Abstract
Introduction
Aspirin is widely used for secondary prevention in patients with hypertension. However, previous studies mainly focused on the preventive effects of aspirin, and there has been a lack of reliable evidence on whether taking aspirin affects blood pressure This study aimed to investigate whether aspirin would affect the blood pressure in patients with hypertension.
Methods
PubMed, Cochrane database, Embase, Scopus and Medline databases were searched until September 2023. For continuous variables (e.g., blood pressure reduction), the mean difference (MD) was selected as the effect magnitude indices. We used the Cochrane Collaboration’s Risk of Bias tool to assess the risk of bias.
Result
A total of five studies were included, comprising 20,312 patients. We found that aspirin did not affect SBP (MD = -0.78, 95% CI: − 2.41, 0.84). A similar result was found for DBP (MD = -0.86, 95% CI: − 2.14, 0.42).
Conclusion
This study showed no significant difference in blood pressure between the aspirin and control groups, suggesting that aspirin does not affect blood pressure.
Similar content being viewed by others
Introduction
Among patients diagnosed with hypertension, only 13.8% were considered controlled [1], and more than 9 million people die each year from hypertension-related diseases [2]. The most severe risk of hypertension is its complications, elevated systolic and diastolic blood pressures are strongly associated with cardiovascular disease risk [3, 4]. A follow-up study of 23,272 patients in the National Health and Nutrition Examination Survey (NHANES) showed that more than 50% of patients who died of coronary heart disease and stroke combined with hypertension [5], Population-based Atherosclerosis Risk in Communities (ARIC) study shows 25% of cardiovascular events are associated with hypertension [6]. However, despite a large number of hypertensive patients and the horrible complications it causes, the treatment of hypertension remains unsatisfactory. Unlike other diseases, hypertension has no apparent symptoms; in other words, hypertension is a so-called silent disease, so fewer patients will request treatment at an early stage or fail to follow prescriptions carefully [7]. Corrao et al. reported that more than 40% of patients would not continue initiating drug therapy within 1 year [8], about 10% of patients forget to take their daily medications [9]. It is therefore not surprising that exploring additional and complementary therapies for hypertension [10].
Aspirin is widely used for the secondary prevention of cardiovascular disease with positive effects [11,12,13]. In some secondary prevention studies, aspirin has been found to reduce the incidence of myocardial infarction and ischemic stroke [14,15,16]. However, previous studies mainly focused on the preventive effects of aspirin, and few studies have focused on the effects of aspirin on blood pressure [17]. There has been a lack of reliable evidence on whether taking aspirin affects blood pressure. Does it lower blood pressure and work in conjunction with other anti-hypertensive medications, or does it have no effect on blood pressure, or is it even more likely to cause fluctuations in blood pressure when taken over a long period? Some studies have found no relationship between aspirin and blood pressure [18,19,20,21,22], while Hermida et al. reported that taking aspirin at bedtime lowered blood pressure [23,24,25,26,27,28]. In their study, untreated hypertensive patients taking aspirin at bedtime reduced SBP and DBP by 6 mmHg and 4 mmHg, respectively. In conclusion, it remains uncertain what effect aspirin has on blood pressure, and therefore, studies are necessary to clarify the relationship between aspirin and blood pressure.
Methods
This study was designed and carried out with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) criteria [29]. The protocol was registered in PROSPERO. (PROSPERO 2022 CRD42022346453).
Eligibility criteria
Inclusion and exclusion criteria were based on population, interventions, comparisons, outcomes (PICO) criteria.
Studies meeting the following criteria will be included: (1) Participants should be at least 18 years old and diagnosed with hypertension (defined as systolic blood pressure greater than 140 mmHg or diastolic blood pressure greater than 90 mmHg, or both), their blood pressure was measured by validation techniques, receiving other anti-hypertensive medications was not restricted during the study period. (2) Participants in the intervention group should take aspirin, and the control group should take placebo or no treatment at all. (3) The study should report BP reduction as an outcome.
