Abstract
Background
Heart failure (HF) and diabetes are associated with increased incidence and worse prognosis of each other. The prognostic value of global longitudinal strain (GLS) measured by cardiovascular magnetic resonance (CMR) has not been established in HF patients with diabetes.
Methods
In this prospective, observational study, consecutive patients (n = 315) with HF underwent CMR at 3T, including GLS, late gadolinium enhancement (LGE), native T1, and extracellular volume fraction (ECV) mapping. Plasma biomarker concentrations were measured including: N-terminal pro B-type natriuretic peptide(NT-proBNP), high-sensitivity troponin T(hs-TnT), growth differentiation factor 15(GDF-15), soluble ST2(sST2), and galectin 3(Gal-3). The primary outcome was a composite of all-cause mortality or HF hospitalisation.
Results
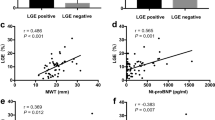
Compared to those without diabetes (n = 156), the diabetes group (n = 159) had a higher LGE prevalence (76 vs. 60%, p < 0.05), higher T1 (1285±42 vs. 1269±42ms, p < 0.001), and higher ECV (30.5±3.5 vs. 28.8±4.1%, p < 0.001). The diabetes group had higher NT-pro-BNP, hs-TnT, GDF-15, sST2, and Gal-3. Diabetes conferred worse prognosis (hazard ratio (HR) 2.33 [95% confidence interval (CI) 1.43–3.79], p < 0.001). In multivariable Cox regression analysis including clinical markers and plasma biomarkers, sST2 alone remained independently associated with the primary outcome (HR per 1 ng/mL 1.04 [95% CI 1.02–1.07], p = 0.001). In multivariable Cox regression models in the diabetes group, both GLS and sST2 remained prognostic (GLS: HR 1.12 [95% CI 1.03–1.21], p = 0.01; sST2: HR per 1 ng/mL 1.03 [95% CI 1.00-1.06], p = 0.02).
Conclusions
Compared to HF patients without diabetes, those with diabetes have worse plasma and CMR markers of fibrosis and a more adverse prognosis. GLS by CMR is a powerful and independent prognostic marker in HF patients with diabetes.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Background
The Asian continent has the highest prevalence of heart failure (HF) cases globally [1]. Diabetes is especially common in South East Asian HF populations, where a unique ‘lean-diabetic’ phenotype with worse outcomes has been identified [2]. The Asian Sudden Cardiac Death in Heart Failure (ASIAN-HF) registry (across 11 Asian countries) reported a 42.5% prevalence of diabetes among HF patients, specifically in higher-income countries such as Singapore, Hong Kong, and Thailand [3]. Patients with HF and diabetes have consistently worse clinical outcomes, including higher risk of hospitalisation for HF and death, compared to those without diabetes [4,5,6]. These findings appear to hold regardless of whether the HF is ischaemic or non-ischaemic in etiology, and regardless of left ventricular (LV) ejection fraction [5, 6].
Cardiac magnetic resonance (CMR) imaging has become the non-invasive reference standard for evaluating HF due to its ability to accurately assess cardiac morphology, function, and myocardial tissue characteristics. In particular, late gadolinium enhancement (LGE) permits visualisation of focal replacement myocardial fibrosis, while T1 mapping pre- and post-gadolinium contrast enables non-invasive measurement of the myocardial extracellular volume fraction (ECV), a quantitative marker of myocardial diffuse interstitial fibrosis. Additionally, global longitudinal strain (GLS), defined as the change in the LV myocardial length between diastole and systole divided by the original end-diastolic length, provides a measure of LV systolic function by CMR that is effectively the same as GLS measured by echocardiography [7, 8].
Patients with diabetes frequently have impaired GLS and have a higher degree of myocardial fibrosis as assessed by ECV and histology [9, 10]. GLS by CMR appears to have prognostic value in HF regardless of ejection fraction and whether the cause of HF is ischemic or non-ischemic [11,12,13]. There is a growing body of literature demonstrating the prognostic value of GLS in asymptomatic patients with diabetes [14, 15]. However, the prognostic utility of GLS by CMR in diabetes patients with established HF is unknown.
Growth differentiation factor-15 (GDF-15), soluble ST2 (sST2), and galectin 3 (Gal-3) are circulating plasma biomarkers associated with inflammation, fibrosis, and cardiac remodelling [16]. Plasma concentrations of these biomarkers appear to provide prognostic information in HF patients beyond established markers including cardiac troponins and natriuretic peptides [16,17,18,19]. The prognostic relevance of these biomarkers has not yet been established in HF patients with diabetes.
This study aimed to assess the prognostic significance of GLS by CMR and novel HF plasma biomarkers associated with inflammation and fibrosis in a cohort of patients with HF and diabetes across the spectrum of LV ejection fraction. We hypothesized that GLS would have an incremental prognostic association in this group, beyond plasma HF biomarkers, LV ejection fraction, and CMR markers of myocardial fibrosis.
Methods
Study population
Patients with HF were recruited prospectively across six tertiary cardiac centres in Singapore (Asian neTwork for Translational Research and Cardiovascular Trials [ATTRaCT], ClinicalTrials.gov NCT02791009). Patients were included if they presented to hospital with a primary diagnosis of HF, or if they attended a hospital clinic within 6 months of an episode of decompensated HF (requiring hospitalization or treatment in an out-patient setting). In all cases, a trained cardiologist adjudicated the clinical diagnosis of HF. The exclusion criteria were: HF primarily due to severe valve disease, HF due to acute coronary syndrome resulting in a transient episode of acute pulmonary oedema, severe renal failure (estimated glomerular filtration rate < 15 mL/min per 1.73m2), specific causes of HF (constrictive pericarditis, complex adult congenital heart disease, hypertrophic cardiomyopathy, eosinophilic myocarditis, cardiac amyloidosis, and acute chemotherapy-induced cardiomyopathy), isolated right HF, and life threatening non-cardiac co-morbidity with life expectancy of < 1 year. All patients underwent clinical assessment at baseline. Diabetes status was identified by baseline questionnaire at recruitment.
