Abstract
Background
The difference in the long-term outcomes of myocardial infarction in patients with non-obstructed coronary arteries (MINOCA) and patients with myocardial infarction with obstructed coronary artery disease (MI-CAD) is not clear. The current study aimed to pool adjusted data to compare long-term outcomes of MINOCA vs MI-CAD.
Methods
Electronic literature search of PubMed, Embase, CENTRAL, and Google Scholar databases was done for publications up to 18th June 2023. Only studies reporting multivariable-adjusted data with > 1 year of follow-up were included.
Results
Sixteen studies met the inclusion criteria. Our meta-analysis revealed no statistically significant difference in the risk of all-cause mortality between MINOCA and MI-CAD patients (HR: 0.90 95% CI 0.68, 1.19 I2 = 94% p = 0.48). Analysis of the limited data showed a reduced combined risk of all-cause mortality and MI (HR: 0.54 95% CI 0.39, 0.76 I2 = 72% p = 0.003) and major adverse cardiac events (MACE) (HR: 0.66 95% CI 0.51, 0.84 I2 = 51% p = 0.0009) in patients with MINOCA vs MI-CAD, and no difference in the risk of cardiovascular mortality (HR: 0.81 95% CI 0.54, 1.22 I2 = 0% p = 0.31) and readmission between the two groups (HR: 0.85 95% CI 0.61, 1.19 I2 = 90% p = 0.35).
Conclusion
A pooled analysis of adjusted outcomes from the available studies indicated that MINOCA and MI-CAD patients have similar long-term all-cause mortality risk. Our conclusions on the risk of cardiovascular mortality, MACE and readmission rates need to be taken with caution due to a lack of adequate studies. Further research is needed to strengthen the evidence on this important subject.
Similar content being viewed by others
Introduction
Acute myocardial infarction (MI) accounts for a significant portion of morbidity and mortality cases around the world [1]. Studies indicate that compared to general population, patients with MI are at 30%-higher risk of mortality and adverse cardiovascular events [2]. The use of coronary angiography during the early management of this disease significantly improves identification of patients with MI and non-obstructed coronary arteries (MINOCA) [3]. A systematic review by Pasupathy et al. [4] indicated that the prevalence of MINOCA is around 6%, ranging between 1 and 14%. Patients with MINOCA tend to be younger, of the female sex, and with lower incidence of hyperlipidaemia compared to patients with MI and obstructed coronary artery disease (MI-CAD) [4].
Based on the guidelines of the European Society of Cardiology, diagnosis of MINOCA requires evidence of MI along with the demonstration of < 50% stenosis on a coronary angiogram [5, 6]. Management of MINOCA is challenging as the apparent reason of MI is not very clear. The disease is heterogeneous without any single pathophysiological mechanism [3]. Studies have reported that factors such as vasospasm of coronary vasculature, thrombosis or embolism, microvascular dysfunction, plaque disturbance, and supply–demand inadequacy may all lead to MI in these patients [7, 8]. Due to the unique nature of the disease and the difference in the mechanism of myocardial injury it is imperative to understand if the prognosis of MINOCA patients differs from that of MI-CAD.
Recently, several publications have compared outcomes of MINOCA and MI-CAD but with variable results. Some authors have reported lower mortality rates in patients with MINOCA [9,10,11], while others indicate no difference in outcomes between the two [12, 13]. A meta-analysis by Pelliccia et al. [14] have attempted to compare mortality rates between the two conditions. However, a significant drawback of this review is that only crude death rates were pooled. The observed difference in outcomes between MINOCA and MI-CAD may be, therefore, due to the difference in clinicopathological features of the diseases or, alternatively, because of the difference in several other risk factors [12]. Therefore, assessing the risk of mortality requires careful accounting for confounding factors. To date, no meta-analysis has compared outcomes of MINOCA and MI-CAD by aggregating multivariable-adjusted data. Current study aims to evaluate if there was a difference in long-term mortality between MINOCA vs MI-CAD by pooling only multi-variable adjusted data.
