Abstract
Background
Hyperuricemia and low level of high-density lipoprotein cholesterol (HDL-C) are both risk factors for coronary artery disease (CAD). The uric acid to HDL-C ratio (UHR) has recently been identified as a new inflammatory and metabolic biomarker. However, the relationship between the UHR and coronary culprit plaques has not been fully investigated in patients with acute coronary syndrome (ACS).
Methods
A total of 346 patients with ACS were enrolled in this study. Culprit lesion characteristics were assessed by optical coherence tomography (OCT). Logistic regression and linear correlation analyses were performed to assess the association between the UHR and culprit plaques. The predictive value of the UHR was investigated by receiver operating characteristic (ROC) curve analysis.
Results
The percentages of typical culprit plaques, including ruptures, erosions and thrombi, were greater in the high-UHR subgroup than those in the low-UHR subgroup. A positive relationship was also found between the UHR and diameter stenosis (r = 0.160, P = 0.003) and between the UHR and area stenosis (r = 0.145, P = 0.007). The UHR was found to be independently associated with plaque rupture, erosion and thrombus. Furthermore, ROC analysis suggested that the UHR had a better predictive value than low-density lipoprotein cholesterol.
Conclusions
An elevated UHR level was independently related to the occurrence rate of culprit plaques. The UHR is a simple and easily acquired parameter for detecting culprit plaques in patients with ACS.
Similar content being viewed by others
Background
Uric acid (UA) is the end product of purine nucleotide metabolism. Previous studies have shown that hyperuricemia is positively correlated with coronary artery disease (CAD), hypertension, atrial fibrillation, heart failure, and even diabetes mellitus (DM) [1]. Moreover, hyperuricemia independently predicts an increased risk of major cardiovascular adverse events (MACEs) and mortality not only in elderly patients but also in young adults with acute coronary syndrome (ACS) [2, 3]. Elevated serum UA promotes an inflammatory response, oxidative stress, and endothelial dysfunction in patients with ACS [4]. Hyperuricemia is also associated with the progression of atherosclerosis [5].
High-density lipoprotein cholesterol (HDL-C) is traditionally considered the “good cholesterol” because it removes and transports excess cholesterol from the periphery to the liver [6]. Low HDL-C levels are a risk factor for atherosclerotic CAD [7]. Recently, the UA to HDL-C ratio (UHR) has been recognized as a biomarker for metabolic disorders and inflammation in many diseases, including CAD [8, 9]. Culprit plaques, such as plaque rupture, erosion and thrombus, are fundamental to the pathogenesis of ACS [10]. However, the association between the UHR and culprit plaques has not been explored. In the present study, we aimed to investigate the association between UHR and culprit plaques by optical coherence tomography (OCT) in patients with ACS.
Patients and methods
Study population
Patients with ACS who underwent percutaneous coronary intervention (PCI) were retrospectively recruited from the Second Affiliated Hospital of Xi’an Jiaotong University between March 2019 and September 2021. OCT was performed to identify the culprit lesions of the target vessels during PCI. The main exclusion criteria were: cardiogenic shock, end-stage renal disease, serious liver dysfunction, allergy to contrast media, left main disease, chronic total occlusion, and heavily calcified or tortuous vessels, which can render OCT examination difficult. Patients with poor OCT imaging quality and in-stent restenosis were also excluded.
The diagnosis and classification of ACS, which includes ST-elevation myocardial infarction (STEMI), non-ST-elevation myocardial infarction (NSTEMI) and unstable angina, were based on clinical symptoms, electrocardiogram findings and laboratory test results according to the guidelines. Blood samples were collected in tubes containing 0.1% EDTA after the patients were hospitalized. The baseline characteristics, medical history and personal habits of the patients were obtained. The laboratory parameters were tested according to standard protocols. The UHR was calculated as the ratio of UA (mg/dl) to HDL-C (mg/dl) and is expressed as a percentage (%).
This retrospective study was conducted in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for cross-sectional studies. The study protocol was approved by the Ethics Committee of the Second Affiliated Hospital of Xi’an Jiaotong University, and this study was also conducted according to the principles of the Helsinki Declaration II. All patients provided written informed consent.
