Abstract
Background
Physiological and pathological cardiomyocyte hypertrophy are important pathophysiological processes of adult congenital heart disease-associated ventricular hypertrophy. Glutamic oxaloacetic transaminase (GOT) is a vital marker of myocardial injury. This study aimed to investigate the changes in GOT levels during physiological and pathological cardiomyocyte hypertrophy in rats.
Methods
RNA-seq analysis and colorimetric methods were used to evaluate the changes in GOT mRNA and activity, respectively. GOT2 protein expression was detected by western blotting and immunofluorescence. Hematoxylin-eosin and wheat germ agglutinin methods were used to observe changes in rat cardiomyocyte morphology.
Results
In juvenile rat hearts, GOT mRNA expression and activity, and GOT2 protein level increased with age-related physiological cardiomyocyte hypertrophy; however, GOT2 protein level was reduced in hypoxia-induced pathological cardiomyocyte hypertrophy.
Conclusions
GOT2 may regulate physiological and pathological myocardial hypertrophy in rats. We speculated that the low GOT2 level contributed to the rapid occurrence of pathological cardiomyocyte hypertrophy, causing strong plasticity of right ventricular cardiomyocytes in the early postnatal period and heart failure in adulthood.
Similar content being viewed by others
Background
Cardiomyocyte hypertrophy, including physiological and pathological cardiomyocyte hypertrophy, is a crucial pathophysiological process of adult congenital heart disease (CHD)-associated ventricular hypertrophy [1,2,3,4]. The most common adult CHD is atrial septal defect (ASD). The typical pathophysiological changes in ASD include right ventricular hypertrophy with less significant alterations in the left ventricle. Previous studies have indicated greater plasticity of the right ventricular myocardium in the early postnatal period. In previous studies, cardiomyocyte volume in the right ventricle increased remarkably in children with Tetralogy of Fallot (TOF) [5,6,7]. A mouse model of pulmonary artery banding, used to simulate changes in the right ventricle of TOF, revealed that cardiomyocyte hypertrophy of younger mice is more evident than older ones further [8]. Recent studies on the regulation of cardiomyocyte hypertrophy unlocked that methyltransferase (METTL) and Akt showed opposing changes during physiological and pathological cardiomyocyte hypertrophy [9,10,11,12]. However, the molecules involved in both physiological and pathological cardiomyocyte hypertrophy are complex and diverse, and their roles have not been still fully elucidated.
Glutamic oxaloacetic transaminase (GOT or AST), closely related to myocardial function, is an essential myocardial injury biomarker [13,14,15]. GOT isozymes, GOT1 and GOT2, are located in the cytoplasm and mitochondrial matrix respectively, and participate in nicotinamide-adenine dinucleotide phosphate (NADPH) oxidative phosphorylation to produce ATP through the malate-aspartate shuttle in cardiac energy metabolism [16, 17]. A clinical study indicated that the activity ratio of serum GOT/alanine transaminase was negatively correlated with the incidence of cardiovascular disease in a healthy Japanese population [18]. The excessive increase in serum GOT activity was associated with a decline in the survival rate of children with cyanotic CHD and right ventricular hypertrophy, such as TOF [14]. In adult animal experiments, angiotensin II (Ang II)-induced pathological cardiomyocyte hypertrophy was associated with decreased GOT2 protein expression, but not with GOT1. Notably, the experimental cardiac hypertrophy may act as a specific stimulus for GOT during myocardial malate-aspartate shuttle [19, 20]. Moreover, hypoxia is not only a typical symptom of cyanotic CHD but a critical cause of disease progression. The results of a perinatal mouse model confirmed that chronic hypoxia inhibited heart development, which presented as an increase in the heart-weight ratio [21, 22]. These studies suggested that GOT2 was correlated with right ventricular hypertrophy; however, GOT2 alterations in age-related physiological and hypoxia-induced pathological cardiomyocyte hypertrophy during childhood remain unexplored.