Studies with the following characteristics will be excluded: (1) Reviews, case reports and, conference abstracts. (2) Studies with less than 50 participants. (3) Literature for which experimental data were not available. (4) Articles for which full text was not available. (5) Non-clinical studies, such as in vivo or in vitro experiments. (6) Non-RCTs, including cohort studies and case-control trials. (7) Studies written in languages other than English.
After reading the title and abstract of the article, articles that met the inclusion criteria and did not conflict with the exclusion criteria will be read in full. Articles that had been read in full and meet the criteria will undergo data extraction. All publications were screened independently by two authors (Li, Xu), any disagreements were resolved after discussions between the two authors or by consulting a third reviewer.
Search strategy
We searched the following databases for relevant papers: PubMed, Cochrane Library, Scopus, Embase, and Medline. We also searched the clinical research registry websites ClinicalTrials (clinicaltrials.gov) and ICTRP (trialsearch.who.int) to ensure no pertinent studies were missed. Searches were conducted up to September 2023.
Data extraction
We utilized a data extraction form based on the Data Extraction Form of the Cochrane Review Group (Cochrane Collaboration) and modified some of its items to suit our study. The data extraction form mainly included the following: age, gender, race, country, blood pressure, intervention method, intervention duration, and primary outcomes. Two authors (Li, Xu) collected the data independently, and any disagreements were resolved through discussions between the two authors or by consulting a third reviewer.
Quality assessment
As only RCTs were included, we used the Cochrane Collaboration’s Risk of Bias tool to assess study quality [30]. We used the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) criteria to assess the quality of evidence.
Statistical analysis
We used Stata17 and Review Manager 5.4 software for data synthesis, with mean difference (MD) selected as the Effect Magnitude Indices for continuous variables (e.g., blood pressure reduction). For studies with more than one intervention group, we combined all intervention groups into one group as recommended by the Cochrane Handbook for the Systematic Review of Interventions [31]. Higgin’s I2 statistics and Cochran’s Q test were used to detect statistical heterogeneity between various studies [32]. When I2 > 50%, a random-effects model was used to evaluate the pooled results; otherwise, a fixed-effects model was used to analyze the pooled results. We used meta-regression to identify any possible sources of heterogeneity. A prespecified subgroup analysis was conducted according to [33] duration of intervention (>3 months vs. <3 months), time of aspirin administration (morning vs. evening), co-administration of other medications (co-administration of other medications vs. aspirin only), and type of control (placebo vs. no treatment). A sensitivity analysis was performed to assess the combined data’s stability and to pinpoint the cause of any heterogeneity. Begg’s and Egger’s linear regression tests were used to investigate publication bias [34].
Result
Study selection
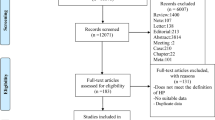
A total of 14,026 studies were identified through the database search. 4608 studies were removed due to duplication. After reading the titles and abstracts, 9383 articles were excluded. 30 articles were excluded after reading the full text for the following reasons: 3 studies could not obtain the full text, 9 were excluded because the intervention group did not meet the criteria, 1 was written in a language other than English, 2 did not provide blood pressure as an outcome, seven participants did not meet the criteria, 5 study type did not meet the criteria, 3 unable to obtain data. After that, 5 studies were included for analysis [22, 25, 35,36,37] (Fig. 1).
Characteristics of the included studies
The characteristics of the eight included studies are shown in Table 1. A total of 20,312 participants were included. All participants were diagnosed with hypertension, mostly from high-income countries (HICs), such as Italy, Spain, and Poland. They were taking small doses of aspirin - usually less than or equal to 100 mg/day. Most studies had less than 3 months of follow-up. One study used placebo as a control; other studies did not have a treatment for the control group. One study prohibited participants from receiving other drugs concurrently, and four studies did not restrict.