Material and methods
This review conforms with the guidelines of the PRISMA statement [15]. Registration of protocol was done on PROSPERO (CRD42023436897). No ethical clearance or patient consent was required for this study.
Search strategy
Two reviewers conducted a literature search for relevant studies in the PubMed, Embase, CENTRAL, and Google Scholar databases. It was completed on 18th June 2023. Keywords used were: “MINOCA”, “myocardial infarction”, “normal”, “non-obstructed”, “absence”, “obstruction”, “coronary artery”, and “coronary stenosis”. The combinations used are shown in Table 1. The retrieved studies were de-duplicated, and titles and abstracts were screened to remove non-relevant publications. Full-text analysis of the selected studies was done, and studies fulfilling all the criteria were included in the final analysis. All disputes were resolved by consultation. Hand search was also done for the bibliography of eligible studies.
Inclusion criteria
The review question according to PICO was: Are the long-term outcomes of patients with MI (population) due to non-obstructed coronary artery disease (intervention) different as compared to those with obstructed coronary artery disease?
The inclusion criteria were then framed based on the above question as follows:
1) All kinds of studies comparing outcomes of MINOCA and MI-CAD. 2) Studies with a follow-up of at least 1 year. 3) Studies reporting multivariable-adjusted outcomes and specifying the factors adjusted for the analysis. 4) Studies were to diagnose MI based on typical symptoms, increase of a minimum of one necrosis biomarker, and ST-segment or T-wave changes on the electrocardiogram. 5) Patients were to be classified into MINOCA or MI-CAD groups based on the angiographic assessment of coronary arteries.
Studies excluded were: 1) Studies wherein angiographic assessment was not carried out. 2) Studies without adjusted outcomes. 3) Studies on Takotsubo syndrome 4) Non-English language studies. 5) Studies with duplicate or overlapping data. In such cases, the study with maximum patients was selected.
Data management and quality assessment
Name of the author, study type, its location, number of patients, age, gender, medical history of patients (hypertension, diabetes mellitus, chronic kidney disease, previous MI or cerebrovascular accident), medications prescribed at discharge (aspirin, P2Y12 inhibitor, statins, beta-blockers), follow-up, and covariates examined were extracted from the included studies. The primary outcome was all-cause mortality after 1 year of follow-up. Secondary outcomes were cardiovascular mortality, the combined risk of mortality and MI, risk of major adverse cardiac events (MACE), and readmission rates between the two groups. MACE was defined as per the included studies. There was no restriction on the cause of readmission; all causes of readmissions were admissible.
Studies were examined for bias using the Newcastle–Ottawa scale (NOS) [16]. The scale judges each study for selection of study participants, comparison of study groups, and outcomes. The score of NOS ranges from 0–9.
Statistical analysis
"Review Manager" (RevMan, version 5.3) was used for all quantitative data analyses. Adjusted hazard ratios (HR) and their 95% confidence intervals (CI) or similar effect sizes were combined by the generic inverse function of Review manager in a random-effects model. Publication bias was examined by visual inspection of funnel plots and Egger’s test. The I2 statistic determined the inter-study heterogeneity. A sensitivity analysis was executed by removing singular studies form the forest plot to check for any outliners. This was done in the software itself to note any change in significance of results.
Results
Search details
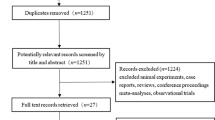
Titles and abstracts of 5292 unique studies, identified by the search across the databases, were examined. Of them, 75 studies were selected for the full-text analysis. A total of 59 studies were excluded. Finally, 16 studies met the inclusion criteria [13, 17,18,19,20,21,22,23,24,25,26,27,28,29,30,31] (Fig. 1, Supplementary material: raw data).