Biomarkers
Blood was collected in dipotassium (K2)-EDTA vacutainer tubes and transported on ice for processing within one hour. Plasma was separated by centrifugation at 3500 g for 10 min at 4oC and stored at -80oC until analysis.
Plasma N-terminal pro B-type natriuretic peptide (NT-proBNP) and high-sensitivity troponin T (hs-TnT) were measured by electrochemiluminescence immunoassay using the Elecsys proBNP G2 V2.1 and Elecsys Troponin T hs V2.1 assays on the Cobas e411 immuno-analyser (Roche Diagnostic GmbH, Mannheim, Germany). The measurement ranges of NT-proBNP and hs-TnT were 10-35000 pg/ml and 3-10000 pg/ml, respectively. Laboratory average concentration and inter-assay coefficient of variation (%CV) of low (NT-proBNP: 147 pg/ml, 4.48%; hs-TnT: 26.8 pg/ml, 5.05%) and high (NT-proBNP: 4679 pg/ml, 4.97%; hs-TnT: 2120 pg/ml, 3.69%) quality control samples of the NT-proBNP and hs-TnT assays were established over 84 and 73 independent assays, respectively.
Human GDF-15 (R&D Systems, Minneapolis, MN, USA; Cat#DGD150), sST2 (Presage ST2 assay, Critical Diagnostics, California, USA) and Gal-3 (R&D Systems, Minneapolis, MN, USA; Cat#DGAL30) were measured by ELISA on the Enspire Multimode Microplate Reader (Perkin Elmer, Waltham, MA, USA). Results were interpolated from standard curves fitted on 5-parameter logistic model (5-PL) using the instrument’s Enspire®software. Laboratory inter-assay %CV of quality control samples were 7.41% at 126 pg/ml, 7.71% at 360 pg/ml and 8.43% at 778 pg/ml for GDF-15 (n = 98), 18.0% at 30.0 ng/ml and 15.2% at 63.6 ng/ml for sST2 (n = 98) and 10.4% at 0.83 ng/ml, 10.6% at 2.41 ng/ml and 12.3% at 4.82 ng/ml for Galectin-3 (n = 56).
Clinical outcomes
The primary outcome was a composite of time to either first hospitalization for HF (regardless of prior history of hospitalization for HF) or all-cause mortality. Follow-up was conducted through a clinic visit at 6 months after baseline assessment and structured phone interviews with the participants at the 1- and 2-year timepoints. Data in patients who were lost to follow-up were censored at the date when the patient was last known to be alive and had not experienced an event.
Cardiovascular magnetic resonance image acquisition
All patients in the ATTRaCT cohort were invited and assessed for suitability for CMR. Those who agreed and were eligible underwent a standardized CMR protocol with a 3 Tesla MRI scanner (Ingenia, Philips Healthcare, Best, The Netherlands). Balanced steady-state free precession cines were acquired in the standard long-axis views and a short-axis stack from base to apex, as described previously [20]. LGE images were acquired at 10 min after 0.1 mmol/kg of gadobutrol (Gadovist®, Bayer Pharma AG, Germany) with a phase sensitive inversion-recovery gradient-echo imaging sequence. Typical parameters were: repetition time (TR) = 6.1 ms; echo time (TE) = 3 ms; time to inversion (TI) = 320–340 ms, flip angle 25°, voxel size = 1.5 × 1.7 × 8 mm, SENSE factor = 2.4, slice thickness 8 mm with 2 mm gap to match short-axis cine slice positions. The inversion time for optimal myocardial nulling was selected from an inversion time scout sequence. T1 maps were acquired at the basal and mid-ventricular short-axis levels, pre- and 15-min post-contrast with modified Look-Locker Inversion-recovery (MOLLI) 5s(3s)3 and 4s(1s)3s(1s)2s acquisition schemes, respectively [21].
CMR analysis
Image analysis was performed using CVI42 software (Circle Cardiovascular Imaging, Calgary, Canada) by trained imaging fellows at the National Heart Research Institute of Singapore CMR Core Laboratory, who were blinded to the clinical information of the patients. Ventricular volumes, mass, and ejection fraction were measured from the short-axis cine stack, using manual contouring of the left ventricle in end-diastole and end-systole, excluding papillary muscles, as detailed previously [20]. LV volumes and mass data were indexed to body surface area. The presence of LGE was assessed qualitatively by two readers according to the recommendations by the Society of CMR [22]. Average native and post-contrast myocardial T1 values were measured by placing a region of interest (ROI) within the middle third of the short-axis myocardial wall at the basal- and mid-ventricular levels, while avoiding regions of focal LGE. The myocardium-blood pool interface was carefully avoided in order to prevent partial volume effects. Pre- and post-contrast blood T1 values were measured in a ROI drawn within the blood pool. ECV was calculated from the pre- and post-contrast average blood and myocardial T1 values, as described previously [23, 24]. Myocardial strain was analysed in the cine images using the Tissue Tracking Plugin [25].
Statistics
Normality was assessed for continuous variables using the Shapiro-Wilk test. Normally distributed data are presented as mean ± standard deviation. Non-normally distributed data are presented as median [interquartile range]. Comparisons were performed for continuous variables using the parametric Student t-test or the non-parametric Mann-Whitney U test. Categorical variables are presented as number (percentage) and compared using the χ2 test.
Univariable Cox regression analysis was performed to identify prognostic variables in the entire cohort and also in the diabetes subgroup. Clinically relevant variables (age, sex, body mass index, diabetes, smoking, coronary artery disease, hypertension, New York Heart Association functional class, and systolic blood pressure), circulating biomarkers (NT-proBNP, hs-TnT, GDF-15, sST2, and Gal-3) and CMR markers of function, remodelling and fibrosis (LV end diastolic volume index, mass index, ejection fraction, GLS, LGE, Native T1, and ECV) were tested in the Cox models. Covariates with a p-value < 0.05 in univariable analyses were entered into the multivariable Cox model to identify independently prognostic variables, using forward stepwise selection (probability to remove threshold ≥0.1). Event-free survival curves were examined using the Kaplan-Meier method and compared with the log-rank test. In the Kaplan-Meier analysis, GLS was treated as a binary variable according to the cohort median, in order to maximise statistical power. Statistical analyses were performed using SPSS Version 28 (Statistical Package for the Social Sciences, International Business Machines, Inc., Armonk, New York, USA) and GraphPad Prism 9.4.1 (GraphPad Software, Inc., San Diego, California, USA). A two-sided p-value < 0.05 was considered as statistically significant.