Risk of bias in included studies
We used the Cochrane Collaboration’s Risk of Bias tool to assess study quality, and the results are shown in Fig. 2. We considered the risk of bias unclear for studies where information supporting the judgment could not be found in the article. Avanzini et al. lost 14.5% of participants during the study, which may be a high risk for incomplete outcome data, especially considering that some of these individuals may quit the study due to side effects. One study was open-label, which we thought would lead to performance bias, so the risk of bias was set to high. One study did not report some of the outcome measures mentioned in the protocol, which we believed would create a high risk of reporting bias.
BP reduction
A total of 5 studies, including 20,312 individuals, were included in this study. Given the significant degree of heterogeneity (I2 = 58.85 and 70.00% for SBP and DBP, respectively), a random effects model was used. In terms of SBP, only one study found a significant decrease in SBP in patients treated with aspirin compared to controls [37]; the other studies showed no difference between the two groups, and the pooled results also suggest that aspirin does not affect SBP (MD = -0.78, 95% CI: − 2.41, 0.84). Similar result was found for DBP (MD = -0.86, 95% CI: − 2.14, 0.42). The forest plot of the synthesis results is shown in Fig. 3. The data are shown in Table 2. The GRADE ratings for both outcomes are low (Table 3).
Meta-regression
Since it can be considered a source of heterogeneity when the P-value is less than 0.05, we may infer that different administration times may be a source of significant heterogeneity. (P = 0.051, 0.001, respectively) (Table 4A). According to the meta-regression results, the remaining various may not be a source of heterogeneity in this study (p = 0.320,0.376 for control, Table 4B; p = 0.628,0.638 for medication, Table 4C; p = 0.320,0.376 for duration, Table 4D).
Subgroup analysis
A subgroup analysis was conducted based on time of aspirin administration (morning vs. evening), duration of intervention (>three months vs. <three months), co-administration of other medications (co-administration vs. aspirin only), and type of control (placebo vs. no treatment). In the subgroup analysis of different administration times, we found heterogeneity decreased in both subgroups. This result suggested that dosing time may be one of the reasons for the heterogeneity (Fig. 4) (Table 5). There were no significant differences in the remaining subgroups (Figs. 5, 6 and 7) (Table 6, 7 and 8).
Publish bias test
We used Begg’s and Egger’s linear regression tests to detect publication bias (Table 9). The results of Egger’s test and Begg’s test are shown in Figs. 8 and 9. No asymmetry was observed in the funnel plot. According to Egger’s test, no publication bias was observed (P = 0.145, P = 0.174). A similar conclusion can be derived from Begg’s test (P = 0.221, P = 0.462).
Analysis of sensitivity
We found that removing studies one by one did not significantly change the results, suggesting that the overall results were not influenced by individual studies. The results for SBP ranged from 0.84 (95% CI: 0.66–1.08) to 1.05 (95% CI: 1.02–1.08), and for DBP from 0.81 (95% CI: 0.61–1.08) to 1.04 (95% CI: 1.01–1.07) (Fig. 10).
Discussion
Some hypertensive patients need to take aspirin for a long time to prevent cardiovascular events, so it is natural to wonder whether aspirin affects blood pressure and the effect of antihypertensive drugs. Aspirin blocks TXA2 and promotes nitric oxide synthesis, which may lead to vasodilation, reduced peripheral vascular resistance, and lower blood pressure [36]. Some studies have also found that aspirin inhibits COX-1 and COX-2. Inhibition of COX-1 leads to vasoconstriction, which in turn leads to increased blood pressure [38]. In addition, some studies have shown that different dosing times or drug interactions can also affect blood pressure [28, 39, 40]. Because of conflicting findings from previous studies, we conducted this meta-analysis and systematic review to clarify what effect aspirin would have on patients with hypertension.