This study aimed to determine the changes in GOT2 during physiological or hypoxia-induced pathological cardiomyocyte hypertrophy. To this end, we assessed age-related changes in GOT2 expression of juvenile rat hearts, the correlation between GOT activity and physiological cardiomyocyte hypertrophy in rat right ventricle, and GOT2 expression changes in hypoxic cardiomyocyte hypertrophy to provide a novel idea and research target for promoting physiological cardiomyocyte hypertrophy, suppressing pathological cardiomyocyte hypertrophy, and accelerating the heart’s transition to left ventricular dominance after birth.
Methods
Day-age choice and tissue processing in immature rats
Previous studies have reported a transition in the rat heart from cardiomyocyte proliferation to cardiomyocyte hypertrophy between the 3rd and 4th days following birth. Subsequently, cytokinesis decreases, and cardiomyocytes primarily grow in a hypertrophic manner. However, the myocardial nuclear division of Sprague Dawley (SD) rats decreased significantly on the 10th day [23]. Compared with previous studies, the positive rates of ki-67, a nuclear division biomarker, were similar in humans within 0–3 months and in SD rats within 28 days (0.55% vs. 0.5%) after birth; thereafter, the ki-67 almost disappeared in humans and rats [8]. In addition, γ- linolenic acid, a type of fatty acid in breast milk, is crucial for the metabolic maturation in the heart development of suckling mice [24]. Therefore, as indicated in Supplementary Fig. 1, we selected 5-, 10-, and 20-day-old suckling mice to represent the three turning points.
Juvenile male SD rats in the 5-, 10-, and 20-day-old groups (n = 3–5 each) were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. The juvenile rats were sacrificed under pentobarbital sodium (5.4 g/kg) treatment [25, 26]. We quickly dissected and opened the abdominal and chest cavities; collected the heart, lung, and liver; and washed them with phosphate-buffered saline (PBS) at 4 °C. After absorbing excess PBS with filter paper, the freshly collected tissues were stored at − 80 °C or fixed in 4% paraformaldehyde solution for 24 h to prepare paraffin sections.
Gene database search
Using the National Center for Biotechnology Information (NCBI) gene database, we obtained the RNA-seq data for GOT1 and GOT2 in 11 organs of female and male Fischer 344 rats aged 2, 6, 21, and 104 weeks old, wherein each week-old group (n = 8) corresponds to infancy, adolescence, adulthood, and old age, respectively [27]. We selected RNA-seq data from the heart, liver, brain, kidney, adrenal gland, and lung for statistical analysis.
GOT2 protein expression levels evaluated by western blotting
Lysis buffer was used to obtain proteins from the tissues and H9C2 cell. Denatured proteins were isolated by an 8% precast gel (Beyotime, China) and transferred onto nitrocellulose membranes. Nitrocellulose membranes were incubated with the specific primary antibodies (anti-GOT2 antibody produced in rabbit, 1:3000, SAB5701225, Sigma-Aldrich; anti-GAPDH antibody produced in mouse, 1:4000, TA-08, ZSGB-BIO) overnight at 4 °C and with secondary antibodies (1:2000) conjugated with horseradish peroxidase for 1 h at 25 °C. Finally, as previously described [28], protein bands were scanned using the FluorChem M MultiFluor System (ProteinSimple, USA).
GOT activity determined using the colorimetric method
The rat tissues stored at − 80 °C were accurately weighed, and physiological saline and magnetic beads were added for homogenization. Subsequently, the homogenized solution was frozen three times at − 80 °C and centrifuged at 4 °C, and the supernatant was isolated for conducting tests. Following the instructions of the aspartate aminotransferase assay kit (Nanjing Jiancheng Bioengineering Institute, China) [29], we divided the samples into test and control wells, and simultaneously prepared standard concentration wells. After 30 min of reaction with the substrate solution, 20 min with the 2,4-dinitrophenylhydrazine solution, and 15 min with NaOH, the absorbance values were measured using a microplate reader at a wavelength of 510 nm.
Hematoxylin-eosin (HE) staining
The rat heart tissue slices were sequentially placed in xylene and ethanol for dewaxing in water, and the hydrated slices were immersed in HE staining solution for 5 min. Subsequently, the stained slices were dehydrated with ethanol and made transparent with xylene. Finally, neutral resin was used to seal the slices. The slides were observed under a light microscope; the nuclei were blue, and the cytoplasm was red [30, 31]. The size of cardiomyocytes indirectly evaluated by counting the number of nuclei in images with the same magnification and area.