Results
Figure 1 shows a flow chart describing patient inclusion. Of the 623 patients enrolled in the ATTRaCT study, 523 patients underwent a baseline CMR study. We excluded 168 studies performed without contrast. An additional 40 patients were excluded due to incomplete baseline clinical or CMR data sets, alternative diagnosis on the basis of CMR, or loss to follow-up. In total, 315 subjects (diabetes, n = 159; without diabetes, n = 156) were included in the study cohort.
The baseline clinical characteristics are shown in Table 1. Compared to patients without diabetes, patients with diabetes were older (60±10 vs. 56±12 years, p < 0.001), more likely to have a history of hypertension (76 vs. 52%, p < 0.001), coronary artery disease (72 vs. 52%, p < 0.001), and had worse NYHA Functional Class (median Class II vs. I, p = 0.04). Patients with diabetes had higher creatinine (96 [81–118] vs. 91 [77–105] µmol/L, p = 0.02) and elevated cardiac biomarkers: NT-proBNP (1091 [326–2272] vs. 579 [232–1136] pg/mL, p < 0.001), hs-TnT (27 [14-41] versus 15 [9-22] ng/L, p < 0.001), GDF-15 (2412 [1603–3331] versus 1039 [753–1470] pg/mL, p < 0.001), sST2 (28 [9-22] versus 26 [21-32] ng/mL, p = 0.038), and Gal-3 (10.0 [8.0–12.0] versus 8.0 [7.0–10.0] ng/mL, p < 0.001). There was no difference in LV ejection fraction and GLS between the groups. The diabetes group had a higher prevalence of LGE (76 vs. 60%, p = 0.002, driven by higher rates of ischaemic LGE (51 vs. 33%, p = 0.003). Prevalence of non-ischaemic LGE did not differ between the groups. The diabetes group had higher native T1 (1285±42 vs. 1269±42 ms, p < 0.001) and ECV (30.5±3.5 vs. 28.8±4.1%, p < 0.001).
After adjustment for potential confounders, including age, sex, hypertension, and coronary artery disease, diabetes remained independently associated with the presence of LGE and increased ECV (p < 0.005 for both analyses). Amongst patients without coronary artery disease, those with diabetes had a numerically higher proportion with ischaemic LGE (21%) compared to those without diabetes (13%), although this difference did not achieve statistical significance (Supplemental Table 1).
Predictors of primary outcome in all patients with heart failure
Over a median follow-up of 23 months, 74 patients experienced the primary outcome (52 hospitalisations for HF, 22 all-cause deaths; Supplemental Table 2). In univariable Cox regression analyses for the entire cohort, clinical markers associated with the primary outcome included diabetes, NYHA functional class, and systolic blood pressure. Circulating biomarkers associated with the primary outcome included NT-proBNP, hs-TnT, GDF-15, sST2, and Gal-3. CMR markers of adverse remodelling (LV mass index and end diastolic volume index), contractile function (LV ejection fraction and GLS), and myocardial fibrosis (presence of LGE, native T1 and ECV) were predictors of worse outcomes. Diabetes, NT-proBNP, and GLS remained independently associated with outcomes in the multivariable analysis (Table 2; Fig. 2).
Event-free survival curves showing: (A) Adverse prognosis in patients with heart failure and diabetes; (B) Patients with diabetes and GLS worse than cohort median (-9.9%) had worst prognosis. Patients with either diabetes or GLS worse than median had similar outcomes. Abbreviations: DM: diabetes mellitus; GLS: global longitudinal strain
Predictors of primary outcome in patients with heart failure and diabetes
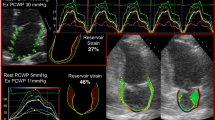
In the diabetes group, 50 patients experienced the primary outcome (35 hospitalisations for HF, 15 all-cause deaths). In univariable analyses, systolic blood pressure, NT-proBNP, hs-TnT, GDF-15, sST2, LV ejection fraction, GLS, and native T1 were associated with the primary outcome (Table 3). GLS and sST2 remained associated with outcomes in the multivariable analysis. Patients with diabetes and worse than median GLS (GLS > -9.9%) had the worst prognosis (log-rank p < 0.001, Fig. 3). Of note, patients with diabetes and GLS better than median had similar outcomes to patients without diabetes and GLS worse than median (Fig. 2B; p = 0.70).
Example of GLS measurement in apical 2-chamber view. Top panel shows a patient with heart failure, diabetes and GLS ≤ -9.9%, at end systole. Bottom panel shows a patient with heart failure, diabetes and GLS > -9.9%, at end systole. Centre panel: event-free survival curves showing adverse prognosis in patients with heart failure, diabetes and GLS worse than cohort median (-9.9%). Abbreviations: DM: diabetes mellitus; GLS: global longitudinal strain
In a multivariable model including clinical markers and plasma biomarkers, only sST2 remained independently associated with the primary outcome, demonstrating stronger prognostic associations than NT-proBNP (Table 4).
The diabetes group was also stratified into four groups based on GLS (above or below the cohort median of -9.9%) and plasma biomarker (above or below median for NT-proBNP, hs-TnT, GDF-15, sST2, and Gal-3). Amongst patients with above median GLS, NT-proBNP and GDF-15 showed additional prognostic value (Supplemental Fig. 1). On the other hand, the combination of below median GLS and sST2 demonstrated particularly adverse prognosis (Supplemental Fig. 2).
Discussion
The main finding of this study is that GLS by CMR is a powerful, independent predictor of adverse outcomes in patients with HF and diabetes, providing incremental prognostic information beyond several circulating plasma biomarkers and CMR markers of cardiac remodelling, inflammation, and fibrosis. In the diabetes group, worse GLS (> median − 9.9%) was associated with adverse prognosis. GLS by CMR is known to have prognostic value in HF, regardless of whether the cause is ischemic or non-ischemic and whether the EF is reduced or preserved [12, 13]. In this study, we have extended those findings to the diabetes subgroup, who are known to have a higher risk of adverse cardiovascular outcomes. Our findings are in agreement with a prior study which showed that GLS by speckle tracking echocardiography has prognostic value in diabetes patients with dilated cardiomyopathy [26]. The results from the current study raise the possibility of GLS-guided risk stratification and management in patients with HF and diabetes. Indeed, there is emerging evidence of improvement in GLS with sodium-glucose cotransporter-2 inhibitors, which may enable this in future [27, 28].