Our study found that aspirin did not change blood pressure levels, either SBP (MD = -0.78, 95% CI: − 2.41, 0.84) or DBP (MD = -0.86, 95% CI: − 2.14, 0.42). We found no significant difference between subgroups after subgroup analysis for factors such as administration time, follow-up, type of control, and concomitant treatment with other medications. However, caution should be exercised in interpreting the findings due to the few included studies. Although some studies have reported that different administration times, especially at bedtime, can lower blood pressure [27, 28], our study showed no significant difference between taking aspirin at bedtime and in the morning. However, the pooled results for the different subgroups do show contrasting trends, and perhaps with more studies included, this difference will be statistically significant. Patients with hypertension often have other co-morbidities that require them to take multiple medications simultaneously. Subgroup analysis showed that concomitant administration of other drugs does not change the result, which could indicate that aspirin does not interfere with other medications, which is consistent with the findings of Johnson et al. [41]. The rest of the subgroup analyses also showed no significant differences between groups. Begg’s test and Egger’s test showed no significant publication bias. However, the publication bias test is not very reliable due to the small number of included studies [34]. Sensitivity analysis showed that the pooled results were not affected by any single studies, and after removing individual studies, the conclusions remained consistent with the main study findings.
In fact, despite some controversy, most people agree that aspirin does not affect blood pressure [20, 21, 42, 43], and our study simply provides more compelling evidence to dispel some of the concerns in clinical practice. Aspirin, primarily used as an antiplatelet drug, may affect blood pressure through certain pathways. For example, it can inhibit the synthesis of the vasodilators PGI2 and PGE2 by blocking COX-1 and COX-2 and causing sodium and water retention [44]. Aspirin can also act in the vascular endothelium to inhibit the expression of pro-inflammatory cytokines and the adhesion of leukocytes, increasing the generation of nitric oxide, which ultimately leads to vasodilatation. This effect becomes more potent as thromboxane A2 and prostaglandins decrease [45,46,47]. We hypothesized that perhaps it is this delicate balance that led to the results of our study, namely that taking aspirin did not affect blood pressure. On the other hand, the effects mentioned above are limited and indirect because aspirin cannot directly affect the mechanisms that regulate blood pressure, which may explain why no blood pressure fluctuations were observed in patients taking aspirin. Our findings are also consistent with some recent studies; for example, in the study by Mirabito Colafella et al., they found that aspirin does not affect vascular function [48]. In a study by Dong et al., they found that aspirin has no significant effect on the gut microbiota of spontaneously hypertensive rats, and alterations in the gut microbiota may be associated with hypertension [49].
Subgroup analyses of different dosing times showed no significant difference in the antihypertensive effect when administered in the morning versus in the afternoon or at bedtime. However, due to the small number of participants in the bedtime dosing subgroup, this conclusion may not be as reliable, especially since some studies did propose that different dosing times result in different antihypertensive effects [50]. Since the secretion of nitric oxide, prostaglandins, angiotensin II, and angiotensin-converting enzyme has a circadian rhythm, different administration times may produce different effects [51, 52]. However, whether these differences are sufficient to alter blood pressure levels remains uncertain. Therefore, we believe there is a need for more trials focusing on this variable.
Limitations
This study also has some limitations. First, most of the included studies had small sample sizes or low study quality, which would lead to unstable and less credible results. Second, the number of included studies was also small, so the assessment of publication bias may lack accuracy, and the overall results may also lack statistical significance. Finally, most of the included studies did not use placebo as a control. Since 20–24% of long-term changes in blood pressure are attributable to the placebo effect [53], the lack of placebo may cause a misinterpretation of the study results.
Future directions
Our findings reduce some of the uncertainty in clinical practice and the process of developing guidelines. Clinicians may not have to worry about whether prescribing aspirin to patients with controlled hypertension will cause blood pressure fluctuations. As aspirin is widely used for secondary prevention of cardiovascular disease, this study may lead to updates in some guidelines. In conclusion, our systematic review and meta-analysis summarizes the results of global studies and draws compelling conclusions about whether aspirin affects blood pressure.