Study details
The studies were published between 2009 and 2023 (Table 2). Five studies were from the North America, two from Asia, two from New Zealand, and the remaining studies were from the European nations. The total number of patients in the MINOCA arm varied between 64 to 16,849. Sample sizes in the MI-CAD arm varied from 412 to 29,931. A total of 29,708 patients with MINOCA were compared with 514,421 patients with MI-CAD in the 16 studies. All studies were retrospective in design, examining data from hospital databases or national registries. Patients was mostly above 60 years old in most studies. Importantly, the study of Magnani et al. [24] had a younger cohort and the age of included patients was 38 and 41 years for MINOCA and MI-CAD groups, respectively. The percentage of hypertensive patients in the study groups ranged from 18.3 to 73.3%, while the percentage of diabetic patients varied from 3.8 to 37.9%. There was inconsistent reporting of data on previous MI and cerebrovascular accidents among the included studies. Medication-related data was also not provided by all included studies. However, a general trend noted was the reduced prescription of anti-platelets, statins, and beta-blockers at discharge in MINOCA patients as compared to MI-CAD patients. The covariates used to assess the outcomes differed across the studies. The follow-up period in the studies ranged from 1 to 19.9 years. All studies were of good quality, with an NOS score of 8.
Outcomes
A total of 28,220 patients with MINOCA were compared with 502,073 patients with MI-CAD in 11 studies reporting all-cause mortality. Our meta-analysis revealed no statistically significant difference in the risk of all-cause mortality between patients with MINOCA and MI-CAD (HR: 0.90 95% CI 0.68, 1.19 I2 = 94% p = 0.48) (Fig. 2). We did not find any gross asymmetry in the funnel plot (Fig. 3). Egger’s test did not indicate any publication bias (p = 0.77). Sensitivity analysis is shown in Table 3. There was no change in the significance of the outcome on the removal of any study and the overall results of all-cause mortality remained non-significant throughout. Data on cardiovascular mortality was reported only by three studies. Meta-analysis showed no statistically significant difference in the risk of cardiovascular mortality between MINOCA and MI-CAD cohorts (HR: 0.81 95% CI 0.54, 1.22 I2 = 0% p = 0.31) (Supplementary Fig. 1).
Four studies were reporting combined risk of death and MI between the two study groups. On pooled analysis of these studies, there was a statistically significant reduced risk of mortality and MI in patients with MINOCA vs MI-CAD (HR: 0.54 95% CI 0.39, 0.76 I2 = 72% p = 0.003) (Fig. 4). Similarly, analysis of five studies showed a significantly reduced risk of MACE in patients with MINOCA as compared to MI-CAD (HR: 0.66 95% CI 0.51, 0.84 I2 = 51% p = 0.0009) (Fig. 5). Lastly, a meta-analysis of data indicated no statistically significant difference in the risk of readmission between the two study groups (HR: 0.85 95% CI 0.61, 1.19 I2 = 90% p = 0.35) (Fig. 6).
Discussion
Due to the widespread prevalence of coronary artery disease, several studies have focussed on assessing long-term outcomes and prognostic factors of MI in the past few years [32,33,34]. Indeed, MI is a well-defined life-threatening disease and the outcomes can differ due to several factors like patient’s age, gender, the severity of disease, presence of risk factors, co-morbidities, and treatment protocol [32]. Thus, assessment of long-term outcomes with any type of MI should also consider the parallel influence of these confounders to present correct scientific evidence. For instance, many studies have explored the impact of gender on outcomes of MI but with variable results based on crude or adjusted data [33, 35]. Bavishi et al. [35] in a comprehensive review have shown that while crude long-term mortality rates may be higher in females as compared to males[Risk ratio (RR) 1.60, 95% CI: 1.46–1.76], the risk is no longer statistically significant when adjusted effect estimates were pooled for a meta-analysis (RR: 1.01, 95% CI: 0.93–1.11). They concluded that baseline clinical differences and different treatment protocols largely contributed to the high crude mortality rates in female patients.