H9C2 culture
The rat embryonic myocardial cell line H9C2 and DMEM cell culture medium were purchased from Shanghai Fuheng Biotechnology Co., Ltd. The complete medium contains 93% basic medium, 5% fetal bovine serum, 1% L-glutamine, and 1% penicillin/streptomycin. H9C2 cells were allowed to adhere for 24 h in the medium, following which the cells were placed in a closed chamber (2.5 L) with a bag of Anaeropack-anaero2.5 (AnaeroPack-Anaero, Japan) for 24 h (oxygen concentration: 1%) to simulate hypoxia [32]. The hypoxic model experiment included the control and hypoxic groups.
Wheat germ agglutinin (WGA) for visualizing H9C2 morphology
After a 24-h exposure to hypoxia, the culture medium was removed by washing the cells with PBS. Next, the cells were fixed with paraformaldehyde, a drop of WGA (Sigma-Aldrich, USA) dye solution was added, and the cells were incubated in the dark for 20 min. Finally, an anti-fluorescence quenching agent containing DAPI (Servicebio, China) was used for sealing [33]. Fluorescence microscopy showed that the nuclei were blue and the WGA was green.
In situ detection of GOT2 protein expression in H9C2 cells using immunofluorescence assay
As previously described [28], after rinsing with PBS, H9C2 cells were fixed with 4% paraformaldehyde solution for 20 min. Then, a 5% bovine serum albumin (BSA) blocking solution (0.5 g BSA, 30 μL TritonX-100 and 10 mL 0.01 M PBS) was used to block and perforate cells. After removing the excess blocking solution, H9C2 cells were incubated with the primary antibody GOT2 (1:200, Sigma-Aldrich, USA) at 4 °C overnight. The next day, the H9C2 cells were incubated with a rabbit-derived red fluorescent secondary antibody (1:500, Thermo Fisher Scientific, USA) in the dark for 90 min. All sections were restained with DAPI. Finally, immunofluorescence microscopy was used for observation and comparison. We used ImageJ to quantitatively evaluate the red immunofluorescence intensity of GOT2 in images with similar cell densities.
Data analysis
GraphPad Prism software (version 8.0.2, USA) was used for statistical processing. Data were expressed as mean ± standard deviation (mean ± SD). An independent sample t-test was used to compare means between two groups. The means among multiple groups were compared by one-way analysis of variance (ANOVA) and the Sidak method when the data were normally distributed or the nonparametric Kruskal-Wallis test when the data were not normally distributed. p values < 0.05 were considered statistically significant [28].
Results
GOT expression in juvenile rat hearts was rapidly increased and GOT2 mRNA level was higher than that of GOT1
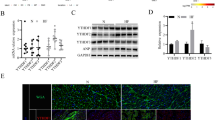
Considering the close correlation between GOT and myocardial function, we obtained RNA-seq data from different rat tissues at different ages (in weeks) by searching the gene database [27] and found that GOT1 and GOT2 mRNA levels in juvenile (< 6-week-old) rat hearts rapidly increased. Moreover, the GOT2 mRNA (74–110 RPKM) level was significantly higher than that of GOT1 (17–35 RPKM) in the rat myocardium (Fig. 1a). The GOT2 protein expression results detected through western blotting were consistent with the RNA-seq findings in juvenile rat hearts. Compared with the 5-day-old group, the protein level of cardiac GOT2 was dramatically enhanced in the 20-day-old group (Fig. 1b). In addition, GOT1 and GOT2 mRNAs were widely distributed in multiple organs, including rat hearts, livers, brains, kidneys, adrenal glands, and lungs, but GOT1 and GOT2 mRNAs in the different tissues other than the rat heart exhibited no significant changes and maintained low levels among the juvenile, adult (21-week-old) and aged (104-week-old) groups (Fig. 1a). These results suggested that the expression and function of GOT (particularly GOT2) were tightly linked to heart development in juvenile rats.