Conclusion
This meta-analysis investigated whether taking aspirin affects blood pressure. The results showed no significant difference in blood pressure between the intervention and control groups, suggesting that aspirin does not lower or raise blood pressure. However, more studies are needed to confirm these findings.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- NSAIDs:
-
Nonsteroidal anti-inflammatory drugs
- COX:
-
Cyclooxygenase
- PG:
-
Prostaglandin
- TXA2:
-
Thromboxane A2
- BP:
-
Blood pressure
- RCT:
-
Randomized controlled trial
- MD:
-
Mean difference
- CI:
-
Confidence intervals
References
Mills KT, Bundy JD, Kelly TN, Reed JE, Kearney PM, Reynolds K, et al. Global disparities of hypertension prevalence and control: a systematic analysis of population-based studies from 90 countries. Circulation. 2016;134(6):441–50.
Norheim OF, Jha P, Admasu K, Godal T, Hum RJ, Kruk ME, et al. Avoiding 40% of the premature deaths in each country, 2010-30: review of national mortality trends to help quantify the UN sustainable development goal for health. Lancet. 2015;385(9964):239–52.
Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360(9349):1903–13.
Rapsomaniki E, Timmis A, George J, Pujades-Rodriguez M, Shah AD, Denaxas S, et al. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life-years lost, and age-specific associations in 1·25 million people. Lancet. 2014;383(9932):1899–911.
Ford ES. Trends in mortality from all causes and cardiovascular disease among hypertensive and nonhypertensive adults in the United States. Circulation. 2011;123(16):1737–44.
Cheng S, Claggett B, Correia AW, Shah AM, Gupta DK, Skali H, et al. Temporal trends in the population attributable risk for cardiovascular disease: the atherosclerosis risk in communities study. Circulation. 2014;130(10):820–8.
Cushman WC, Evans GW, Byington RP, Goff DC, Grimm RH, Cutler JA, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362(17):1575–85.
Corrao G, Zambon A, Parodi A, Poluzzi E, Baldi I, Merlino L, et al. Discontinuation of and changes in drug therapy for hypertension among newly-treated patients: a population-based study in Italy. J Hypertens. 2008;26(4):819–24.
Lee JK, Grace KA, Taylor AJ. Effect of a pharmacy care program on medication adherence and persistence, blood pressure, and low-density lipoprotein cholesterol: a randomized controlled trial. JAMA. 2006;296(21):2563–71.
Rahman K, Lowe GM. Garlic and cardiovascular disease: a critical review. J Nutr. 2006;136(3 Suppl):736S-740S.
Gu Q, Dillon CF, Eberhardt MS, Wright JD, Burt VL. Preventive aspirin and other antiplatelet medication use among U.S. adults aged ≥ 40 years: data from the National Health and nutrition examination survey, 2011-2012. Public Health Rep. 2015;130(6):643–54.
Duffy D, Kelly E, Trang A, Whellan D, Mills G. Aspirin for cardioprotection and strategies to improve patient adherence. Postgrad Med. 2014;126(1):18–28.
Shinohara Y, Katayama Y, Uchiyama S, Yamaguchi T, Handa S, Matsuoka K, et al. Cilostazol for prevention of secondary stroke (CSPS 2): an aspirin-controlled, double-blind, randomised non-inferiority trial. Lancet Neurol. 2010;9(10):959–68.
Baigent C, Blackwell L, Collins R, Emberson J, Godwin J, Peto R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373(9678):1849–60.
Gorelick PB, Weisman SM. Risk of hemorrhagic stroke with aspirin use: an update. Stroke. 2005;36(8):1801–7.
Hennekens CH, Buring JE, Sandercock P, Collins R, Peto R. Aspirin and other antiplatelet agents in the secondary and primary prevention of cardiovascular disease. Circulation. 1989;80(4):749–56.
Lip GY, Felmeden DC, Dwivedi G. Antiplatelet agents and anticoagulants for hypertension. Cochrane Database Syst Rev. 2011;12:CD003186.
Polónia J, Boaventura I, Gama G, Camões I, Bernardo F, Andrade P, et al. Influence of non-steroidal anti-inflammatory drugs on renal function and 24h ambulatory blood pressure-reducing effects of enalapril and nifedipine gastrointestinal therapeutic system in hypertensive patients. J Hypertens. 1995;13(8):925–31.