In this study, we attempted to extrapolate the same theory in assessing the long-term outcomes of patients with MINOCA compared to MI-CAD patients. Many of the studies comparing MINOCA and MI-CAD, reported a favourable prognosis in patients with MINOCA [9,10,11]. Bossard et al. [9] compared data of 1599 MINOCA patients with 22,184 MI-CAD patients, and have demonstrated significantly lower all-cause mortality, cardiovascular mortality, repeat MI and major bleeding episodes in MINOCA patients. Eggers et al. [36] in a retrospective analysis of a Swedish registry have shown lower rates of all-cause mortality, cardiovascular mortality, and MACE events in patients with MINOCA as compared to MI-CAD. Other studies from Germany [37] and Egypt [38] have also demonstrated more favourable outcomes in patients with MINOCA. In the prior review on this topic, Pelliccia et al. [14] have reported annual long-term mortality rates of 2.2% in patients with MINOCA and 5% in patients with MI-CAD. By compiling evidence from 26 studies, the authors reported a statistically significant 40% lower risk of all-cause mortality in patients with MINOCA as compared to MI-CAD (RR 0.60, 95% CI: 0.46 to 0.78).
While Pelliccia et al. [14] pooled only crude data, our review synthesized data of only adjusted effect estimates and presents contrasting results. Our analysis shows that long-term mortality does not significantly differ between the two disease types after adjusting for confounding factors. We acknowledge that the statistical power of our analysis would be lower as compared to the previous review as only 11 studies were available in our primary analysis despite extending the literature search by five more years and adding recent studies. However, the sample size of our analysis was large, with data of 28,220 patients with MINOCA and 502,073 patients with MI-CAD. Furthermore, sensitivity analysis demonstrated that no study in our analysis had a disproportionate impact on the overall outcome. Forest plot analysis showed that the study of Dreyer et al. [21], with its significantly large sample size, may be considered an outliner. The authors of this study noted a significant higher risk of all-cause mortality in MINOCA after adjusting for past cardiovascular history and comorbidities. These difference in their results as compared to other studies may be attributable to two reasons. First, a study by Dreyer et al. only included elderly patients (≥ 65 years). Secondly, over 50% of patients were eventually excluded due to incompleteness or lack of data linkage. This suggests possible selection bias, and may impact the generalizability of the results.
The outcomes of our study should be interpreted while considering the differences in the factors adjusted in the included studies. The most common adjusted confounders were age, gender, and comorbidities like diabetes, hypertension, and dyslipidaemia. Several studies have demonstrated that patients with MINOCA are younger and more often of female gender [4, 12]. Consistent with younger age, these patients may also have a lower prevalence of other risk factors such as diabetes, smoking, hypertension, renal disease, history of MI, and stroke [39]. However, a systematic review had indicated that cardiovascular risk factors are not different in MINOCA and MI-CAD patients [4]. This could explain the lack of difference in all-cause mortality between MINOCA and MI-CAD in the current meta-analysis. Moreover, unlike MI-CAD, no clear management strategy exist for MINOCA, and it differs from case to case. Similar to heart failure, MINOCA is considered a working diagnosis that requires further evaluation to identify the underlying cause. Further investigations like transthoracic echocardiography and magnetic resonance imaging are needed to tailor the treatment based on the underlying pathology [3]. Research also indicates that secondary prevention strategies are less commonly utilized in MINOCA as compared to MI-CAD. Renin-angiotensin inhibitors may have a beneficial role but dual antiplatelet therapy and statins offer no advantage in MINOCA patients [40]. Thus, it is evident that the outcomes of these conditions may be influenced only by the differences in the baseline characteristics but also by the variability in the management protocols. The lack of clear management strategy and lower utilization of prevention protocols could be another reason for similar mortality of MINOCA and MI-CAD despite the younger age of the MINOCA group.
In our secondary analysis, we noted a significantly reduced risk of combined mortality and MI as well as MACE in patients with MINOCA as compared to MI-CAD. These results should be interpreted with extreme caution due to limited data and small sample size of the studies. There is a need for further research to explore the differences in the risk of cardiovascular mortality, MACE and readmission rates between MINOCA and MI-CAD patients.
