Abstract
Background
To achieve potential financial savings and avoid exposing the patients to unnecessary risk, an optimal diagnostic strategy to identify low risk individual who may derive minimal benefit from further cardiac imaging testing (CIT) is important for patients with stable chest pain (SCP) suggestive of chronic coronary syndrome (CCS). Although several diagnostic strategies have been recommended by the most recent guidelines, few randomized controlled trials (RCTs) have prospectively investigated the actual effect of applying these strategies in clinical practice.
Methods
OPERATE (OPtimal Evaluation of stable chest pain to Reduce unnecessAry utilization of cardiac imaging TEsting) trial is an investigator-initiated, multicenter, coronary computed tomography angiography (CCTA)-based, 2-arm parallel-group, double-blind, pragmatic and confirmative RCT planning to include 800 subjects with SCP suggestive of CCS. After enrollment, all subjects will be randomized to two arms (2016 U.K. National Institute of Health and Care Excellence guideline-determined and 2019 European Society of Cardiology guideline-determined diagnostic strategy) on a 1:1 basis. According to each strategy, CCTA should be referred and deferred for a subject in high and low risk group, respectively. The primary (effectiveness) endpoint is CCTA without obstructive coronary artery disease. Safety of each strategy will be mainly assessed by 1-year major adverse cardiovascular event rates.
Discussion
The OPERATE trial will provide comparative effectiveness and safety evidences for two different diagnostic strategies for patients with SCP suggestive of CCS, with the intension of improving the diagnostic yield of CCTA at no expense of safety.
Clinical trial registration
ClinicalTrial.org Identifier NCT05640752.
Similar content being viewed by others
Background
In daily clinical routine, the evaluation of new-onset stable chest pain (SCP) suggestive of chronic coronary syndrome (CCS) remains a challenge for physicians, in consideration of the decreasing prevalence of coronary artery disease (CAD) and dramatically rising costs related to these patients [1, 2]. Although coronary computed tomography angiography (CCTA) has been the first-line cardiac imaging testing (CIT) according to current recommendations [3,4,5], an increasing body of evidence pointed to the fact that most of patients referred to CCTA as well as other CIT had normal results and no adverse clinical events [6,7,8,9,10]. Thus, an optimal diagnostic strategy to identify low risk patients who may derive minimal benefit from further CIT is the cornerstone of clinical management for SCP, which has been proposed and reiterated by most recent guidelines to achieve potential financial savings and avoid exposing patients to unnecessary risk [3,4,5].
Different diagnostic strategies have been developed to defer unnecessary CIT. The 2016 U.K. National Institute of Health and Care Excellence (NICE) guideline abandoned pretest probability (PTP)-based strategy and recommended the referrals of CCTA in patients with angina or abnormal ECG [5]. On the contrary, the 2019 European Society of Cardiology (ESC) guideline suggested an updated PTP model to rule out CAD in patients with ESC-PTP ≤ 5% and recommended noninvasive CIT for patients with ESC-PTP ≥ 5% [3]. For patients with borderline ESC-PTP (5–15%), Winther et al. developed the risk factor-weighted clinical likelihood (RF-CL) model, which was also proven to improve the prediction of CAD [11]. Both ESC-PTP and RF-CL model were recommended by the latest guideline for evaluation and diagnosis of chest pain [4].
2016 NICE guideline-determined diagnostic strategy (NICE strategy) [12, 13] and ESC guideline-determined diagnostic strategy (ESC strategy) [11, 14, 15] were externally validated and compared in different CCTA-based cohorts of SCP patients, as well as our previous studies from the CCTA Improves Clinical Management of Stable Chest Pain (CICM-SCP) registry [16,17,18]. While evidences from these observational cohorts and post hoc analyses of randomized controlled trial (RCT) suggested that the PTP-based strategy offered more effective deferral for CIT than the symptom-focused strategy did [16], they only examined patients who had underwent CIT and indications for CIT relied on a nonrandomized and complicated fashion, resulting in substantial biases. In fact, few RCTs have prospectively determined the actual effect of applying these strategies in clinical practice [19, 20].
Therefore, the OPERATE (OPtimal Evaluation of stable chest pain to Reduce unnecessAry utilization of cardiac imaging TEsting) study is designed to compare the effectiveness and safety of two proposed diagnostic strategies, ESC and NICE strategy, in identification of low risk individual who may derive minimal benefit from CCTA among patients with SCP suggestive of CCS in a pragmatic RCT.
