216(1):212–6.
Cederström S, Lundman P, Alfredsson J, Hagström E, Ravn-Fischer A, Söderberg S, et al. Association between high-sensitivity C-reactive protein and coronary atherosclerosis in a general middle-aged population. Sci Rep. 2023;13(1):12171.
Erdöl MA, Gayretli Yayla K. Relationship between C-reactive protein to albumin ratio and coronary artery calcium score and CAD-RADS scores with coronary computed tomography angiography. Turk J Med Sci. 2021;51:2674–82.
Arps K, Rifai MA, Blaha MJ, Michos ED, Nasir K, Yeboah J, et al. Usefulness of coronary artery calcium to identify adults of sufficiently high risk for Atherothrombotic Cardiovascular events to consider low-dose Rivaroxaban Thromboprophylaxis (from MESA). Am J Cardiol. 2019;124(8):1198–206.
Harada PH, Benseñor IM, Bittencourt MS, Nasir K, Blaha MJ, Jones SR, et al. Composite acute phase glycoproteins with coronary artery calcification depends on metabolic syndrome presence - the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil). J Cardiol. 2019;73(5):408–15.
Mehta A, Patel J, Al Rifai M, Ayers CR, Neeland IJ, Kanaya AM, et al. Inflammation and coronary artery calcification in South asians: the mediators of atherosclerosis in South asians living in America (MASALA) study. Atherosclerosis. 2018;270:49–56.
Pesaro AE, Katz M, Liberman M, Pereira C, Mangueira CLP, de Carvalho AEZ, et al. Circulating osteogenic proteins are associated with coronary artery calcification and increase after myocardial infarction. PLoS ONE. 2018;13(8):e0202738.
Wang Y, Zhou BY, Zhu CG, Guo YL, Wu NQ, Qing P, et al. Distribution of ABO blood groups and coronary artery calcium. Heart Lung Circ. 2017;26(6):593–8.
Kaplan H, Thompson RC, Trumble BC, Wann LS, Allam AH, Beheim B, et al. Coronary atherosclerosis in indigenous south American tsimane: a cross-sectional cohort study. Lancet. 2017;389(10080):1730–9.
Kim BJ, Kang JG, Lee SH, Lee JY, Sung KC, Kim BS, Kang JH. Relationship of echocardiographic Epicardial Fat Thickness and Epicardial Fat volume by computed tomography with coronary artery calcification: data from the CAESAR Study. Arch Med Res. 2017;48(4):352–9.
Kommuri NV, Zalawadiya SK, Veeranna V, Kollepara SLS, Ramesh K, Briasoulis A, Afonso L. Association between various anthropometric measures of obesity and markers of subclinical atherosclerosis. Expert Rev Cardiovasc Ther. 2016;14(1):127–35.
Yu K, Min X, Lin Y, Huang Y, Huang S, Liu L, et al. Increased IL-37 concentrations in patients with arterial calcification. Clin Chim Acta. 2016;461:19–24.
Wu GY, Xu BD, Wu T, Wang XY, Wang TX, Zhang X, et al. Correlation between serum parathyroid hormone levels and coronary artery calcification in patients without renal failure. Biomedical Rep. 2016;5(5):601–6.
Sorensen MH, Gerke O, Eugen-Olsen J, Munkholm H, Lambrechtsen J, Sand NPR, et al. Soluble urokinase plasminogen activator receptor is in contrast to high-sensitive C-reactive-protein associated with coronary artery calcifications in healthy middle-aged subjects. Atherosclerosis. 2014;237(1):60–6.
Navaravong L, Steenson C, Sigurdsson G. Coronary plaque type and burden by computed tomography angiography without association to C-reactive protein. North Am J Med Sci. 2014;6(6):21–6.
Rhee EJ, Seo MH, Kim JD, Jeon WS, Park SE, Park CY, et al. Metabolic health is more closely associated with coronary artery calcification than obesity. PLoS ONE. 2013;8(9):e74564.