Our review has limitations. Firstly, only eleven studies provided data on long-term all-cause mortality. We had to exclude many studies due to the lack of adjusted data. Thus, our analysis does not encompass the entirety of evidence available in the literature. Secondly, we noted high heterogeneity in the meta-analysis which partly could be due to differences among the included studies for the factors adjusted in the multivariable analysis. It is possible that other measured and unmeasured factors in the included studies could have affected the outcomes. Thirdly, all included studies were retrospective with inherent bias associated with these types of studies. Fourthly, we could include maximum studies only in the primary outcome analysis. We were unable to comprehensively analyse other important outcomes like cardiovascular mortality, recurrent MI, MACE, and readmission rates due to limited data. Lastly, the software RevMan used in our meta-analysis uses the DerSimonian & Laird Method to calculate error rates and can result in false positive results with scarce data.
A major strength of our study is that this is the first meta-analysis comparing mortality rates between MINOCA and MI-CAD by pooling adjusted data. The consistency of the outcomes on leave-one-out analysis lends credibility to our conclusions. The contrasting results presented by our study as compared to the previous review [14] have important clinical implications as they suggest that MINOCA should not be considered a benign entity as compared to MI-CAD. Clinicians should aggressively search for the underlying pathology to adequately manage this disease. Further research should be conducted to identify specific risk factors associated with poor outcomes with MINOCA.
To conclude, this is a large meta-analysis of 16 studies, reporting only adjusted and long-term differences between MINOCA and MI-CAD patients. We show that there is no difference in the risk of all-cause mortality between the two types of disease. The consistency of the results on sensitivity analysis indicated robustness of our evidence. No difference between MINOCA and MI-CAD was detected in terms of the cardiovascular mortality. Limited evidence also showed reduced risk of MACE in MINOCA vs MI-CAD but no difference in the risk of readmissions between the two conditions. Our results should be interpreted cautiously due to the high heterogeneity in the meta-analysis and limited data on cardiovascular mortality, MACE, and readmissions. Further research is needed to strengthen the evidence on this topic.
Availability of data and materials
All data generated or analysed during this study are included in this published article and its supplementary information.
References
Reed GW, Rossi JE, Cannon CP. Acute myocardial infarction. The Lancet. 2017;389:197–210. https://doi.org/10.1016/S0140-6736(16)30677-8.
Johansson S, Rosengren A, Young K, Jennings E. Mortality and morbidity trends after the first year in survivors of acute myocardial infarction: A systematic review. BMC Cardiovasc Disord. 2017;17. https://doi.org/10.1186/s12872-017-0482-9.
Poku N, Noble S. Myocardial infarction with non obstructive coronary arteries (MINOCA): a whole new ball game. Expert Rev Cardiovasc Ther. 2017;15:7–14. https://doi.org/10.1080/14779072.2017.1266256.
Pasupathy S, Air T, Dreyer RP, Tavella R, Beltrame JF. Systematic review of patients presenting with suspected myocardial infarction and nonobstructive coronary arteries. Circulation. 2015;131:861–70. https://doi.org/10.1161/CIRCULATIONAHA.114.011201.
Agewall S, Beltrame JF, Reynolds HR, Niessner A, Rosano G, Caforio ALP, et al. ESC working group position paper on myocardial infarction with non-obstructive coronary arteries. Eur Heart J. 2017;38:143–53. https://doi.org/10.1093/eurheartj/ehw149.
Niccoli G, Scalone G, Crea F. Acute myocardial infarction with no obstructive coronary atherosclerosis: Mechanisms and management. Eur Heart J. 2015;36:475–81. https://doi.org/10.1093/eurheartj/ehu469.