GLS by CMR is well-suited for routine clinical use. The technique relies on software packages which track the endocardial and epicardial borders, and reference values are specific for the software used for analysis. Speckle tracking echocardiography has a number of limitations, including dependence on high quality 2D images, and results are often affected by foreshortening, dropout of the apical and anterolateral segments on apical views, and/or suboptimal acoustic windows [13, 29, 30]. GLS by CMR overcomes these limitations and can be measured from routinely acquired bright-blood, steady-state free precession long-axis cine imaging. Disadvantages of GLS by CMR include its susceptibility to through-plane motion artefacts, limited temporal resolution for quantification of strain rate, and limitations in patients with contraindications to CMR [13]. Furthermore, GLS by CMR may be affected by inter-vendor differences in algorithms, similar to speckle tracking echocardiography, which has resulted in difficulties establishing reference values [30]. Nonetheless, GLS by CMR has shown close correlation with GLS by speckle tracking echocardiography, and has become an important component of the CMR examination alongside volumetric analysis and tissue characterisation in the assessment of HF [31].
In this study, sST2 demonstrated incremental prognostic value over other biomarkers including NT-proBNP and hs-TnT in HF patients with diabetes. sST2 is produced by cardiomyocytes and cardiac fibroblasts in response to myocardial stress, inflammation or injury [32,33,34]. Other sources of sST2 include endothelial cells of the aorta and coronary arteries as well as certain immune cells [32]. It acts as a decoy receptor for interleukin-33, attenuating its cardioprotective biological effects which include blunting myocardial hypertrophy and fibrosis, and inhibiting cardiomyocyte apoptosis [16, 32, 35]. We have confirmed that sST2 levels are higher in HF patients with diabetes [36, 37]. Furthermore, sST2 is known to have prognostic value in acute and chronic HF, independent of plasma natriuretic peptides [38,39,40]. We have provided further evidence that these findings also apply to the subgroup of HF patients with diabetes [41]. Our findings therefore suggest a possible role for sST2 in risk stratification amongst HF patients with diabetes and in monitoring response to treatment [42,43,44,45,46,47].
In this study, HF patients with diabetes had worse CMR markers of myocardial injury, inflammation, and fibrosis. The association between diabetes and elevated ECV remained after adjusting for potential confounders that differed between the diabetic and non-diabetic groups including age, hypertension, coronary artery disease and presence of LGE. These findings are in agreement with prior studies, including a recent meta-analysis, which showed an association between diabetes and a higher degree of myocardial fibrosis as assessed by histology as well as ECV by CMR [9]. Furthermore, diabetes remained associated with the presence of LGE in logistic regression models, even accounting for age, sex, hypertension and coronary artery disease. This was an interesting finding given that the higher rate of LGE in the diabetes group was primarily driven by higher rates of ischemic LGE. These results may reflect a higher frequency of silent myocardial infarction in diabetes patients, which has been demonstrated previously using a variety of imaging modalities, including CMR [48, 49]. Although our diabetes group had a numerically higher proportion with ischemic LGE in the absence of coronary artery disease, that difference did not reach statistical significance.
Both focal and diffuse myocardial fibrotic processes are known to occur in patients with diabetes independently of co-morbid conditions, including coronary atherosclerosis and hypertension. Diffuse interstitial and perivascular fibrosis are structural hallmarks of diabetic cardiomyopathy, but focal replacement fibrosis can also be seen, even in the absence of coronary artery disease [50]. Several mechanisms may explain the fibrosis burden in diabetes. Hyperglycaemia is thought to upregulate the expression of profibrotic factors such as transforming growth factor beta 1 and down-regulate the activity of the matrix metalloproteinases [51]. Hyperglycaemia is also known to promote the formation of advanced glycation end products (AGEs) which can cross-link collagen in the interstitium, increasing their resistance to degradation. AGEs can result in generation of reactive oxygen species and oxidative stress which further promotes a pro-fibrotic state [9]. Pro-inflammatory cytokines and chemokines, as well as increased renin-angiotensin-aldosterone system activity in diabetes have also been implicated in the development of myocardial fibrosis.
Native T1 values were higher in the diabetes group and may reflect interstitial expansion due to myocardial fibrosis as well as myocardial oedema affecting the cellular and interstitial compartments [52]. There is some disagreement in the literature regarding whether native T1 values are increased in diabetes. Several studies have shown an association between diabetes and increased T1 [53,54,55,56]. Although a positive association was found between diabetes and native T1 in a recent meta-analysis, the result was not statistically significant [9]. The authors of that study suggested that the lack of statistical significance may have resulted from limited sample size of the included studies. Indeed, the diabetes cohort in the present study was larger than any of those in the included studies. Furthermore, T1 values are known to be dependent on a variety of factors, including field strength, pulse sequence, and region of measurement within the myocardium. In the present study, T1 maps were obtained at 3T using the same MOLLI sequence for all patients, and with experienced observers performing standardized analyses that may improve reproducibility and eliminate technical cofounders [57, 58]. Whilst it does appear that diabetes is associated with higher T1, larger studies controlling for the variability in T1 are required to more conclusively answer this question.
Limitations
One limitation of this study is the absence of measures of glycaemic control, which are known to be prognostic in patients with HF and diabetes [59]. However, GLS by speckle tracking echocardiography is known to have prognostic value independent of glycaemic control in diabetes cohorts with preserved and reduced ejection fraction, and therefore this is unlikely to have altered the prognostic associations for GLS by CMR [14, 26]. Our HF cohort included patients with both ischaemic and non-ischaemic etiologies, as well as both preserved and reduced ejection fraction. Unfortunately, the study was not powered for analyses of these subgroups. Documented history of coronary artery disease did not associate with outcomes in the univariable analysis and therefore etiology of HF is unlikely to have affected the results. Finally, this was a single centre study using feature tracking software from a single vendor, limiting generalizability of the results.