Methods/design
Overall design and objectives
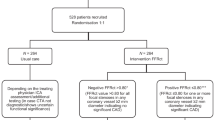
Figure 1 shows an overview of the study design. OPERATE trial is an investigator-initiated, multicenter, CCTA-based, 2-arm 1:1 parallel-group, double-blind, pragmatic and confirmative RCT planning to include 800 subjects with SCP suggestive of CCS (ClinicalTrials.gov Identifier NCT05640752). This study protocol is developed in accordance with the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) [21] and the SPIRIT checklist is available in Additional file 1. The primary objective of OPERATE trial is to compare the rates of CCTA without obstructive CAD according to NICE and ESC strategy. The key secondary objective is to assess whether the two strategies have no significant difference in terms of major adverse cardiac events (MACEs). We hypothesize that when comparing with NICE strategy, ESC strategy which sequentially incorporated the ESC-PTP model with RF-CL model will decrease the probability of CCTA without obstructive CAD but not at the expense of safety and cost over a follow-up period of 1 year.
Study design
SCP: stable chest pain; CCS: chronic coronary syndrome; CAD: coronary artery disease; ECG: electrocardiogram; NICE strategy: 2016 National Institute of Health and Care Excellence guideline-determined strategy; ESC strategy: 2019 European Society of Cardiology guideline-determined strategy; MACE: major adverse cardiovascular event; CCTA: coronary computed tomography angiography; RF-CL: risk factor-weighted clinical likelihood; PTP: pretest probability
Enrollment and screening
Subjects will be enrolled competitively from five major cardiac centers recognized as tertiary A level (Tianjin Chest Hospital, Tianjin First Central Hospital, Beijing Chaoyang Hospital, Tianjin Second Teaching Hospital of Tianjin University of Traditional Chinese and Hebei Petrochina Central Hospital) in Beijing-Tianjin-Hebei region, which has more than 100 million population. The total annual number of patients presenting with SCP suggestive of CCS and referred to CIT in these high-volume sites is more than 30,000 and 20,000, respectively. Subjects are considered eligible for inclusion if they suffer SCP suggestive of CCS, are more than 30 years of age, and willing and able to give informed consent. Eligible subjects are clinically stable, have no history of CAD and will undergo routine electrocardiogram (ECG) and echocardiography according to the latest guideline [3, 4]. Trained research nurses introduce the trial to subjects and investigators are advised not to randomize subjects who expressed a clear preference for undergoing CIT or not during the informed consent process. More details about inclusion and exclusion criteria are listed in Table 1.
Baseline clinical data
Baseline characteristics are defined and collected as described previously [16, 18, 22]. Hypertension is defined as blood pressure of ≥ 140/90 mmHg or the use of anti-hypertension medication. Hyperlipidemia is defined as total cholesterol of ≥ 220 mg/dL, low-density lipoprotein cholesterol of ≥ 140 mg/dL, fasting triglycerides of ≥ 150 mm/dL or receiving treatment with oral lipid-lowering agents. Diabetes is defined as fasting glucose levels over 7 mmol/L or treatment currently with either diet, oral glucose lowering agents or insulin. Smoking is defined as current smoking or smoking in past 6 months. Abnormal ECG is defined pathological Q waves or ST-segment and T wave abnormalities in at least two adjacent leads. A family history of premature CAD is defined as diagnosis of the disease in a male first-degree relative before 55 years of age or in a female first-degree relative before 65 years of age. Typical angina is defined as having 3 characteristics: (1) substernal discomfort of characteristic quality, (2) precipitated by physical exertion or emotion, and (3) relieved with rest or nitroglycerin within 10 min. Atypical angina is defined as having 2 of the 3 definition characteristics. Nonanginal chest pain is characterized as chest pain or discomfort that meets 1 or 0 of the 3 definition characteristics [23].
Randomization and blinding
After collection of baseline clinical data, eligible subjects will be randomized (in blocks of 2) sequentially 1:1 to start double-blind diagnostic strategy with 1 of 2 regimens: NICE and ESC. The computerized randomization list is generated independently by a statistician from the statistical and data coordinating group who will not participate rest of the study. Both investigators and subjects are blinded to the allocation process until the end of trial (sequentially numbered, opaque, sealed envelope will be used to conceal allocation). Once the subject is ready for randomization, the corresponding envelope will be opened by the independent strategy assignment expert panel consisting of cardiologists who will not participate rest of the study. The expert panel determine whether the subject should be referred to CCTA according to the given diagnostic strategy (see below) and only send the final recommendation about referral or deferral of CCTA to the physician and subject.