Culprit lesion identification
The transradial access was the preferred approach for coronary angiography and PCI. The culprit vessel was identified according to the results of angiography and electrocardiogram, and an OCT examination was conducted to determine the culprit lesion in the target vessels. All patients received standard dual antiplatelet medication according to guidelines, that is, initial oral treatment with aspirin (300 mg) and ticagrelor (180 mg) or clopidogrel (300 mg) followed by aspirin (100 mg) daily and ticagrelor (90 mg) twice daily or clopidogrel daily (75 mg). An intravascular infusion of 100 IU/kg of heparin was administered prior to PCI. After the drug-eluted stent was implanted, glycoprotein IIb/IIIa receptor inhibitors were administered intravenously when necessary.
OCT image acquisition
Intravascular OCT images were obtained using a commercial frequency-domain OCT system (OCT Mobile Dragonfly, St. Jude Medical/Abbott, St. Paul, MN, USA). An OCT image catheter was advanced distal to the culprit lesion after flushing with 0.2 mg of nitroglycerin. A virtually blood-free environment was created by continuous injection of the contrast agent, and the OCT catheter was immediately pulled back manually or automatically at a rate of 20 mm/s. The total length of the OCT pullback was 75 mm during every single examination.
OCT image analysis
All OCT images were analyzed on a St. Jude OCT Review Workstation by three independent physicians who were blinded to the study conditions. The culprit plaque was classified according to established criteria as we previously described [11]. Fibrous plaque features with homogeneous and highly backscattering regions. Lipid-rich plaques have low signal regions and diffuse borders. The fibrous cap thickness (FCT) of fibrous plaques or lipid-rich plaques was measured in triplicate, and the average value was calculated. The lipid arc was measured at the largest lipid region across the entire lesion. Thin-cap fibroatheroma (TCFA) was defined as a lipid-rich plaque with the thinnest part of the fibrous cap (< 65 μm). Plaque rupture was defined as disruption of the fibrous cap with cavity formation. Plaque erosion was identified as a thrombus attached to an irregular but intact luminal surface. Thrombi were classified as white, red, or mixed type according to the light scattering rate. Calcification was characterized by sharply delineated and low-backscattering heterogeneous regions. The arc, depth, and length of the calcification lesion were measured three times. Macrophages were defined as signal-rich regions with heterogeneous backward shadows. Cholesterol crystals were recognized as linear and highly backscattering regions within a plaque, especially in fibrous plaques. The minimal lumen area (MLA) and minimal lumen diameter (MLD) were automatically calculated along the length of the culprit lesion. The percentage of area of stenosis (AS) and diameter of stenosis (DS) were also recorded at the same time.
Statistical analysis
Continuous data are presented as the mean ± standard deviation (SD). One-way analysis of variance (ANOVA) was used to compare groups. Categorical data are presented as frequencies and percentages (%), and the results were compared using the chi-square test or Fisher’s exact test. Correlations among variables were measured using the Spearman’s rank test or Pearson’s test. Univariate and multivariate logistic regression analyses were conducted to determine associations between culprit lesions and target variables. The predictive values of variables were examined by receiver operating characteristic (ROC) curve analysis and the area under the curve (AUC) was calculated. A two-tailed P < 0.05 was considered to indicate statistical significance. Analyses were conducted using SPSS 26.0 statistical software (SPSS Inc, Chicago, IL, USA).
Results
Baseline demographic and clinical characteristics
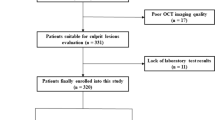
Between March 2019 and September 2021, a total of 562 patients with ACS were recruited and underwent OCT examination. A total of 216 patients were excluded due to different reasons for exclusion. Finally, 346 patients with ACS were enrolled in this study and the samples were divided into two groups by the median of UHR (13.64%). The flow chart of this study is shown in Fig. 1. The average age of the patients was 58.83 years, and 76.9% of them were male. The percentages of patients with different types of ACS were compared, and no significant differences were found (P = 0.103). Significant differences were found in the proportion of males, current smoking status, alcohol consumption status, age, number of smoking years, hemoglobin (Hb) level, thyroid stimulating hormone (TSH) level, triglyceride (TG) level, HDL-C level, UHR, very low-density lipoprotein cholesterol (VLDL) level, estimated glomerular filtration rate (eGFR), apolipoprotein (Apo) A1 level and lipoprotein(a) [Lp(a)] level (Table 1). Furthermore, the proximal part of the left anterior descending artery accounted for the majority of the culprit vessels.