Changes in rat GOT expression in multiple tissues. a Age-related changes in GOT mRNA expression in different rat tissues by RNA-seq (n = 8). b GOT2 protein expression detected by western blotting in rat heart tissue (n = 5), full-length blots were presented in Supplementary Fig. 2. GOT: glutamic oxaloacetic transaminase. Data were expressed as mean ± SD, *p < 0.05
GOT activity increased with the right ventricle cardiomyocyte volume in juvenile rat hearts
GOT primarily participates in NADPH oxidative phosphorylation to supply energy via transaminase activity. To study the relationship between GOT function and physiological cardiomyocyte hypertrophy in juvenile rats, we further used a colorimetric method to determine the GOT activity in rat heart, liver, and lung tissues and HE staining to observe the normal age-related physiological cardiomyocyte hypertrophy in the rat right ventricle after birth. The results showed that the GOT activities in rat lungs and livers at the ages of 5, 10, and 20 days differed insignificantly; however, compared with the 5-day-old group, the cardiac GOT activity in the 20-day-old group rats was significantly increased (Fig. 2a), while the size of cardiomyocytes and heart volume were significantly increased, and hearts gradually shifted towards the left ventricular dominance (Fig. 2b and c). The above-mentioned results indicated an association between increased GOT activity and juvenile rat heart right ventricular cardiomyocyte volume, highlighting the role of GOT transaminase activity in rat age-related physiological cardiomyocyte hypertrophy.
Changes in GOT activity and cardiomyocyte volume in juvenile rat hearts. a The colorimetric method was performed to detect the GOT activities in rat heart, liver and lung tissues (n = 3–5). b, c HE staining was used to observe the age-related physiological cardiomyocyte hypertrophy in the juvenile rat right ventricle. (n = 3–5). GOT: glutamic oxaloacetic transaminase; HE: hematoxylin-eosin staining. Data were expressed as mean ± SD, **p < 0.01, scale bar: 50 μm
Reduced GOT2 protein expression in hypoxia-induced hypertrophic cardiomyocytes
Hypoxia is vital in the pathological hypertrophy of the right ventricular myocardium in patients with cyanotic CHD [21, 34]. By building a hypoxic cell model, using WGA staining, immunofluorescence, and western blotting, we found that compared with the control group, the surface area of H9C2 cells exposed to hypoxia increased by 2-fold (Fig. 3a), while the red fluorescence intensity representing the GOT2 protein decreased significantly in accordance with the results of western blotting (Fig. 3b and c). These results suggested that cardiomyocyte-derived GOT2 protein expression was inhibited during hypoxic pathological cardiomyocyte hypertrophy. In other words, the reduction in GOT2 protein may be a key factor contributing to the hypoxia-induced pathological cardiomyocyte hypertrophy.
Cell surface area and GOT2 protein expression in myocardial cell line H9C2. a WGA staining was used to outline the edges of H9C2 cells (N = 3, n = 3), scale bar: 50 μm. b In situ immunofluorescence detection of GOT2 protein expression in H9C2 cells; red fluorescence represents GOT2 protein, scale bar: 200 μm. c Western blotting was employed to detect the GOT2 protein expression in H9C2 cells (N = 3, n = 3), full-length blots were presented in Supplementary Fig. 3. WGA: wheat germ agglutinin, a specific cardiomyocyte membrane dye; GOT2: glutamic oxaloacetic transaminase 2. N: the number of independent experiments, n: the number of duplicates. Data were expressed as mean ± SD, **p < 0.01
Discussion
In this study, we initially found a positive correlation between myocardial GOT2 and age in juvenile rats through the lens of GOT, a hallmark of myocardial injury. Under physiological conditions, GOT2 may inhibit excessive cardiomyocyte hypertrophy in juvenile rats by continuously enhancing its mRNA and protein expression. Hypoxia-induced GOT2 reduction in CHD may contribute to pathological cardiomyocyte hypertrophy in children and adults with CHD.