Diagnostic strategies
For a subject in high risk group according to each strategy, CCTA should be referred as the first-line CIT and other CIT, including noninvasive functional testing (NFT) and invasive coronary angiography (ICA), will be considered as an alternative. In both diagnostic strategies, subjects determined to be at low risk will be referred to optimal medication treatment (OMT) with no immediate CIT. The decisions regarding OMT will be done at discretion of the referring physicians according to recent primary prevention guidelines [24, 25], and is not a part of the study protocol. Thus, in cases where symptoms don’t resolve with maximum OMT, additional cardiac or non-cardiac diagnostic testing could be pursued as an escalation of the diagnostic strategy. Based on current SCP guidelines [3,4,5] and results of proposed researches [11,12,13,14,15,16,17], details of risk groups are illustrated in Fig. 1; Table 2 and as follows:
According to NICE strategy, subjects with nonanginal chest pain and normal ECG are classified into low risk group. Subjects with typical and atypical angina or nonanginal chest pain with abnormal ECG are classified into high risk group [5].
For each subject, ESC-PTP is calculated using age, sex and type of chest pain according to 2019 ESC guideline for the diagnosis and management of CCS [3] and RF-CL is calculated using age, sex, type of chest pain, hypertension, dyslipidemia, diabetes, smoking and family history of CAD based on the publication of Winther et al. [11], respectively. According to ESC strategy, subjects with ESC-PTP ≤ 5% are classified into low risk group and ones with ESC-PTP ≥ 15% are classified into high risk group [3]. For subjects with ESC-PTP of 5-15%, ones with RF-CL ≥ 15% are classified into high risk group and ones with RF-CL < 15% are classified into low risk group. The cut-off of 15% for RF-CL is chosen because RF-CL < 15% was associated with an extremely low prevalence of obstructive CAD and risk of clinical events [11, 16].
CCTA and other CIT
All participating sites will use standard equipment, procedure and interpretation for CIT, as defined by current practice guidelines [3, 4, 26,27,28,29] and local protocols as previously described in the CICM-SCP registry [16, 22, 30]. All results will be reported by independent radiology/cardiology consultants with a minimum 5-year experience in the imaging modality and provided to the local physicians for subsequent decision making. The site interpretation of CIT was verified by central review of the clinical reports with review of the primary images as needed [31, 32]. The exercise electrocardiography criterion for a positive test was greater ≥ 1.5 mm of horizontal or down sloping ST segment deviation (depression or elevation) in any 2 leads except aVR for at least 60 to 80 ms after the end of the QRS complex, either during or after exercise. The criterion of single-photon emission computed tomography or positron emission tomography for a positive test was area of ischaemia ≥ 10% of the left ventricle myocardium. The stress echocardiography criterion for a positive test was ≥ 3 of 16 segments with stress-induced hypokinesia or akinesia. The criterion of cardiac magnetic resonance for a positive test was ≥ 2 of 16 segments with stress perfusion defects or ≥ 3 dobutamine-induced dysfunctional segments. The fractional flow reserve (FFR) derived from CCTA criterion for a positive test was FFR < 0.8.
Specially, CCTA images are acquired using 64-slice, or greater, multidetector CT scanners and all DICOM imaging data will be sent to the CCTA Core Laboratory (Tianjin Chest Hospital) for further analysis. In the CCTA Core Laboratory, all CCTA images will be analyzed by three experienced observers, 2 radiologists and 1 cardiologist, who are blinded to the other data. In image analyses, all segments ≥ 2 mm in diameter are identified and analyzed using the updated Coronary Artery Disease–Reporting and Data System (CAD-RADS) [29]. The percent diameter stenosis in every segment is categorized as 0% (no stenosis), 1–24% (minimal stenosis), 25–49% (mild stenosis), 50–69% (moderate stenosis), 70–99% (severe stenosis), and 100% (total occlusion). Interobserver disagreements are resolved by consensus.
The result of CCTA, as well as other CIT, will be provided to the local physician and he or she will make subsequent clinical decisions for the subject, such as additional CIT and clinical interventions like cardiac rehabilitation, OMT and coronary revascularization (CR), based on recommendations from clinical guidelines [3, 4, 26,27,28,29] and his or her cumulative clinical assessment of the subject.