Smith SR, Coffman TM, Svetkey LP. Effect of low-dose aspirin on thromboxane production and the antihypertensive effect of captopril. J Am Soc Nephrol. 1993;4(5):1133–9.
Zanchetti A, Hansson L, Leonetti G, Rahn K-H, Ruilope L, Warnold I, et al. Low-dose aspirin does not interfere with the blood pressure-lowering effects of antihypertensive therapy. J Hypertens. 2002;20(5):1015–22.
Nawarskas JJ, Townsend RR, Cirigliano MD, Spinler SA. Effect of aspirin on blood pressure in hypertensive patients taking enalapril or losartan. Am J Hypertens. 1999;12(8 Pt 1):784–9.
Avanzini F, Palumbo G, Alli C, Roncaglioni MC, Ronchi E, Cristofari M, et al. Effects of low-dose aspirin on clinic and ambulatory blood pressure in treated hypertensive patients. Collaborative Group of the Primary Prevention Project (PPP)--Hypertension study. Am J Hypertens. 2000;13(6 Pt 1):611–6.
Hermida RC, Ayala DE, Fernández JR, Mojón A, Alonso I, Silva I, et al. Administration time-dependent effects of aspirin in women at differing risk for preeclampsia. Hypertension. 1999;34(4 Pt 2):1016–23.
Hermida RC, Ayala DE, Iglesias M. Administration time-dependent influence of aspirin on blood pressure in pregnant women. Hypertension. 2003;41(3 Pt 2):651–6.
Hermida RC, Ayala DE, Calvo C, López JE, Fernández JR, Mojón A, et al. Administration time-dependent effects of aspirin on blood pressure in untreated hypertensive patients. Hypertension. 2003;41(6):1259–67.
Hermida RC, Fernández JR, Ayala DE, Mojón A, Iglesias M. Influence of aspirin usage on blood pressure: dose and administration-time dependencies. Chronobiol Int. 1997;14(6):619–37.
Hermida RC, Ayala DE, Calvo C, López JE, Mojón A, Rodríguez M, et al. Differing administration time-dependent effects of aspirin on blood pressure in dipper and non-dipper hypertensives. Hypertension. 2005;46(4):1060–8.
Hermida RC, Ayala DE, Mojón A, Fernández JR. Ambulatory blood pressure control with bedtime aspirin administration in subjects with prehypertension. Am J Hypertens. 2009;22(8):896–903.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ (Clinical Research ed). 2009;339:b2535.
Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ (Clinical Research ed). 2011;343:d5928.
Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration; 2011. Available from handbook.cochrane.org.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60.
Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane handbook for systematic reviews of interventions. Cochrane Database Syst Rev. 2019;10:ED000142.
Hayashino Y, Noguchi Y, Fukui T. Systematic evaluation and comparison of statistical tests for publication bias. J Epidemiol. 2005;15(6):235–43.
Hansson L, Zanchetti A, Carruthers SG, Dahlöf B, Elmfeldt D, Julius S, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the hypertension optimal treatment (HOT) randomised trial. HOT Study Group Lancet. 1998;351(9118):1755–62.
Hermida RC, Ayala DE, Calvo C, López JE. Aspirin administered at bedtime, but not on awakening, has an effect on ambulatory blood pressure in hypertensive patients. J Am Coll Cardiol. 2005;46(6):975–83.
Krasinska B, Paluszkiewicz L, Miciak-Lawicka E, Krasinski M, Rzymski P, Tykarski A, et al. The impact of acetylsalicylic acid dosed at bedtime on circadian rhythms of blood pressure in the high-risk group of cardiovascular patients-a randomized, controlled trial. Eur J Clin Pharmacol. 2021;77(1):35–43.
Zanchetti A, Hansson L, Dahlöf B, Julius S, Ménard J, Warnold I, et al. Benefit and harm of low-dose aspirin in well-treated hypertensives at different baseline cardiovascular risk. J Hypertens. 2002;20(11):2301–7.