Tamis-Holland JE, Jneid H, Reynolds HR, Agewall S, Brilakis ES, Brown TM, et al. Contemporary Diagnosis and Management of Patients With Myocardial Infarction in the Absence of Obstructive Coronary Artery Disease: A Scientific Statement From the American Heart Association. Circulation. 2019;139:E891-908. https://doi.org/10.1161/CIR.0000000000000670.
Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, et al. 2015 ACC/AHA/SCAI focused update on primary percutaneous coronary intervention for patients with ST-elevation myocardial infarctionAn update of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention and the 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction a report of the American college of cardiology/American Heart Association task force on clinical practice guidelines and the society for cardiovascular angiography and interventions. Circulation. 2016;133:1135–47. https://doi.org/10.1161/CIR.0000000000000336.
Bossard M, Gao P, Boden W, Steg G, Tanguay JF, Joyner C, et al. Antiplatelet therapy in patients with myocardial infarction without obstructive coronary artery disease. Heart. 2021. https://doi.org/10.1136/heartjnl-2020-318045.
Dwyer JP, Redfern J, Freedman SB. Low utilisation of cardiovascular risk reducing therapy in patients with acute coronary syndromes and non-obstructive coronary artery disease. Int J Cardiol. 2008;129:394–8. https://doi.org/10.1016/j.ijcard.2007.12.023.
Montenegro Sá F, Ruivo C, Santos LG, Antunes A, Saraiva F, Soares F, et al. Myocardial infarction with nonobstructive coronary arteries: A single-center retrospective study. Coron Artery Dis. 2018;29:511–5. https://doi.org/10.1097/MCA.0000000000000619.
Safdar B, Spatz ES, Dreyer RP, Beltrame JF, Lichtman JH, Spertus JA, et al. Presentation, clinical profile, and prognosis of young patients with myocardial infarction with nonobstructive coronary arteries (MINOCA): Results from the VIRGO study. J Am Heart Assoc. 2018;7. https://doi.org/10.1161/JAHA.118.009174.
Vranken NPA, Pustjens TFS, Kolkman E, Hermanides RS, Bekkers SCAM, Smulders MW, et al. MINOCA: The caveat of absence of coronary obstruction in myocardial infarction. IJC Hear Vasc. 2020;29. https://doi.org/10.1016/j.ijcha.2020.100572.
Pelliccia F, Pasceri V, Niccoli G, Tanzilli G, Speciale G, Gaudio C, et al. Predictors of Mortality in Myocardial Infarction and Nonobstructed Coronary Arteries: A Systematic Review and Meta-Regression. Am J Med. 2020;133:73-83.e4. https://doi.org/10.1016/j.amjmed.2019.05.048.
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009;6:e1000097. https://doi.org/10.1371/journal.pmed.1000097.
Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 30 Oct 2020.
Lawless M, Appelman Y, Beltrame JF, Navarese EP, Ratcovich H, Wilkinson C, et al. Sex differences in treatment and outcomes amongst myocardial infarction patients presenting with and without obstructive coronary arteries: a prospective multicentre study. Eur Hear J open. 2023;3:oead033. https://doi.org/10.1093/EHJOPEN/OEAD033.
Magnani G, Bricoli S, Ardissino M, Maglietta G, Nelson A, Malagoli Tagliazucchi G, et al. Long-term outcomes of early-onset myocardial infarction with non-obstructive coronary artery disease (MINOCA). Int J Cardiol. 2022;354:7–13. https://doi.org/10.1016/J.IJCARD.2022.02.015.
Cortell A, Sanchis J, Bodí V, Núñez J, Mainar L, Pellicer M, et al. Non-ST-Elevation Acute Myocardial Infarction With Normal Coronary Arteries: Predictors and Prognosis. Rev Española Cardiol (English Ed. 2009;62:1260–6. https://doi.org/10.1016/s1885-5857(09)73353-5.
Andersson HB, Pedersen F, Engstrøm T, Helqvist S, Jensen MK, Jørgensen E, et al. Long-term survival and causes of death in patients with ST-elevation acute coronary syndrome without obstructive coronary artery disease. Eur Heart J. 2018;39:102–10. https://doi.org/10.1093/eurheartj/ehx491.