Study endpoint
The primary outcome, main secondary outcomes and other outcomes are described below and all outcomes are included Table 3. The primary endpoint of the study which indicates effectiveness of the diagnostic strategy is CCTA without obstructive CAD, defined as the summary of nonobstructive CAD, no sign of CAD and nondiagnostic result detected by CCTA according to each strategy during initial management. Obstructive CAD is defined as present if a patient had at least one lesion with ≥ 50% diameter stenosis at CCTA.
The key secondary endpoint indicating the safety of diagnostic strategy is the time to first MACE using composite of the following clinical events: All-cause death is used rather than cardiovascular death to eliminate the need for possibly difficult adjudication of causes of death, especially given the relatively low mortality; Nonfatal myocardial infarction (MI), including spontaneous and procedure-related, is defined according to the Fourth Universal Definition of Myocardial Infarction [33]; Hospitalization due to unstable angina is defined as an event in which the final diagnosis is myocardial ischemia (at least one of the following must be present: dynamic ST depression, ischemia on stress testing or significant epicardial coronary artery stenosis) and either of the following criteria is present: ischemic discomfort or equivalent symptoms requiring hospitalization within 48 h of symptoms and lasting at least 10 min at rest, or occurring in an accelerated pattern within 48 h of hospitalization. Only symptomatic events are defined as MACE. Asymptomatic events, such as silent MI is defined as incidental findings–if they will be detected at all.
In addition to the individual components of MACE, other secondary endpoints indicating the safety of diagnostic strategy include the followings: All-cause death, nonfatal MI, hospitalization due to unstable angina, overall exposure to radiation and complications (classified as major and minor) related to cardiovascular procedures which occur within 72 h. The estimated cumulative radiation exposure over the entire trial will be measured in milliSieverts using administered dose on CCTA, converted contrast agent dose for nuclear and administered radiation dose (kerma air product or dose length product) or fluoroscopy time for angiography [34].
Another secondary endpoint indicating the effectiveness of diagnostic strategy is resource use patterns (cumulative number of cardiovascular procedures during both initial management and follow-up, such as noninvasive and invasive CIT or CR). The diagnostic metrics of CCTA during initial management, including diagnostic yield proportion of normal CCTA and necessary CCTA, will be also calculated. Necessary CCTA is defined as obstructive CAD or alteration of OMT due to nonobstructive CAD on CCTA. Other effectiveness outcomes include time length of initial management and health-related quality of life (HRQOL).
Study period and follow-up
The planned duration of the study is 24 months: 12 months for the enrollment period, defined as first visit of first subject to first visit of last subject and 12 months for the follow-up period. Study will preliminarily end when the following have occurred: at least 800 subjects have been randomized and 12 ± 1 month have elapsed since the last subject is randomized. All subjects will be followed for a minimum of 1 year and we will also try to continue the further follow-up after 1 year at 12-month intervals until death or withdrawal. Follow-up will be performed at 1st month (± 7 days), 3rd moth (± 14 days), 6th month (± 14 days), and 12th month (± 28 days) after randomization. The information of clinical visits is shown in Table 4. For outcome evaluation and recording of test complications, subjects have either a telephone call or clinic visit and the medical records are collected by trained interviewers. Data collected for suspected events are provided to the independent clinical event adjudication committee masked to the strategy assignment and intervention.
Sample size
The sample size calculation is performed using R (version 3.6.4; R Foundation for Statistical Computing, Vienna, Austria). To compute a weighted average rate as the projected rate of CCTA without obstructive CAD for a diagnostic strategy, data from previous literatures used in the sample size calculation include rate of CCTA without obstructive CAD in high risk group and distribution of subjects in low and high risk group.
For NICE strategy, theses parameters fluctuated over a wide range in different cohorts [12, 13, 16]. Thus, the distribution of subjects and the rate of CCTA without obstructive CAD in high risk group is estimated to be approximately 2:3 for low/high risk group and 62%, respectively, resulting in a projected rate of 3/(2 + 3)×57%=37.2%. Similarly, we assume that the projected rate of primary endpoint is 2/(2 + 3)×57%=22.8% for ESC strategy, based on the results of the original study [11] as well as our previous studies including one which investigated the RF-CL model in subjects with borderline PTP [16, 17].
For the analysis of primary endpoint, on 2-sided test 5% significance level and for the 1:1 allocation ratio, a sample size of 800 (allowing for 10% noncompletion) will provide more than 99% power between NICE and ESC arm. Thus, the final sample size is chosen to be 800 subjects (400 in each randomization group).