In previous studies, the right ventricle myocardium of children with cyanotic CHD in the early postnatal period demonstrated strong plasticity [5,6,7,8]. Although some studies have shown that METTL can simultaneously regulate physiological and pathological hypertrophy, leading to significant cardiomyocyte hypertrophy [9,10,11,12], the related regulatory molecules about physiological and pathological cardiomyocyte hypertrophy of the right ventricle in the early postnatal period require further exploration. Recent studies have revealed that GOT2 was related to Ang II-induced pathological cardiomyocyte hypertrophy in adult mice. To clarify GOT2 changes in age-related physiological cardiomyocyte hypertrophy, we first observed changes in GOT during rat normal heart development. RNA-seq results showed that unlike the stable GOT mRNA expression in other organs, GOT mRNA in juvenile rat hearts increased rapidly but was relatively stable in adulthood. These results suggested that GOT played a basic and common role in rat heart, liver, brain, kidney, adrenal gland and lung cell activities and was tightly linked to heart development in juvenile rats. Moreover, GOT2 mRNA expression was higher than that of GOT1 in rat hearts. In addition, Rat heart GOT mRNA was highly expressed at all ages, which was consistent with clinical research results. GOT derived from acute injured myocardium in children after CHD surgery can cause a sharp increase in serum GOT activity [14]. Western blotting experiments further confirmed that the expression pattern of myocardial GOT2 protein was similar to that of GOT2 mRNA in juvenile rats.
To further explore the relationship between GOT function and physiological cardiomyocyte hypertrophy, we compared the changes in GOT transaminase activity and age-related physiological cardiomyocyte hypertrophy in rats and found that GOT activity increased with physiological cardiomyocyte hypertrophy. These results suggested that GOT2 may regulate physiological cardiomyocyte hypertrophy or the rapid GOT2 change in juvenile rat hearts may be related to the plasticity of cardiomyocytes.
Finally, we explored the relationship between GOT2 expression and hypoxic pathological cardiomyocyte hypertrophy. The results of cell experiments showed that hypoxia induced a decrease in GOT2 protein expression of rat myocardial cell line H9C2 and an increase in the myocardial cell surface, which was familiar with Ang II-induced GOT2 reduction in adult mice with cardiac hypertrophy [19]. Similarly, Romanowicz et al. found that chronic hypoxia inhibited juvenile mouse heart development, characterized by increased cardiac mass [21]. The above-mentioned results indicated that the inhibition of GOT2 expression may be a mechanism underlying hypoxia-induced pathological cardiomyocyte hypertrophy in cyanotic CHD. Some researchers found reactive oxygen species (ROS), could potentially modulate cardiac maturation. Considering that GOT2 is distributed in mitochondria, which are the chief consumers of oxygen and the potential source of ROS induced by hypoxia, we hypothesized that the increased ROS induced by hypoxia may be the underlying mechanism for the reduction in GOT2 expression [35,36,37].
Conclusions
Based on the results of the present study, we preliminary explored GOT2 changes in age-related physiological and hypoxia-induced pathological cardiomyocyte hypertrophy in rats, providing new insights and research targets for promoting physiological and inhibiting pathological cardiomyocyte hypertrophy. Considering that physiological and pathological hypertrophy is controlled by distinct cellular signaling pathways [3], further animal experiments should be performed to delineate the specific criteria used to determine physiological and pathological hypertrophy. Further, GOT2 expression intervention experiments should be conducted to confirm the protective effect of GOT2 on cardiomyocyte hypertrophy and to provide a novel theoretical basis for the treatment of cyanotic CHD in children and adults.
Availability of data and materials
All data required to evaluate the conclusions are presented in the manuscript and/or Supplementary Materials. The datasets analyzed in the current study are available from the NCBI repository, (https://www.ncbi.nlm.nih.gov/gene/?term=GOT2 and https://www.ncbi.nlm.nih.gov/gene/?term=GOT1) [27]. The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Abbreviations
- Ang II:
-
Angiotensin II
- BSA:
-
Bovine serum albumin
- CHD:
-
Congenital heart disease
- GOT or AST:
-
Glutamic-oxaloacetic transaminase
- HE:
-
Hematoxylin-eosin staining
- METTL:
-
Methyltransferase
- NADPH:
-
Nicotinamide-adenine dinucleotide phosphate
- NCBI:
-
National Center for biotechnology information
- PBS:
-
Phosphate-buffered saline
- ROS:
-
Reactive oxygen species
- SD:
-
Sprague Dawley
- TOF:
-
Tetralogy of Fallot
- WGA:
-
Wheat germ agglutinin
References
Liu Y, Chen J, Fontes SK, Bautista EN, Cheng Z. Physiological and pathological roles of protein kinase a in the heart. Cardiovasc Res. 2022;118(2):386–98.