Bainey KR, Welsh RC, Alemayehu W, Westerhout CM, Traboulsi D, Anderson T, et al. Population-level incidence and outcomes of myocardial infarction with non-obstructive coronary arteries (MINOCA): Insights from the Alberta contemporary acute coronary syndrome patients invasive treatment strategies (COAPT) study. Int J Cardiol. 2018;264:12–7. https://doi.org/10.1016/j.ijcard.2018.04.004.
Planer D, Mehran R, Ohman EM, White HD, Newman JD, Xu K, et al. Prognosis of patients with non-ST-segment-elevation myocardial infarction and nonobstructive coronary artery disease: Propensity-matched analysis from the acute catheterization and urgent intervention triage strategy trial. Circ Cardiovasc Interv. 2014;7:285–93. https://doi.org/10.1161/CIRCINTERVENTIONS.113.000606.
Barr PR, Harrison W, Smyth D, Flynn C, Lee M, Kerr AJ. Myocardial Infarction Without Obstructive Coronary Artery Disease is Not a Benign Condition (ANZACS-QI 10). Hear Lung Circ. 2018;27:165–74. https://doi.org/10.1016/j.hlc.2017.02.023.
Raparelli V, Elharram M, Shimony A, Eisenberg MJ, Cheema AN, Pilote L. Myocardial Infarction With No Obstructive Coronary Artery Disease: Angiographic and Clinical Insights in Patients With Premature Presentation. Can J Cardiol. 2018;34:468–76. https://doi.org/10.1016/j.cjca.2018.01.004.
Williams MJA, Barr PR, Lee M, Poppe KK, Kerr AJ. Outcome after myocardial infarction without obstructive coronary artery disease. Heart. 2019;105:524–30. https://doi.org/10.1136/heartjnl-2018-313665.
Abdu FA, Liu L, Mohammed AQ, Luo Y, Xu S, Auckle R, et al. Myocardial infarction with non-obstructive coronary arteries (MINOCA)in Chinese patients: Clinical features, treatment and 1 year follow-up. Int J Cardiol. 2019;287:27–31. https://doi.org/10.1016/j.ijcard.2019.02.036.
Choo EH, Chang K, Lee KY, Lee D, Kim JG, Ahn Y, et al. Prognosis and Predictors of Mortality in Patients Suffering Myocardial Infarction With Non-Obstructive Coronary Arteries. J Am Heart Assoc. 2019;8. https://doi.org/10.1161/JAHA.119.011990.
Schmitz K, Groth N, Mullvain R, Renier C, Oluleye O, Benziger C. Prevalence, Clinical Factors, and Outcomes Associated with Myocardial Infarction with Non-Obstructive Coronary Artery (MINOCA). Crit Pathways Cardiol A J Evidence-Based Med. 2020;Publish Ah. https://doi.org/10.1097/hpc.0000000000000249.
Dreyer RP, Tavella R, Curtis JP, Wang Y, Pauspathy S, Messenger J, et al. Myocardial infarction with non-obstructive coronary arteries as compared withmyocardial infarction and obstructive coronary disease: Outcomes in aMedicare population. Eur Heart J. 2020;41:870–8. https://doi.org/10.1093/eurheartj/ehz403.
Gasior P, Desperak A, Gierlotka M, Milewski K, Wita K, Kalarus Z, et al. Clinical Characteristics, Treatments, and Outcomes of Patients with Myocardial Infarction with Non-Obstructive Coronary Arteries (MINOCA): Results from a Multicenter National Registry. J Clin Med. 2020;9:2779. https://doi.org/10.3390/jcm9092779.
Lopez-Pais J, Izquierdo Coronel B, Galán Gil D, Espinosa Pascual MJ, Alcón Durán B, Martinez Peredo CG, et al. Clinical characteristics and prognosis of myocardial infarction with non-obstructive coronary arteries (MINOCA): A prospective single-center study. Cardiol J. 2020. https://doi.org/10.5603/cj.a2020.0146.