Statistical plan
All statistical analyses are performed by the statistical and data coordinating group using R (version 3.6.4; R Foundation for Statistical Computing, Vienna, Austria) or MedCalc (version 15.2.2; MedCalc Software, Mariakerke, Belgium). Two-tailed P < 0.05 is considered statistically significant. The primary analysis is conducted according to a modified intention-to-treat (ITT) principle. All subjects who undergone randomization and don’t withdraw before assigned intervention (CCTA or no further testing) or other CIT during the initial management will be included. Categorical variables will be described by counts and proportions and compared by chi-square tests or fisher exact test. Continuous variables will be described by mean and standard deviation or median and interquartile range and compared through t test or Mann-Whitney U tests.
The comparison of primary outcome between two diagnostic strategy regimens was expressed as risk ratio, along with 95% confidence interval and number needed to treat. The per-protocol analysis excluding subjects with insufficient compliance or with other major protocol deviation(s) and the traditional ITT analysis including all subjects who are randomized will be performed as prespecified sensitivity analyses. Different diagnostic metrics, such as initial CIT without positive result, unnecessary CCTA and CCTA with no sign of CAD, will also be considered as sensitivity analyses. To explore the consistency of strategy effectiveness according to specific subject characteristics, a limited number of prespecified subgroup analyses for the primary endpoint are conducted using interaction terms in log-binomial regression models. We will also undertake a hierarchical analysis (giving priority to clinical importance of the components of the composite outcome rather than time to first event: Death > nonfatal MI > hospitalization due to unstable angina > CCTA without obstructive CAD > referral to CIT) using the matched win ratio method [35].
Kaplan-Meier curves for cumulative event-free estimates survival from the first of the following: endpoints of concern, death, the end of 1-year follow-up period or loss to follow-up, are presented graphically and the Log-rank test will be used to calculate the corresponding p. A Cox proportional hazards model will be used to compare the time to study endpoint and to account for heterogeneity among subjects, the models will be adjusted for pre-specified and minimization covariates, which are selected on the basis of their established importance in other CCS cohorts, highly complete data capture, and a sufficient range of values for risk to vary among subjects meeting study eligibility criteria.
Multiple imputation using the R package Multivariate Imputation by Chained Equations algorithm will be used for missing data in ITT analysis of primary endpoint to ensure all subjects could be included in the ITT analysis, as well as HRQOL analysis [36]. Strategy comparisons of HRQOL are performed by using a mixed-effects regression model to account for repeated measures within a subject, with strategy, time point, baseline HRQOL and minimization covariates as predictor variables. If the analyses above show significant differences in study endpoints, information from the study and other sources, including medical costs, resource use, HRQOL and clinical outcomes will be used for the potential cost-effectiveness analyses to quantify the incremental cost per quality-adjusted life year gained.
Discussion
Recent studies have demonstrated that performance of CIT was significantly influenced by PTP [37,38,39] and supported different diagnostic strategies to defer unnecessary CIT in patients at low risk [11, 14, 15]. However, only 32% of imaging centers in Europe selected optimal CIT based on estimation of PTP while 31% proceed directly to CCTA [40] and routine utilization of CCTA in low risk, even clinically healthy population were common in China [41]. Although current guidelines regarding the evaluation and management of SCP symptoms suggestive of CCS all emphasize the identification of patients unlikely to benefit from further CIT by structured and evidence-based diagnostic strategies [3,4,5], ample evidence of inappropriate referrals of CIT persists [6,7,8,9], which might be mainly attributed to the deficiency of consensus among clinicians and researchers on the preferred diagnostic strategy at the basis of specialized RCTs. Thus, the rationale and design outlined above are unique and desiderated in that the OPERATE trial will systematically compare two different diagnostic strategies in patients with SCP suggestive of CCS.