Oldfield CJ, Duhamel TA, Dhalla NS. Mechanisms for the transition from physiological to pathological cardiac hypertrophy. Can J Physiol Pharmacol. 2020;98(2):74–84.
Nakamura M, Sadoshima J. Mechanisms of physiological and pathological cardiac hypertrophy. Nat Rev Cardiol. 2018;15(7):387–407.
Shimizu I, Minamino T. Physiological and pathological cardiac hypertrophy. J Mol Cell Cardiol. 2016;97:245–62.
El Khoudary SR, Fabio A, Yester JW, Steinhauser ML, Christopher AB, Gyngard F, Adams PS, Morell VO, Viegas M, Da Silva JP, Da Silva LF, Castro-Medina M, McCormick A, Reyes-Múgica M, Barlas M, Liu H, Thomas D, Ammanamanchi N, Sada R, Cuda M, Hartigan E, Groscost DK, Kühn B. Design and rationale of a clinical trial to increase cardiomyocyte division in infants with tetralogy of Fallot. Int J Cardiol. 2021;339:36–42.
Xie Y, Ma A, Wang B, Peng R, Jing Y, Wang D, Finnell RH, Qiao B, Wang Y, Wang H, Zheng Y. Rare mutations of ADAM17 from TOFs induce hypertrophy in human embryonic stem cell-derived cardiomyocytes via HB-EGF signaling. Clinical science (London, England: 1979). 2019;133(2):225–238.
Xia Y, Hong H, Ye L, Wang Y, Chen H, Liu J. Label-free quantitative proteomic analysis of right ventricular remodeling in infant tetralogy of Fallot patients. J Proteome. 2013;84:78–91.
Ye L, Wang S, Xiao Y, Jiang C, Huang Y, Chen H, Zhang H, Zhang H, Liu J, Xu Z, Hong H. Pressure overload greatly promotes neonatal right ventricular Cardiomyocyte proliferation: a new model for the study of heart regeneration. J Am Heart Assoc. 2020;9(11):e015574.
Wang L, Wang J, Yu P, Feng J, Xu GE, Zhao X, Wang T, Lehmann HI, Li G, Sluijter JPG, Xiao J. METTL14 is required for exercise-induced cardiac hypertrophy and protects against myocardial ischemia-reperfusion injury. Nat Commun. 2022;13(1):6762.
Li H, Trager LE, Liu X, Hastings MH, Xiao C, Guerra J, To S, Li G, Yeri A, Rodosthenous R, Silverman MG, Das S, Ambardekar AV, Bristow MR, González-Rosa JM, Rosenzweig A. lncExACT1 and DCHS2 regulate physiological and pathological cardiac growth. Circulation. 2022;145(16):1218–33.
Gao R, Wang L, Bei Y, Wu X, Wang J, Zhou Q, Tao L, Das S, Li X, Xiao J. Long noncoding RNA cardiac physiological hypertrophy-associated regulator induces cardiac physiological hypertrophy and promotes functional recovery after myocardial ischemia-reperfusion injury. Circulation. 2021;144(4):303–17.
Moc C, Taylor AE, Chesini GP, Zambrano CM, Barlow MS, Zhang X, Gustafsson ÅB, Purcell NH. Physiological activation of Akt by PHLPP1 deletion protects against pathological hypertrophy. Cardiovasc Res. 2015;105(2):160–70.
Beal RW. Serum glutamic oxaloacetic transaminase (S.G.O.T.) in the diagnosis and prognosis of myocardial infarction. Med J Aust. 1961;48(2):301–5.
Shteyer E, Yatsiv I, Sharkia M, Milgarter E, Granot E. Serum transaminases as a prognostic factor in children post cardiac surgery. Pediatr Int. 2011;53(5):725–8.
Toraih EA, Elshazli RM, Hussein MH, Elgaml A, Amin M, El-Mowafy M, El-Mesery M, Ellythy A, Duchesne J, Killackey MT, Ferdinand KC, Kandil E, Fawzy MS. Association of cardiac biomarkers and comorbidities with increased mortality, severity, and cardiac injury in COVID-19 patients: a meta-regression and decision tree analysis. J Med Virol. 2020;92(11):2473–88.