OCT findings in different groups
Representative OCT images of plaque rupture and erosion with thrombi are shown in Fig. 2. The percentages of typical culprit plaques, including rupture, erosion and thrombus, were greater in the high-UHR group than those in the low-UHR group (Table 1). The percentage of plaque rupture was also greater than that of plaque erosion and calcified nodules (25.4% versus 17.7% versus 3.5%). Furthermore, the percentage of white thrombi was greater than that of red or mixed thrombi. No significant differences were found in other plaque types, such as fibrous plaques, lipid plaques, cholesterol crystals and macrophages.
Correlation of the UHR with plaque parameters
To further investigate the associations between the UHR and plaque parameters, a series of correlation studies were performed (Table 2). Among these parameters, white blood cells (r = 0.143, P = 0.008), Hb (r = 0.151, P = 0.005), and TG (r = 0.298, P < 0.001) was positively correlated with the UHR. A positive relationship was also found between UHR and DS (r = 0.160, P = 0.003) and between the UHR and AS (r = 0.145, P = 0.007). In addition, the age (r = -0.311, P < 0.001), eGFR (r = -0.161, P = 0.003), TC (r = -0.106, P = 0.049), Apo A1 (r = -0.556, P < 0.001), Lp(a) (r = -0.155, P = 0.004), TSH (r = -0.152, P = 0.006) and left ventricular ejection fraction (LVEF) (r = -0.184, P = 0.001) were negatively correlated with the UHR.
Univariate and multivariate logistic regression analyses
Univariate logistic regression analysis revealed that the UHR (continuous variable or categorical variable) was closely related with plaque rupture. After adjusting for different confounding parameters, including age, sex, medical history, smoking status, alcohol consumption, statins and UA-lowering drugs in four different models, the UHR was found to be independently associated with plaque rupture. Furthermore, the UHR was divided into four quartiles in each model and multivariate logistic regression analysis was also performed. The UHR was also significantly associated with the odds of plaque rupture (Table S1). Similar results were found for erosion (Table S2) and thrombi (Table S3).
ROC curve analysis
To investigate the predictive capacity of the UHR for culprit plaques, a series of ROC curve analyses were conducted. In plaque rupture group, the AUC value for the UHR was 0.625 (95% CI: 0.560–0.690, P < 0.001), which was greater than that for LDL-C [0.568 (95% CI: 0.501–0.636, P = 0.568)] (Fig. 3A). Similar results were found for plaque erosion and thrombus. In the plaque erosion group, the AUC was 0.624 (95% CI: 0.554–0.695, P = 0.002) for UHR, and 0.530 (95% CI: 0.452–0.609, P = 0.445) for LDL-C (Fig. 3B). In the thrombus group, the AUC was 0.631 (95% CI: 0.567–0.694, P < 0.001) for the UHR, and 0.571 (95% CI: 0.504–0.638, P = 0.037) for LDL-C (Fig. 3C).
Discussion
In the present study, the relationship between the UHR and the characteristics of culprit plaques determined by OCT in patients with ACS was investigated. We found that the UHR, a novel parameter combining UA and HDL-C, was significantly associated with culprit plaques and showed excellent predictive value for culprit plaques and thrombi in patients with ACS. To the best of our knowledge, this is the first study concerning the relationship between the UHR and culprit plaques in patients with ACS.
The concentration of UA is controlled by xanthine oxidase. A high level of UA is associated with short- or long-term adverse MACEs and mortality in patients with ACS who are undergoing PCI [12]. For each 1 mg/dL increase in UA level, the risk of death from atherosclerotic disease increased by 48% in men and 126% in women [13]. A cross-sectional study with a sample of 1 947 hospitalized patients with DM showed that the serum UA levels were closely associated with metabolic syndrome but not with carotid atherosclerosis [14]. However, in another study, a high level of UA was associated with metabolic syndrome and was an independent risk factor for carotid atherosclerosis in patients with DM [15]. A linear association between high UA variability and the development of CAD was found in a retrospective cross-sectional study, and patients with high levels of UA were older and had higher mortality from CAD [16]. Furthermore, hyperuricemia was found to be an independent risk factor for a high atherogenic index of plasma levels [17].
Regarding the possible mechanism, hyperuricemia significantly promotes the development of atherosclerotic plaques, downregulates the protein levels of inflammatory genes involved in signaling pathway, promotes the formation of foam cells, and increases lipid peroxidation in macrophages treated with oxidized LDL [18, 19]. A previous study also indicated that UA levels are positively associated with inflammatory cytokines, such as interleukin (IL)-6, IL-1β, C-reactive protein, and tumor necrosis factor, and may subsequently induce metabolic abnormalities [20, 21]. Overall, UA represents one of the indicators of oxidative homeostasis and plays a central role in the development of coronary plaques [22].