Snoep JD, Hovens MMC, Pasha SM, Frölich M, Pijl H, Tamsma JT, et al. Time-dependent effects of low-dose aspirin on plasma renin activity, aldosterone, cortisol, and catecholamines. Hypertension. 2009;54(5):1136–42.
Hermida RC, Crespo JJ, Domínguez-Sardiña M, Otero A, Moyá A, Ríos MT, et al. Bedtime hypertension treatment improves cardiovascular risk reduction: the Hygia chronotherapy trial. Eur Heart J. 2020;41(48):4565–76.
Johnson AG, Nguyen TV, Day RO. Do nonsteroidal anti-inflammatory drugs affect blood pressure? A meta-analysis. Ann Intern Med. 1994;121(4):289–300.
Bautista LE, Vera LM. Antihypertensive effects of aspirin: what is the evidence? Curr Hypertens Rep. 2010;12(4):282–9.
Wu R, Laplante M-A, De Champlain J. Prevention of angiotensin II-induced hypertension, cardiovascular hypertrophy and oxidative stress by acetylsalicylic acid in rats. J Hypertens. 2004;22(4):793–801.
Whelton A. Nephrotoxicity of nonsteroidal anti-inflammatory drugs: physiologic foundations and clinical implications. Am J Med. 1999;106(5B):13S-24S.
Monobe H, Yamanari H, Nakamura K, Ohe T. Effects of low-dose aspirin on endothelial function in hypertensive patients. Clin Cardiol. 2001;24(11):705–9.
Buga GM, Gold ME, Fukuto JM, Ignarro LJ. Shear stress-induced release of nitric oxide from endothelial cells grown on beads. Hypertension. 1991;17(2):187–93.
Madajka M, Korda M, White J, Malinski T. Effect of aspirin on constitutive nitric oxide synthase and the biovailability of NO. Thromb Res. 2003;110(5–6):317–21.
Mirabito Colafella KM, van Dorst DCH, Neuman RI, Lv D, Neves KB, Montezano AC, et al. Differential effects of cyclo-oxygenase 1 and 2 inhibition on angiogenesis inhibitor-induced hypertension and kidney damage. Clin Sci (Lond). 2022;136(9):675–94.
Dong S, Liu Q, Zhou X, Zhao Y, Yang K, Li L, et al. Effects of Losartan, Atorvastatin, and Aspirin on Blood Pressure and Gut Microbiota in Spontaneously Hypertensive Rats. Molecules. 2023;28(2).
Cortés-Ríos J, Rodriguez-Fernandez M. Understanding the dosing-time-dependent antihypertensive effect of valsartan and aspirin through mathematical modeling. Front Endocrinol (Lausanne). 2023;14:1110459.
Cermakian N, Westfall S, Kiessling S. Circadian clocks and inflammation: reciprocal regulation and shared mediators. Arch Immunol Ther Exp. 2014;62(4):303–18.
Kaur G, Phillips CL, Wong K, McLachlan AJ, Saini B. Timing of administration: for commonly-prescribed medicines in Australia. Pharmaceutics. 2016;8(2).
Staessen JA, Thijs L, Bieniaszewski L, O’Brien ET, Palatini P, Davidson C, et al. Ambulatory monitoring uncorrected for placebo overestimates long-term antihypertensive action. Systolic hypertension in Europe (SYST-EUR) trial investigators. Hypertension. 1996;27(3 Pt 1):414–20.
Acknowledgements
Not applicable.
Funding
This study was not funded.
Author information
Authors and Affiliations
Contributions
Jiang developed the search strategy for the review and assisted with the method description. Li and Xu completed article screening and completed data extraction. Li created the PRISMA diagram. Li and Jiang created the tables. Li performed the meta-analysis. Li, Jiang, and Xu prepared the manuscript. Lin Chen helped interpret the results and correct grammatical errors in the article. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors do not have conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, Z., Xu, S., Chen, L. et al. Effect of aspirin on blood pressure in hypertensive patients: a systematic review and meta-analysis. BMC Cardiovasc Disord 24, 90 (2024). https://doi.org/10.1186/s12872-024-03737-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-024-03737-x