Conclusions
Patients with HF and diabetes had worse CMR and plasma markers of injury, inflammation, and fibrosis, and an adverse prognosis. sST2 showed incremental prognostic value beyond NT-proBNP in HF patients with diabetes. GLS is an important and independent prognostic marker in this group. Future studies should explore whether GLS-guided risk stratification and management can improve outcomes in this group of patients.
Data availability
The datasets generated and analysed for the current study are not publicly available. Please contact the corresponding author for data requests.
Abbreviations
- HF:
-
heart failure
- GLS:
-
global longitudinal strain
- CMR:
-
cardiovascular magnetic resonance
- LGE:
-
late gadolinium enhancement
- ECV:
-
extraceullar volume
- NT-proBNP:
-
N-terminal pro B-type natriuretic peptide
- hs-TnT:
-
high-sensitivity troponin T
- GDF-15:
-
growth differentiation factor 15
- sST2:
-
soluble ST2
- Gal-3:
-
galectin 3
- LV:
-
left ventricle
References
Lam CS, Teng TK, Tay WT, Anand I, Zhang S, Shimizu W, et al. Regional and ethnic differences among patients with heart failure in Asia: the Asian sudden cardiac death in heart failure registry. Eur Heart J. 2016;37(41):3141–53. https://doi.org/10.1093/eurheartj/ehw331. Epub 2016/08/10.
Ang N, Chandramouli C, Yiu K, Lawson C, Tromp J. Heart failure and multimorbidity in Asia. Curr Heart Fail Rep. 2023;20(1):24–32. https://doi.org/10.1007/s11897-023-00585-2. Epub 2023/02/23.
Yap J, Tay WT, Teng TK, Anand I, Richards AM, Ling LH, et al. Association of Diabetes Mellitus on Cardiac Remodeling, Quality of Life, and clinical outcomes in heart failure with reduced and preserved ejection fraction. J Am Heart Assoc. 2019;8(17):e013114. PubMed PMID: 31431116; PubMed Central PMCID: PMCPMC6755825.
Rosano GM, Vitale C, Seferovic P. Heart failure in patients with diabetes Mellitus. Card Fail Rev. 2017;3(1):52–5. Epub 2017/08/09. doi: 10.15420/cfr.2016:20:2. PubMed PMID: 28785476; PubMed Central PMCID: PMCPMC5494155.
Johansson I, Dahlstrom U, Edner M, Nasman P, Ryden L, Norhammar A. Prognostic implications of type 2 diabetes Mellitus in ischemic and nonischemic heart failure. J Am Coll Cardiol. 2016;68(13):1404–16. https://doi.org/10.1016/j.jacc.2016.06.061. Epub 2016/09/24.
MacDonald MR, Petrie MC, Varyani F, Ostergren J, Michelson EL, Young JB, et al. Impact of diabetes on outcomes in patients with low and preserved ejection fraction heart failure: an analysis of the Candesartan in Heart failure: Assessment of reduction in mortality and morbidity (CHARM) programme. Eur Heart J. 2008;29(11):1377–85. https://doi.org/10.1093/eurheartj/ehn153. Epub 2008/04/17.
Chamsi-Pasha MA, Zhan Y, Debs D, Shah DJ. CMR in the Evaluation of Diastolic Dysfunction and Phenotyping of HFpEF: Current Role and Future Perspectives. JACC Cardiovasc Imaging. 2020;13(1 Pt 2):283 – 96. Epub 2019/06/17. https://doi.org/10.1016/j.jcmg.2019.02.031. PubMed PMID: 31202753.
Kinno M, Nagpal P, Horgan S, Waller AH. Comparison of Echocardiography, Cardiac magnetic resonance, and computed tomographic imaging for the evaluation of left ventricular myocardial function: part 1 (Global Assessment). Curr Cardiol Rep. 2017;19(1):9. https://doi.org/10.1007/s11886-017-0815-4. Epub 2017/02/09.
Salvador DB Jr., Gamba MR, Gonzalez-Jaramillo N, Gonzalez-Jaramillo V, Raguindin PFN, Minder B, et al. Diabetes and myocardial fibrosis: a systematic review and Meta-analysis. JACC Cardiovasc Imaging. 2022;15(5):796–808. https://doi.org/10.1016/j.jcmg.2021.12.008. Epub 2022/05/06.
Marwick TH, Ritchie R, Shaw JE, Kaye D. Implications of underlying mechanisms for the Recognition and Management of Diabetic Cardiomyopathy. J Am Coll Cardiol. 2018;71(3):339–51. PubMed PMID: 29348027.
Frojdh F, Fridman Y, Bering P, Sayeed A, Maanja M, Niklasson L, et al. Extracellular volume and global longitudinal strain both associate with outcomes but correlate minimally. JACC Cardiovasc Imaging. 2020;13(11):2343–54. https://doi.org/10.1016/j.jcmg.2020.04.026. Epub 2020/06/22.
Romano S, Judd RM, Kim RJ, Kim HW, Klem I, Heitner JF, et al. Feature-tracking global longitudinal strain predicts death in a Multicenter Population of patients with ischemic and nonischemic dilated cardiomyopathy incremental to Ejection Fraction and Late Gadolinium Enhancement. JACC Cardiovasc Imaging. 2018;11(10):1419–29. PubMed PMID: 29361479; PubMed Central PMCID: PMCPMC6043421.
Kammerlander AA, Dona C, Nitsche C, Koschutnik M, Schonbauer R, Duca F, et al. Feature Tracking of Global Longitudinal strain by using Cardiovascular MRI improves risk stratification in Heart failure with preserved ejection fraction. Radiology. 2020;296(2):290–8. https://doi.org/10.1148/radiol.2020200195. Epub 2020/06/03.
Holland DJ, Marwick TH, Haluska BA, Leano R, Hordern MD, Hare JL, et al. Subclinical LV dysfunction and 10-year outcomes in type 2 diabetes mellitus. Heart. 2015;101(13):1061–6. https://doi.org/10.1136/heartjnl-2014-307391. Epub 2015/05/04.