Tanaka M, Fukui M, Tomiyasu K, Akabame S, Nakano K, Yamasaki M, et al. Eosinophil count is positively correlated with coronary artery calcification. Hypertens Res. 2012;35(3):325–8.
Atar AI, Yilmaz OC, Akin K, Selcoki Y, Er O, Eryonucu B. Association between gamma-glutamyltransferase and coronary artery calcification. Int J Cardiol. 2013;167(4):1264–7.
Bian L-Q, Zhang Z-Y, Kim S-J, Zhou C-C, Choi Y-H. Gamma glutamyltransferase as a novel marker of coronary artery calcification in women. J Cardiovasc Med. 2012;13(11):684–90.
Raaz-Schrauder D, Klinghammer L, Baum C, Frank T, Lewczuk P, Achenbach S, et al. Association of systemic inflammation markers with the presence and extent of coronary artery calcification. Cytokine. 2012;57(2):251–7.
Jenny NS, Brown ER, Detrano R, Folsom AR, Saad MF, Shea S, et al. Associations of inflammatory markers with coronary artery calcification: results from the multi-ethnic study of atherosclerosis. Atherosclerosis. 2010;209(1):226–9.
Ramadan MM, Mahfouz EM, Gomaa GF, El-Diasty TA, Alldawi L, Ikrar T, et al. Evaluation of coronary calcium score by multidetector computed tomography in relation to endothelial function and inflammatory markers in asymptomatic individuals. Circ J. 2008;72(5):778–85.
Khera A, de Lemos JA, Peshock RM, Lo HS, Stanek HG, Murphy SA, et al. Relationship between C-reactive protein and subclinical atherosclerosis: the Dallas Heart Study. Circulation. 2006;113(1):38–43.
Huang PH, Chen LC, Leu HB, Ding PY, Chen JW, Wu TC, Lin SJ. Enhanced coronary calcification determined by electron beam CT is strongly related to endothelial dysfunction in patients with suspected coronary artery disease. Chest. 2005;128(2):810–5.
Kullo IJ, McConnell JP, Bailey KR, Kardia SL, Bielak LF, Peyser PA, et al. Relation of C-reactive protein and fibrinogen to coronary artery calcium in subjects with systemic hypertension. Am J Cardiol. 2003;92(1):56–8.
Reilly MP, Wolfe ML, Localio AR, Rader DJ. C-reactive protein and coronary artery calcification: the study of inherited risk of coronary atherosclerosis (SIRCA). Arterioscler Thromb Vasc Biol. 2003;23(10):1851–6.
Vazirian F, Sadeghi M, Wang D, Javidi Dashtbayaz R, Gholoobi A, Samadi S, Mohammadpour AH. Correlation between osteoprotegerin and coronary artery calcification in diabetic subjects: a systematic review of observational studies. BMC Cardiovasc Disord. 2023;23(1):1–10.
Vazirian F, Sadeghi M, Kelesidis T, Budoff MJ, Zandi Z, Samadi S, Mohammadpour AH. Predictive value of lipoprotein (a) in coronary artery calcification among asymptomatic cardiovascular disease subjects: a systematic review and meta-analysis. Nutr Metab Cardiovasc Dis. 2023;33(11):2055–2066.
Abedi F, Sadeghi M, Omidkhoda N, Kelesidis T, Ramezani J, Samadi S, Mohammadpour AH. HDL-cholesterol concentration and its association with coronary artery calcification: a systematic review and meta-analysis. Lipids Health Dis. 2023;22(1):1–16.
Samadi S, Sadeghi M, Dashtbayaz RJ, Nezamdoost S, Mohammadpour AH, Jomehzadeh V. Prognostic role of osteoprotegerin and risk of coronary artery calcification: a systematic review and meta-analysis. Biomark Med. 2022;17(3):171–80.
Mohammadpour AH, Shamsara J, Nazemi S, Ghadirzadeh S, Shahsavand S, Ramezani M. Evaluation of RANKL/OPG serum concentration ratio as a new biomarker for coronary artery calcification: a pilot study. Thrombosis. 2012;2012:306263.
Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice guidelines. Circulation. 2014;129(25suppl2):S1–45.
Polonsky TS, McClelland RL, Jorgensen NW, Bild DE, Burke GL, Guerci AD, Greenland P. Coronary artery calcium score and risk classification for coronary heart disease prediction. JAMA. 2010;303(16):1610–6.
Kavousi M, Elias-Smale S, Rutten JH, Leening MJ, Vliegenthart R, Verwoert GC, et al. Evaluation of newer risk markers for coronary heart disease risk classification: a cohort study. Ann Intern Med. 2012;156(6):438–44.
Kavousi M, Desai CS, Ayers C, Blumenthal RS, Budoff MJ, Mahabadi A-A, et al. Prevalence and prognostic implications of coronary artery calcification in low-risk women: a meta-analysis. JAMA. 2016;316(20):2126–34.
Budoff MJ, Young R, Burke G, Jeffrey Carr J, Detrano RC, Folsom AR, et al. Ten-year association of coronary artery calcium with atherosclerotic cardiovascular disease (ASCVD) events: the multi-ethnic study of atherosclerosis (MESA). Eur Heart J. 2018;39(25):2401–8.
Aguiar FJ, Ferreira-Júnior M, Sales MM, Cruz-Neto LM, Fonseca LA, Sumita NM, et al. Proteína C reativa: aplicações clínicas e propostas para utilização racional. Rev Assoc Méd Bras. 2013;59(1):85–92.
da Silva SH, Moresco RN. Biomarcadores cardíacos na avaliação da síndrome coronariana aguda. Scientia Med. 2011;21(3):132–142.
Du Clos TW, Mold C. Pentraxins (CRP, SAP) in the process of complement activation and clearance of apoptotic bodies through Fcγ receptors. Curr Opin Organ Transplant. 2011;16(1):15.
Pitthan E, Martins OMO, Barbisan JN. Novos biomarcadores inflamatórios E De disfunção Endotelial: predição de risco cardiovascular. Rev AMRIGS. 2014;58(1):69–77.
Rader DJ. Inflammatory markers of coronary risk. N Engl J Med; 2000; 19;343(16)1179–82.
Ridker PM. High-sensitivity C-reactive protein, inflammation, and cardiovascular risk: from concept to clinical practice to clinical benefit. Am Heart J. 2004;148(1):S19–26.
Abdelbaky A, Corsini E, Figueroa AL, Fontanez S, Subramanian S, Ferencik M, et al. Focal arterial inflammation precedes subsequent calcification in the same location: a longitudinal FDG-PET/CT study. Circ Cardiovasc Imaging. 2013;6(5):747–54.
Paul A, Ko KW, Li L, Yechoor V, McCrory MA, Szalai AJ, Chan L. C-reactive protein accelerates the progression of atherosclerosis in apolipoprotein E–deficient mice. Circulation. 2004;109(5):647–55.
Tennent GA, Hutchinson WL, Kahan MC, Hirschfield GM, Gallimore JR, Lewin J, et al. Transgenic human CRP is not pro-atherogenic, pro-atherothrombotic or pro-inflammatory in apoE–/– mice. Atherosclerosis. 2008;196(1):248–55.
Acknowledgements
Not applicable.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Amirhossein Tajani and Navid Omidkhoda independently screened and extracted the data from the articles. The first draft of the manuscript was written by Seyed Amirhossein Tajani, Navid Omid khoda and Amir Hooshang Mohammadpour. Masoumeh Sadeghi performed the statistical analysis and revised the manuscript. Sara Samadi and Vahid Jomehzadeh resolved any discrepancies during screening and data extraction and revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Tajani, A., Sadeghi, M., Omidkhoda, N. et al. The association between C-reactive protein and coronary artery calcification: a systematic review and meta-analysis. BMC Cardiovasc Disord 24, 204 (2024). https://doi.org/10.1186/s12872-024-03856-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-024-03856-5