Recasens M, Delaunoy JP. Immunological properties and immunohistochemical localization of cysteine sulfinate or aspartate aminotransferase-isoenzymes in rat CNS. Brain Res. 1981;205(2):351–61.
Scholz TD, Koppenhafer SL, tenEyck CJ, Schutte BC. Ontogeny of malate-aspartate shuttle capacity and gene expression in cardiac mitochondria. Am J Phys. 1998;274(3):C780–8.
Higashitani M, Mizuno A, Kimura T, Shimbo T, Yamamoto K, Tokioka S, Kobayashi D. Low aspartate aminotransferase (AST)/alanine aminotransferase (ALT) ratio associated with increased cardiovascular disease and its risk factors in healthy Japanese population. J Gastrointestinal Liver Dis. 2022;31(4):429–36.
Chen Q, Zhang L, Chen S, Huang Y, Li K, Yu X, Wu H, Tian X, Zhang C, Tang C, Du J, Jin H. Downregulated endogenous sulfur dioxide/aspartate aminotransferase pathway is involved in angiotensin II-stimulated cardiomyocyte autophagy and myocardial hypertrophy in mice. Int J Cardiol. 2016;225:392–401.
Mavrides C, Korecky B. Subcellular distribution of the enzymes of the malate-aspartate shuttle in rat heart and effect of experimental cardiac hypertrophy. Biosci Rep. 1985;5(2):95–100.
Romanowicz J, Guerrelli D, Dhari Z, Mulvany C, Reilly M, Swift L, Vasandani N, Ramadan M, Leatherbury L, Ishibashi N, Posnack NG. Chronic perinatal hypoxia delays cardiac maturation in a mouse model for cyanotic congenital heart disease. Am J Physiol Heart Circ Physiol. 2021;320(5):H1873–h86.
Thandavarayan RA, Chitturi KR, Guha A. Pathophysiology of acute and chronic right heart failure. Cardiol Clin. 2020;38(2):149–60.
Li F, Wang X, Capasso JM, Gerdes AM. Rapid transition of cardiac myocytes from hyperplasia to hypertrophy during postnatal development. J Mol Cell Cardiol. 1996;28(8):1737–46.
Paredes A, Justo-Méndez R, Jiménez-Blasco D, Núñez V, Calero I, Villalba-Orero M, Alegre-Martí A, Fischer T, Gradillas A, Sant'Anna VAR, Were F, Huang Z, Hernansanz-Agustín P, Contreras C, Martínez F, Camafeita E, Vázquez J, Ruiz-Cabello J, Area-Gómez E, Sánchez-Cabo F, Treuter E, Bolaños JP, Estébanez-Perpiñá E, Rupérez FJ, Barbas C, Enríquez JA, Ricote M. γ-Linolenic acid in maternal milk drives cardiac metabolic maturation. Nature. 2023;618(7964):365–73.
Laferriere CA, Leung VS, Pang DS. Evaluating intrahepatic and intraperitoneal sodium pentobarbital or ethanol for mouse euthanasia. J Am Assoc Lab Anim Sci. 2020;59(3):264–8.
Tobar Leitão SA, Soares DDS, Carvas Junior N, Zimmer R, Ludwig NF, Andrades M. Study of anesthetics for euthanasia in rats and mice: a systematic review and meta-analysis on the impact upon biological outcomes (SAFE-RM). Life Sci. 2021;284:119916.
Yu Y, Fuscoe JC, Zhao C, Guo C, Jia M, Qing T, Bannon DI, Lancashire L, Bao W, Du T, Luo H, Su Z, Jones WD, Moland CL, Branham WS, Qian F, Ning B, Li Y, Hong H, Guo L, Mei N, Shi T, Wang KY, Wolfinger RD, Nikolsky Y, Walker SJ, Duerksen-Hughes P, Mason CE, Tong W, Thierry-Mieg J, Thierry-Mieg D, Shi L, Wang C. A rat RNA-Seq transcriptomic BodyMap across 11 organs and 4 developmental stages. Nat Commun. 2014;5:3230.