Wang Y, Yang H, Huynh Q, Nolan M, Negishi K, Marwick TH. Diagnosis of nonischemic stage B Heart failure in type 2 diabetes Mellitus: optimal parameters for prediction of heart failure. JACC Cardiovasc Imaging. 2018;11(10):1390–400. https://doi.org/10.1016/j.jcmg.2018.03.015. Epub 2018/05/21.
Meijers WC, Bayes-Genis A, Mebazaa A, Bauersachs J, Cleland JGF, Coats AJS, et al. Circulating heart failure biomarkers beyond natriuretic peptides: review from the Biomarker Study Group of the Heart Failure Association (HFA), European Society of Cardiology (ESC). Eur J Heart Fail. 2021;23(10):1610–32. https://doi.org/10.1002/ejhf.2346. Epub 2021/09/10.
Chan MM, Santhanakrishnan R, Chong JP, Chen Z, Tai BC, Liew OW, et al. Growth differentiation factor 15 in heart failure with preserved vs. reduced ejection fraction. Eur J Heart Fail. 2016;18(1):81–8. https://doi.org/10.1002/ejhf.431. Epub 2015/10/27.
Emdin M, Aimo A, Vergaro G, Bayes-Genis A, Lupon J, Latini R, et al. sST2 predicts Outcome in Chronic Heart failure beyond NT-proBNP and high-sensitivity troponin T. J Am Coll Cardiol. 2018;72(19):2309–20. 2165. PubMed PMID: 30384887.
Anand IS, Rector TS, Kuskowski M, Adourian A, Muntendam P, Cohn JN. Baseline and serial measurements of galectin-3 in patients with heart failure: relationship to prognosis and effect of treatment with valsartan in the Val-HeFT. Eur J Heart Fail. 2013;15(5):511–8. https://doi.org/10.1093/eurjhf/hfs205. Epub 2013/01/08.
Le TT, Tan RS, De Deyn M, Goh EP, Han Y, Leong BR, et al. Cardiovascular magnetic resonance reference ranges for the heart and aorta in Chinese at 3T. J Cardiovasc Magn Reson. 2016. https://doi.org/10.1186/s12968-016-0236-3. 18:21. Epub 2016/04/14.
Zhang S, Le TT, Kabus S, Su B, Hausenloy DJ, Cook SA, et al. Cardiac magnetic resonance T1 and extracellular volume mapping with motion correction and co-registration based on fast elastic image registration. MAGMA. 2018;31(1):115–29. https://doi.org/10.1007/s10334-017-0668-2. Epub 2017/12/23.
Schulz-Menger J, Bluemke DA, Bremerich J, Flamm SD, Fogel MA, Friedrich MG, et al. Standardized image interpretation and post processing in cardiovascular magnetic resonance: Society for Cardiovascular Magnetic Resonance (SCMR) board of trustees task force on standardized post processing. J Cardiovasc Magn Reson. 2013;15(1):35. https://doi.org/10.1186/1532-429X-15-35. Epub 2013/05/03.
Ugander M, Oki AJ, Hsu LY, Kellman P, Greiser A, Aletras AH, et al. Extracellular volume imaging by magnetic resonance imaging provides insights into overt and sub-clinical myocardial pathology. Eur Heart J. 2012;33(10):1268–78. https://doi.org/10.1093/eurheartj/ehr481. Epub 2012/01/27.
Arheden H, Saeed M, Higgins CB, Gao DW, Bremerich J, Wyttenbach R, et al. Measurement of the distribution volume of gadopentetate dimeglumine at echo-planar MR imaging to quantify myocardial infarction: comparison with 99mTc-DTPA autoradiography in rats. Radiology. 1999;211(3):698–708. https://doi.org/10.1148/radiology.211.3.r99jn41698. Epub 1999/06/03.
Cai J, Bryant JA, Le TT, Su B, de Marvao A, O’Regan DP, et al. Fractal analysis of left ventricular trabeculations is associated with impaired myocardial deformation in healthy Chinese. J Cardiovasc Magn Reson. 2017;19(1):102. https://doi.org/10.1186/s12968-017-0413-z. Epub 2017/12/16.
Tanaka H, Tatsumi K, Matsuzoe H, Matsumoto K, Hirata KI. Impact of diabetes mellitus on left ventricular longitudinal function of patients with non-ischemic dilated cardiomyopathy. Cardiovasc Diabetol. 2020;19(1):84. https://doi.org/10.1186/s12933-020-01063-y. Epub 2020/06/15.
Gamaza-Chulian S, Diaz-Retamino E, Gonzalez-Teston F, Gaitero JC, Castillo MJ, Alfaro R, et al. Effect of sodium-glucose cotransporter 2 (SGLT2) inhibitors on left ventricular remodelling and longitudinal strain: a prospective observational study. BMC Cardiovasc Disord. 2021;21(1):456. https://doi.org/10.1186/s12872-021-02250-9. Epub 2021/09/23.
Tanaka H, Soga F, Tatsumi K, Mochizuki Y, Sano H, Toki H, et al. Positive effect of dapagliflozin on left ventricular longitudinal function for type 2 diabetic mellitus patients with chronic heart failure. Cardiovasc Diabetol. 2020;19(1):6. https://doi.org/10.1186/s12933-019-0985-z. Epub 2020/01/09.
Kalam K, Otahal P, Marwick TH. Prognostic implications of global LV dysfunction: a systematic review and meta-analysis of global longitudinal strain and ejection fraction. Heart. 2014;100(21):1673–80. https://doi.org/10.1136/heartjnl-2014-305538. Epub 2014/05/27.
Taylor RJ, Moody WE, Umar F, Edwards NC, Taylor TJ, Stegemann B, et al. Myocardial strain measurement with feature-tracking cardiovascular magnetic resonance: normal values. Eur Heart J Cardiovasc Imaging. 2015;16(8):871–81. https://doi.org/10.1093/ehjci/jev006. Epub 2015/02/26.
Onishi T, Saha SK, Delgado-Montero A, Ludwig DR, Onishi T, Schelbert EB, et al. Global longitudinal strain and global circumferential strain by speckle-tracking echocardiography and feature-tracking cardiac magnetic resonance imaging: comparison with left ventricular ejection fraction. J Am Soc Echocardiogr. 2015;28(5):587–96. https://doi.org/10.1016/j.echo.2014.11.018. Epub 2015/01/13.