As an important component of lipid metabolism in the human body, HDL-C is composed of cholesterol, triglycerides, phospholipids and apolipoproteins, especially Apo A1. It has been demonstrated that HDL-C plays an important role in promoting cholesterol efflux in arterial wall cells to the liver and preventing arteriosclerosis in circulation through its anti-inflammatory and antioxidant effects [23, 24]. Although there is controversy regarding the relationship between HDL-C and CAD, the metabolism of HDL-C is far more complex and HDL-C itself remains a standard marker of CAD risk [25, 26]. More mechanistic and clinical studies are needed in the future to explore the exact relationship between HDL-C and cardiovascular risk.
Recently, the UHR has attracted clinical attention clinically as a novel biomarker for different diseases. A high UHR was found to be positively associated with the incidence of ischemic heart diseases without DM [8]. The UHR could serve as a promising predictor of diabetes control in men with DM [27]. Other studies have also shown that UA and HDL-C may interact with each other and have synergistic effects on atherosclerosis and CAD [28, 29]. However, no previous study has explored the relationship between UHR status and coronary artery culprit plaques in patients with ACS. In this study, we reported that a high UHR is associated with a greater incidence of plaque rupture and erosion, followed by the formation and presentation of a thrombus. This may be related to the high concentration of UA and low concentration of HDL-C, as we have shown in the manuscript. These synergistic adverse effects on CAD may be mediated through oxidative damage to endothelial cells and inflammation. It is also worth noting that there was no difference in the UHR level among patients with unstable angina, NSTEMI or STEMI. However, there may be differences in patients with ACS with those with chronic coronary syndrome if these patients are compared.
ACS mostly arises from the rupture or erosion of a culprit plaque [30, 31]. LDL-C is an independent predictor of culprit plaques. In the present study, we compared the performance of the UHR with that of LDL-C, and found that the UHR had a better AUC than LDL-C. In this study, we also predicted the presence of culprit plaques or thrombi in patients with ACS by simply calculating the index of the UHR. For patients with a greater UHR, we have more reasons to perform OCT examinations via PCI to specify and treat culprit plaques.
Study limitations.
This study has several limitations. First, this retrospective study included only has a small number of patients with ACS. Second, the blood samples were acquired when patients were admitted to the hospital, and clinical results were obtained only once. Third, the OCT examinations were mainly conducted in the culprit vessel according to coronary angiography results instead of all three coronary vessels. The need for good-quality images in OCT excluded distal lesion and high-risk patients, such as those with tortuous and high-calcified vessels, which made this study population too selected. Fourth, the MACE incidence and follow up of the patients were not further investigated. More work should be done and more patients and more vessels should be studied in the future.
Conclusions.
We found that plaque rupture, erosion and thrombus were more prevalent in the high-UHR group than in the low-UHR group, and the UHR could serve as an independent predictor for culprit or culprit plaques in patients with ACS.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- HDL-C:
-
high-density lipoprotein cholesterol
- CAD:
-
coronary artery disease
- UA:
-
uric acid
- UHR:
-
the uric acid to HDL-C ratio
- ACS:
-
acute coronary syndrome
- OCT:
-
optical coherence tomography
- ROC:
-
receiver operator characteristic
- AUC:
-
area under the curve
- LDL-C:
-
low-density lipoprotein cholesterol
- DM:
-
diabetes mellitus
- MACEs:
-
major cardiovascular adverse events
- PCI:
-
percutaneous coronary intervention
- STEMI:
-
ST-elevation myocardial infarction
- NSTEMI:
-
non-ST-elevation myocardial infarction
- FCT:
-
fibrous cap thickness
- TCFA:
-
thin-cap fibroatheroma
- MLA:
-
minimal lumen area
- MLD:
-
minimal lumen diameter
- AS:
-
area stenosis
- DS:
-
diameter stenosis
- ANOVA:
-
one-way analysis of variance
- Hb:
-
hemoglobin
- TSH:
-
thyroid stimulating hormone
- TG:
-
triglyceride
- VLDL:
-
very low-density lipoprotein cholesterol
- eGFR:
-
estimated glomerular filtration rate
- Lp(a):
-
lipoprotein(a)
- IL:
-
interleukin
References
Yu W, Cheng JD. Uric Acid and Cardiovascular Disease: an update from molecular mechanism to clinical perspective. Front Pharmacol. 2020;11:582680.