Liu X, Zhang S, Wang X, Wang Y, Song J, Sun C, Chen G, Yang G, Tao Y, Hu Y, Bu D, Huang Y, Du J, Jin H. Endothelial cell-derived SO2 controls endothelial cell inflammation, smooth muscle cell proliferation, and collagen synthesis to inhibit hypoxic pulmonary vascular Remodelling. Oxidative Med Cell Longev. 2021;2021:5577634.
Wu L, Fiet MD, Raaijmakers DR, Woudstra L, van Rossum AC, Niessen HWM, Krijnen PAJ. Transient atrial inflammation in a murine model of Coxsackievirus B3-induced myocarditis. Int J Exp Pathol. 2022;103(4):149–55.
Jin L, Piao ZH, Liu CP, Sun S, Liu B, Kim GR, Choi SY, Ryu Y, Kee HJ, Jeong MH. Gallic acid attenuates calcium calmodulin-dependent kinase II-induced apoptosis in spontaneously hypertensive rats. J Cell Mol Med. 2018;22(3):1517–26.
Fan S, Xiao G, Ni J, Zhao Y, Du H, Liang Y, Lv M, He S, Fan G, Zhu Y. Guanxinning injection ameliorates cardiac remodeling in HF mouse and 3D heart spheroid models via p38/FOS/MMP1-mediated inhibition of myocardial hypertrophy and fibrosis. Biomed Pharmacother. 2023;162:114642.
Liu H, Wang Y, Yue Y, Zhang P, Sun Y, Chen Q. [Periostin inhibits hypoxia-induced oxidative stress and apoptosis in human periodontal ligament fibroblasts via p38 MAPK signaling pathway]. Nan fang yi ke da xue xue bao =. J South Med Univ. 2020;40(7):942–8.
Lai TC, Lee TL, Chang YC, Chen YC, Lin SR, Lin SW, Pu CM, Tsai JS, Chen YL. MicroRNA-221/222 mediates ADSC-exosome-induced Cardioprotection against ischemia/reperfusion by targeting PUMA and ETS-1. Front Cell Dev Biol. 2020;8:569150.
Johnson J, Yang Y, Bian Z, Schena G, Li Y, Zhang X, Eaton DM, Gross P, Angheloiu A, Shaik A, Foster M, Berretta R, Kubo H, Mohsin S, Tian Y, Houser SR. Systemic hypoxemia induces Cardiomyocyte hypertrophy and right ventricular specific induction of proliferation. Circ Res. 2023;132(6):723–40.
Tian X, Zhang Q, Huang Y, Chen S, Tang C, Sun Y, Du J, Jin H. Endothelin-1 downregulates sulfur dioxide/aspartate aminotransferase pathway via reactive oxygen species to promote the proliferation and migration of vascular smooth muscle cells. Oxidative Med Cell Longev. 2020;2020:9367673.
Fuhrmann DC, Brüne B. Mitochondrial composition and function under the control of hypoxia. Redox Biol. 2017;12:208–15.
Momtahan N, Crosby CO, Zoldan J. The role of reactive oxygen species in in vitro cardiac maturation. Trends Mol Med. 2019;25(6):482–93.
Acknowledgements
We thank Professor Hongfeng Jiang for his expert technical advice and Editage (http://www.editage.cn) for English language editing.
Funding
This research was funded by the Science and Technology Development Fund of Beijing Anzhen Hospital, Capital Medical University (grant number AZYZR202203).
Author information
Authors and Affiliations
Contributions
Xin Liu performed the majority of the experiments, wrote and revised the article. Xiaolu Li and Haotan Zhou assisted with the experiment. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study is reported in accordance with ARRIVE guidelines (https:// arriveguidelines. org). The animal experiments were approved by the Laboratory Animal Ethics Committee of Beijing Anzhen Hospital, Capital Medical University and the article did not contain any studies with human participants performed by any of the authors.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liu, X., Li, X. & Zhou, H. Changes in glutamic oxaloacetic transaminase 2 during rat physiological and pathological cardiomyocyte hypertrophy. BMC Cardiovasc Disord 23, 595 (2023). https://doi.org/10.1186/s12872-023-03648-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-023-03648-3