Sciatti E, Merlo A, Scangiuzzi C, Limonta R, Gori M, D’Elia E et al. Prognostic Value of sST2 in Heart Failure. J Clin Med. 2023;12(12). Epub 2023/06/28. https://doi.org/10.3390/jcm12123970. PubMed PMID: 37373664; PubMed Central PMCID: PMCPMC10299183.
Pascual-Figal DA, Januzzi JL. The biology of ST2: the International ST2 Consensus Panel. Am J Cardiol. 2015;115(7 Suppl):3B-7B. Epub 2015/02/11. https://doi.org/10.1016/j.amjcard.2015.01.034. PubMed PMID: 25665766.
Berezin AE, Berezin AA. Circulating Cardiac biomarkers in diabetes Mellitus: a New Dawn for Risk Stratification-A Narrative Review. Diabetes Ther. 2020;11(6):1271–91. https://doi.org/10.1007/s13300-020-00835-9. Epub 2020/05/21.
Aimo A, Januzzi JL Jr., Bayes-Genis A, Vergaro G, Sciarrone P, Passino C, et al. Clinical and prognostic significance of sST2 in Heart failure: JACC Review topic of the Week. J Am Coll Cardiol. 2019;74(17):2193–203. https://doi.org/10.1016/j.jacc.2019.08.1039. Epub 2019/10/28.
Ky B, French B, McCloskey K, Rame JE, McIntosh E, Shahi P, et al. High-sensitivity ST2 for prediction of adverse outcomes in chronic heart failure. Circ Heart Fail. 2011;4(2):180–7. https://doi.org/10.1161/CIRCHEARTFAILURE.110.958223. Epub 2010/12/24.
AbouEzzeddine OF, McKie PM, Dunlay SM, Stevens SR, Felker GM, Borlaug BA et al. Suppression of Tumorigenicity 2 in Heart Failure With Preserved Ejection Fraction. J Am Heart Assoc. 2017;6(2). Epub 2017/02/20. https://doi.org/10.1161/JAHA.116.004382. PubMed PMID: 28214792; PubMed Central PMCID: PMCPMC5523750.
Demissei BG, Cotter G, Prescott MF, Felker GM, Filippatos G, Greenberg BH, et al. A multimarker multi-time point-based risk stratification strategy in acute heart failure: results from the RELAX-AHF trial. Eur J Heart Fail. 2017;19(8):1001–10. https://doi.org/10.1002/ejhf.749. Epub 2017/01/31.
Aimo A, Vergaro G, Passino C, Ripoli A, Ky B, Miller WL, et al. Prognostic value of Soluble suppression of Tumorigenicity-2 in Chronic Heart failure: a Meta-analysis. JACC Heart Fail. 2017;5(4):280–6. https://doi.org/10.1016/j.jchf.2016.09.010. Epub 2016/11/07.
Aimo A, Vergaro G, Ripoli A, Bayes-Genis A, Pascual Figal DA, de Boer RA, et al. Meta-analysis of Soluble suppression of Tumorigenicity-2 and prognosis in Acute Heart failure. JACC Heart Fail. 2017;5(4):287–96. https://doi.org/10.1016/j.jchf.2016.12.016. Epub 2017/02/13.
Castiglione V, Chiriaco’ M, Aimo A, Januzzi J, Richards AM, Lam CSP, et al. Prognostic value of sST2 in heart failure patients with diabetes. Eur Heart J. 2021;42(Supplement1). https://doi.org/10.1093/eurheartj/ehab724.0869.
Gurgoze MT, van Vark LC, Baart SJ, Kardys I, Akkerhuis KM, Manintveld OC, et al. Multimarker Analysis of serially measured GDF-15, NT-proBNP, ST2, GAL-3, cTnI, Creatinine, and prognosis in Acute Heart failure. Circ Heart Fail. 2023;16(1):e009526. PubMed PMID: 36408685; PubMed Central PMCID: PMCPMC9833118.
van Vark LC, Lesman-Leegte I, Baart SJ, Postmus D, Pinto YM, Orsel JG, et al. Prognostic value of serial ST2 measurements in patients with Acute Heart failure. J Am Coll Cardiol. 2017;70(19):2378–88. https://doi.org/10.1016/j.jacc.2017.09.026. Epub 2017/11/04.
Weir RA, Miller AM, Murphy GE, Clements S, Steedman T, Connell JM, et al. Serum soluble ST2: a potential novel mediator in left ventricular and infarct remodeling after acute myocardial infarction. J Am Coll Cardiol. 2010;55(3):243–50. PubMed PMID: 20117403.
Gaggin HK, Motiwala S, Bhardwaj A, Parks KA, Januzzi JL. Jr. Soluble concentrations of the interleukin receptor family member ST2 and beta-blocker therapy in chronic heart failure. Circ Heart Fail. 2013;6(6):1206–13. https://doi.org/10.1161/CIRCHEARTFAILURE.113.000457. Epub 2013/10/12.
Maisel A, Xue Y, van Veldhuisen DJ, Voors AA, Jaarsma T, Pang PS, et al. Effect of spironolactone on 30-day death and heart failure rehospitalization (from the COACH Study). Am J Cardiol. 2014;114(5):737–42. https://doi.org/10.1016/j.amjcard.2014.05.062. Epub 2014/08/19.
Bayes-Genis A, Aimo A, Jhund P, Richards M, de Boer RA, Arfsten H, et al. Biomarkers in heart failure clinical trials. A review from the Biomarkers Working Group of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2022;24(10):1767–77. https://doi.org/10.1002/ejhf.2675. Epub 2022/09/09.
Chiariello M, Indolfi C. Silent myocardial ischemia in patients with diabetes mellitus. Circulation. 1996;93(12):2089–91. https://doi.org/10.1161/01.cir.93.12.2089. Epub 1996/06/15.
Nham E, Kim SM, Lee SC, Chang SA, Sung J, Cho SJ, et al. Association of cardiovascular disease risk factors with left ventricular mass, biventricular function, and the presence of silent myocardial infarction on cardiac MRI in an asymptomatic population. Int J Cardiovasc Imaging. 2016;32 Suppl 1:173–81. https://doi.org/10.1007/s10554-016-0885-1. Epub 2016/05/23. PubMed PMID: 27209284.