Lv S, Liu W, Zhou Y, Liu Y, Shi D, Zhao Y, et al. Hyperuricemia and severity of coronary artery disease: an observational study in adults 35 years of age and younger with acute coronary syndrome. Cardiol J. 2019;26(3):275–82.
Tai S, Li X, Zhu Z, Tang L, Yang H, Fu L, et al. Hyperuricemia is a risk factor for one-year overall survival in Elderly Female patients with Acute Coronary Syndrome. Cardiovasc Ther. 2020;2020:2615147.
Mandurino-Mirizzi A, Cornara S, Somaschini A, Demarchi A, Galazzi M, Puccio S, et al. Elevated serum uric acid is associated with a greater inflammatory response and with short- and long-term mortality in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Nutr Metab Cardiovasc Dis. 2021;31(2):608–14.
Kobayashi N, Hata N, Tsurumi M, Shibata Y, Okazaki H, Shirakabe A, et al. Relation of coronary culprit lesion morphology determined by Optical Coherence Tomography and Cardiac outcomes to serum uric acid levels in patients with Acute Coronary Syndrome. Am J Cardiol. 2018;122(1):17–25.
von Eckardstein A, Nordestgaard BG, Remaley AT, Catapano AL. High-density lipoprotein revisited: biological functions and clinical relevance. Eur Heart J. 2023;44(16):1394–407.
Julve J, Escola-Gil JC. High-density lipoproteins and Cardiovascular Disease: the Good, the bad, and the future. Int J Mol Sci. 2021;22(14).
Park B, Jung DH, Lee YJ. Predictive value of serum uric acid to HDL cholesterol ratio for Incident Ischemic Heart Disease in non-diabetic koreans. Biomedicines. 2022;10(6).
Park B, Jung DH, Lee HS, Lee YJ. Triglyceride to HDL-Cholesterol ratio and the Incident Risk of Ischemic Heart Disease among koreans without diabetes: a Longitudinal Study Using National Health Insurance Data. Front Cardiovasc Med. 2021;8:716698.
Tomaniak M, Katagiri Y, Modolo R, de Silva R, Khamis RY, Bourantas CV, et al. Vulnerable plaques and patients: state-of-the-art. Eur Heart J. 2020;41(31):2997–3004.
Deng F, Li D, Lei L, Yang Q, Li Q, Wang H, et al. Association between apolipoprotein B/A1 ratio and coronary plaque vulnerability in patients with atherosclerotic cardiovascular disease: an intravascular optical coherence tomography study. Cardiovasc Diabetol. 2021;20(1):188.
Tscharre M, Herman R, Rohla M, Hauser C, Farhan S, Freynhofer MK, et al. Uric acid is associated with long-term adverse cardiovascular outcomes in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Atherosclerosis. 2018;270:173–9.
Li M, Hu X, Fan Y, Li K, Zhang X, Hou W, et al. Hyperuricemia and the risk for coronary heart disease morbidity and mortality a systematic review and dose-response meta-analysis. Sci Rep. 2016;6:19520.
Li W, Wang Y, Ouyang S, Li M, Liu R, Zhang Y, et al. Association between serum uric acid level and carotid atherosclerosis and metabolic syndrome in patients with type 2 diabetes Mellitus. Front Endocrinol (Lausanne). 2022;13:890305.
Li Q, Yang Z, Lu B, Wen J, Ye Z, Chen L, et al. Serum uric acid level and its association with metabolic syndrome and carotid atherosclerosis in patients with type 2 diabetes. Cardiovasc Diabetol. 2011;10:72.
Kim HK, Lee M, Lee YH, Lee BW, Cha BS, Kang ES. Uric acid variability as a predictive marker of newly developed Cardiovascular events in type 2 diabetes. Front Cardiovasc Med. 2021;8:775753.
Duan Y, Chang X, Ding X, An Y, Wang G, Liu J. Association of hyperuricemia with apolipoprotein AI and atherogenic index of plasma in healthy Chinese people: a cross-sectional study. BMC Cardiovasc Disord. 2022;22(1):372.
He B, Nie Q, Wang F, Wang X, Zhou Y, Wang C, et al. Hyperuricemia promotes the progression of atherosclerosis by activating endothelial cell pyroptosis via the ROS/NLRP3 pathway. J Cell Physiol. 2023;238(8):1808–22.