Sulo G, Igland J, Sulo E, Øverland S, Egeland GM, Vollset SE, et al. Mortality following first-time hospitalization with acute myocardial infarction in Norway, 2001–2014: Time trends, underlying causes and place of death. Int J Cardiol. 2019;294:6–12. https://doi.org/10.1016/j.ijcard.2019.07.084.
Alabas OA, Gale CP, Hall M, Rutherford MJ, Szummer K, Lawesson SS, et al. Sex differences in treatments, relative survival, and excess mortality following acute myocardial infarction: National cohort study using the SWEDEHEART registry. J Am Heart Assoc. 2017;6. https://doi.org/10.1161/JAHA.117.007123.
Ajam T, Devaraj S, Fudim M, Ajam S, Soleimani T, Kamalesh M. Lower Post Myocardial Infarction Mortality Among Women Treated at Veterans Affairs Hospitals Compared to Men. Am J Med Sci. 2020;360:537–42. https://doi.org/10.1016/j.amjms.2019.12.005.
Bavishi C, Bangalore S, Patel D, Chatterjee S, Trivedi V, Tamis-Holland JE. Short and long-term mortality in women and men undergoing primary angioplasty: A comprehensive meta-analysis. Int J Cardiol. 2015;198:123–30. https://doi.org/10.1016/j.ijcard.2015.07.001.
Eggers KM, Hjort M, Baron T, Jernberg T, Nordenskjöld AM, Tornvall P, et al. Morbidity and cause-specific mortality in first-time myocardial infarction with nonobstructive coronary arteries. J Intern Med. 2019;285:419–28. https://doi.org/10.1111/joim.12857.
Ohlow MA, Wong V, Brunelli M, Von Korn H, Farah A, Memisevic N, et al. Acute coronary syndrome without critical epicardial coronary disease: Prevalence, characteristics, and outcome. Am J Emerg Med. 2015;33:150–4. https://doi.org/10.1016/j.ajem.2014.10.048.
Abdelmonem YY, Bakr AA, El-Hossary HG, Ghany MMA. Patients with non-obstructive coronary artery disease admitted with acute myocardial infarction carry a better outcome compared to those with obstructive coronary artery disease. Egypt Hear J. 2017;69:191–9. https://doi.org/10.1016/j.ehj.2017.03.001.
Rakowski T, De Luca G, Siudak Z, Plens K, Dziewierz A, Kleczyński P, et al. Characteristics of patients presenting with myocardial infarction with non-obstructive coronary arteries (MINOCA) in Poland: data from the ORPKI national registry. J Thromb Thrombolysis. 2019;47:462–6. https://doi.org/10.1007/s11239-018-1794-z.
Paolisso P, Bergamaschi L, Saturi G, D’Angelo EC, Magnani I, Toniolo S, et al. Secondary prevention medical therapy and outcomes in patients with myocardial infarction with non-obstructive coronary artery disease. Front Pharmacol. 2020;10. https://doi.org/10.3389/fphar.2019.01606.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Xueli Lu, Shengnan Zhu, Yanjiao Lu and Yanming Li: Conceptualization, Methodology. Xueli Lu and Yanjiao Lu: Data curation, Writing- Original draft preparation. Xueli Lu, Shengnan Zhu and Yanming Li: Visualization, Investigation. Xueli Lu: Supervision. Xueli Lu and Yanming Li: Software, Validation. Xueli Lu: Writing—Reviewing and Editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 2.
Supplementary material: Raw data.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lu, X., Zhu, S., Lu, Y. et al. Long term all-cause mortality after myocardial infarction with non-obstructed vs obstructed coronary artery disease: a meta-analysis of adjusted data. BMC Cardiovasc Disord 24, 9 (2024). https://doi.org/10.1186/s12872-023-03674-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-023-03674-1