Russo I, Frangogiannis NG. Diabetes-associated cardiac fibrosis: Cellular effectors, molecular mechanisms and therapeutic opportunities. J Mol Cell Cardiol. 2016;90:84–93. https://doi.org/10.1016/j.yjmcc.2015.12.011. Epub 2015/12/26.
Borghetti G, von Lewinski D, Eaton DM, Sourij H, Houser SR, Wallner M. Diabetic Cardiomyopathy: current and future therapies. Beyond Glycemic Control Front Physiol. 2018;9:1514. https://doi.org/10.3389/fphys.2018.01514. Epub 2018/11/15.
Haaf P, Garg P, Messroghli DR, Broadbent DA, Greenwood JP, Plein S. Cardiac T1 mapping and extracellular volume (ECV) in clinical practice: a comprehensive review. J Cardiovasc Magn Reson. 2016;18(1):89. https://doi.org/10.1186/s12968-016-0308-4. Epub 2016/12/03.
Swoboda PP, McDiarmid AK, Erhayiem B, Ripley DP, Dobson LE, Garg P, et al. Diabetes Mellitus, Microalbuminuria, and subclinical Cardiac Disease: identification and monitoring of individuals at risk of heart failure. J Am Heart Assoc. 2017;6(7). https://doi.org/10.1161/JAHA.117.005539. PubMed PMID: 28716801; PubMed Central PMCID: PMCPMC5586286. Epub 2017/07/19.
Cao Y, Zeng W, Cui Y, Kong X, Wang M, Yu J, et al. Increased myocardial extracellular volume assessed by cardiovascular magnetic resonance T1 mapping and its determinants in type 2 diabetes mellitus patients with normal myocardial systolic strain. Cardiovasc Diabetol. 2018;17(1):7. https://doi.org/10.1186/s12933-017-0651-2. Epub 2018/01/06.
Lam B, Stromp TA, Hui Z, Vandsburger M. Myocardial native-T1 times are elevated as a function of hypertrophy, HbA1c, and heart rate in diabetic adults without diffuse fibrosis. Magn Reson Imaging. 2019;61:83 – 9. Epub 2019/05/28. https://doi.org/10.1016/j.mri.2019.05.029. PubMed PMID: 31125612; PubMed Central PMCID: PMCPMC6663625.
Kucukseymen S, Neisius U, Rodriguez J, Tsao CW, Nezafat R. Negative synergism of diabetes mellitus and obesity in patients with heart failure with preserved ejection fraction: a cardiovascular magnetic resonance study. Int J Cardiovasc Imaging. 2020;36(10):2027–38. https://doi.org/10.1007/s10554-020-01915-4. Epub 2020/06/14.
Kellman P, Hansen MS. T1-mapping in the heart: accuracy and precision. J Cardiovasc Magn Reson. 2014;16(1):2. Epub 2014/01/07. https://doi.org/10.1186/1532-429X-16-2. PubMed PMID: 24387626; PubMed Central PMCID: PMCPMC3927683.
Treibel TA, Fridman Y, Bering P, Sayeed A, Maanja M, Frojdh F, et al. Extracellular volume associates with outcomes more strongly than native or post-contrast myocardial T1. JACC Cardiovasc Imaging. 2020;13(1 Pt 1):44–54. https://doi.org/10.1016/j.jcmg.2019.03.017. Epub 2019/05/20.
Aguilar D, Bozkurt B, Ramasubbu K, Deswal A. Relationship of hemoglobin A1C and mortality in heart failure patients with diabetes. J Am Coll Cardiol. 2009;54(5):422–8. https://doi.org/10.1016/j.jacc.2009.04.049. Epub 2009/07/25.
Acknowledgements
The contribution of all the site investigators and clinical co-ordinators is acknowledged.
Funding
The ATTRaCT study was supported by research grants from A*STAR Biomedical Research Council ATTRaCT program [SPF2014/003, SPF2014/004, SPF2014/005] (A*STAR, Singapore). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Consortia
Contributions
CSPL and AMR designed the study. PJC, FE, RF and CWLC made substantial contributions to the study design. NRI, SPC, OWL, JPCC, JAB and TTL collected the data. NRI analysed the data. NRI, MU and CWLC interpreted the data and wrote the manuscript. CC and AMR made substantial revisions to the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethics approval was obtained from the SingHealth Centralized Institutional Review Board in Singapore, and all participants provided written informed consent. The study was conducted in accordance with the principles of the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
CSPL is supported by a Clinician Scientist Award from the National Medical Research Council of Singapore; has received research support from Boston Scientific, Bayer, Roche Diagnostics, AstraZeneca, Medtronic, and Vifor Pharma; has served as consultant or on the Advisory Board/ Steering Committee/ Executive Committee for Abbott Diagnostics, Amgen, Applied Therapeutics, AstraZeneca, Bayer, Biofourmis, Boehringer Ingelheim, Boston Scientific, Corvia Medical, Cytokinetics, Darma Inc., Us2.ai, JanaCare, Janssen Research & Development LLC, Medtronic, Menarini Group, Merck, MyoKardia, Novartis, Novo Nordisk, Radcliffe Group Ltd., Roche Diagnostics, Sanofi, Stealth BioTherapeutics, The Corpus, Vifor Pharma and WebMD Global LLC; and serves as co-founder & non-executive director of Us2.ai. MU is principal investigator for an institutional research and development agreement regarding cardiovascular magnetic resonance imaging between Karolinska University Hospital and Siemens. AMR receives research grants, consultancy fees, advisory board fees and/or laboratory support-in-kind from Roche Diagnostics, Abbott Laboratories, Thermo Fisher, Novo Nordisk, Sphyngotec and Novartis and holds the New Zealand Heart Foundation Chair in Cardiovascular Studies. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Iyer, N.R., Chan, SP., Liew, O.W. et al. Global longitudinal strain and plasma biomarkers for prognosis in heart failure complicated by diabetes: a prospective observational study. BMC Cardiovasc Disord 24, 141 (2024). https://doi.org/10.1186/s12872-024-03810-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-024-03810-5