Lee TS, Lu TM, Chen CH, Guo BC, Hsu CP. Hyperuricemia induces endothelial dysfunction and accelerates atherosclerosis by disturbing the asymmetric dimethylarginine/dimethylarginine dimethylaminotransferase 2 pathway. Redox Biol. 2021;46:102108.
Ruggiero C, Cherubini A, Ble A, Bos AJ, Maggio M, Dixit VD, et al. Uric acid and inflammatory markers. Eur Heart J. 2006;27(10):1174–81.
Kushiyama A, Nakatsu Y, Matsunaga Y, Yamamotoya T, Mori K, Ueda K, et al. Role of uric acid metabolism-related inflammation in the pathogenesis of metabolic Syndrome Components such as atherosclerosis and Nonalcoholic Steatohepatitis. Mediators Inflamm. 2016;2016:8603164.
Cortese F, Scicchitano P, Cortese AM, Meliota G, Andriani A, Truncellito L, et al. Uric acid in metabolic and cerebrovascular disorders: a review. Curr Vasc Pharmacol. 2020;18(6):610–8.
Hu J, Xi D, Zhao J, Luo T, Liu J, Lu H, et al. High-density lipoprotein and inflammation and its significance to atherosclerosis. Am J Med Sci. 2016;352(4):408–15.
Sorrentino SA, Besler C, Rohrer L, Meyer M, Heinrich K, Bahlmann FH, et al. Endothelial-vasoprotective effects of high-density lipoprotein are impaired in patients with type 2 diabetes mellitus but are improved after extended-release niacin therapy. Circulation. 2010;121(1):110–22.
Sirtori CR, Corsini A, Ruscica M. The role of high-density lipoprotein cholesterol in 2022. Curr Atheroscler Rep. 2022;24(5):365–77.
Franczyk B, Rysz J, Lawinski J, Rysz-Gorzynska M, Gluba-Brzozka A. Is High HDL-Cholesterol Level Always Beneficial? Biomedicines. 2021;9(9).
Aktas G, Kocak MZ, Bilgin S, Atak BM, Duman TT, Kurtkulagi O. Uric acid to HDL cholesterol ratio is a strong predictor of diabetic control in men with type 2 diabetes mellitus. Aging Male. 2020;23(5):1098–102.
Hu X, Liu J, Li W, Wang C, Li G, Zhou Y, et al. Elevated serum uric acid was associated with pre-inflammatory state and impacted the role of HDL-C on carotid atherosclerosis. Nutr Metab Cardiovasc Dis. 2022;32(7):1661–9.
Yang Y, Zhang J, Jia L, Su J, Ma M, Lin X. The interaction between uric acid and high-density lipoprotein cholesterol on the prognosis of patients with acute myocardial infarction. Front Cardiovasc Med. 2023;10:1226108.
Naghavi M, Libby P, Falk E, Casscells SW, Litovsky S, Rumberger J, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: part I. Circulation. 2003;108(14):1664–72.
Naghavi M, Libby P, Falk E, Casscells SW, Litovsky S, Rumberger J, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: part II. Circulation. 2003;108(15):1772–8.
Acknowledgements
The authors thank the study subjects for their participation and support of this study.
Funding
This work was supported by the Natural Science Foundation of Shaanxi Province (Grant No. 2021JM-291 to W.J., No. 2020JQ-538 to Q.Y.) and the Key Research and Development Program of Shaanxi Province (Grant No. 2020SF-248 to R.H.). The authors gratefully acknowledge all those who took part in this study.
Author information
Authors and Affiliations
Contributions
W.J. contributed to the study design, data acquisition, and data analysis and wrote the manuscript. F.D. and F.J. contributed to the study design and data analysis. Y.S., L.Z., J.H., and D.L. contributed to the data acquisition. Q.Y. and R.H. reviewed and edited the intellectual content. All authors gave final approval for this version to be published. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics statement
The study protocol was approved by the Ethics Committee of the Second Affiliated Hospital of Xi’an Jiaotong University, and the study was conducted according to the principles of the Helsinki Declaration II. All patients provided written informed consent.
Consent for publication
Written informed consent for publication has been obtained from the participants in this study.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Deng, F., Jia, F., Sun, Y. et al. Predictive value of the serum uric acid to high-density lipoprotein cholesterol ratio for culprit plaques in patients with acute coronary syndrome. BMC Cardiovasc Disord 24, 155 (2024). https://doi.org/10.1186/s12872-024-03824-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-024-03824-z