The risk of incorrectly deferral of CIT for patients with severe CAD should emerge as particularly strong candidates accounting for the overuse of CIT in clinical practice [1]. In fact, a risk of false negative will always be present for any testing and current guidelines recommended no further CIT in patients with low probability of obstructive CAD and cardiovascular events [3,4,5]. Thereby, identifying a proportion of patients at low risk to avoid CIT which may lead to false positive results and extra exposure of accompanying risk is acceptable, especially in an era of excess CIT use [42]. Data from more than 30, 000 CCS patients demonstrated that on the basis of contemporary OMT, most patients with angina were likely to experience resolution of symptoms without events or CR [43]. Moreover, both NICE and ESC strategy were externally validated in the CICM-SCP registry composed of patients from similar sites [16,17,18], which maximized the generalizabilities and reliabilities of them. Indeed, according to data from CICM-SCP registry composed of patients who underwent CCTA based on the decisions of local physicians, the proportion of patients who were classified into low risk group by NICE and ESC strategy but suffered MACE during a 17-month follow-up was only 1.1% and 0.7%, respectively [16]. On the other hand, the principle of OPERATE trial which left major decisions to local heart team members and referring physicians is intended to allow considerable leeway for clinical judgment in keeping with the pragmatic design. Also, this design in combination with ITT analysis can potentially elucidate the effectiveness of diagnostic strategies in routine clinical practice.
Although PROMISE minimal risk tool (PMRT) has been also validated in the CICM-SCP registry and demonstrated a robust risk assessment of SCP in several other validation studies [16, 44,45,46], OPERATE trial does not includes an arm of PMRT-based diagnostic strategy for the reasons below. First, the cutoff derivations of PMRT to identify low risk individual vary considerably among different cohorts [16, 44,45,46,47]. Second, instead of concerning about obstructive CAD, the PMRT defined minimal CAD risk as no coronary plaque or calcification and no MACE during follow-up. Third, the PMRT included a parameter from blood test, the high-density lipoprotein cholesterol level, as an independent variable. Except for ESC-PTP and RF-CL model, the coronary artery calcium score (CACS)-weighted clinical likelihood (CACS-CL) model which integrated CACS into estimation of PTP [11] was also recommended by the latest guideline for evaluation and diagnosis of chest pain [4] and externally validated in the CICM-SCP registry [16]. However, CACS scan is an extra test with radiation exposure and cost that potentially delays diagnosis and violates the hypothesis of identifying a subgroup for whom no further test is needed [18, 46]. As a result, a CACS-CL model-based diagnostic strategy has not been included by OPERATE trial so far.
Several representative RCTs [7, 9, 48, 49] and observational registry [16, 43] has provided obvious evidences that patients referred to CIT for suspected CCS had a very low rate of clinical event, resulting in a urgent demand to conservatively improve utilization of resources [1]. Meanwhile, the low event rate will limit our strength of evidence and causal statements inferred from an underpowered trial with a predefined primary outcome of MACE [1, 27]. To overcome this problem, we choose CCTA without obstructive CAD as primary endpoint to indicate effectiveness of the diagnostic strategy, which is, in turn, the main concerned outcome of PTP models, and introduce the approach of win ratio to analyze composite endpoint of MACE, CCTA without obstructive CAD and referral to CIT in the order of clinically importance. Similar designs using the imaging-based primary endpoints have also been selected by other comparative effectiveness trials of diagnostic strategies for SCP [20, 32, 50]. In addition, although it has been proposed that cardiac ischemia or plaque characteristics detected by CCTA to target intervention may improve clinical outcome, a detailed evaluation and treatment pathway have not been established [32, 51, 52]. Thus, anatomy severity remains the primary arbiter of post-CCTA management [4].
In patients with SCP suggestive of CCS, CCTA provided excellent performance for diagnoses and prognosis of CAD in a noninvasive and feasible way [27] and major guideline bodies have started to endorse incorporation of CCTA more definitively than before [3,4,5, 28]. Thus, we uniformly give CCTA the first-line status in subjects at high risk according to NICE and ESC strategy for several reasons. First, recent researches indicated that in terms of major clinical outcomes, CCTA-first strategy was superior or not inferior to NFT- or ICA-first strategy [7, 9, 49, 53, 54]. Second, CCTA are broadly available because of relatively low technical and personnel demands, while NFT, like positron emission tomography and stress cardiac magnetic resonance, although powerful, are much less available and their applicability is still limited by infrastructural and capacity requirements, especially in China [10]. Third, models constituting the ESC strategy were established in CCTA-based cohorts [3, 11]. Last, the post-CCTA clinical management paradigm based on CAD-RADS has been demonstrated to be associated with lower risk of MACEs and fewer invasive procedures [30].
In conclusion, we anticipate that the OPERATE study will investigate the real effects of symptom-focused (NICE) and PTP-based (ESC) diagnostic strategy on decisions of clinical management and subsequent clinical outcomes in patients with SCP suggestive of CCS.
Data Availability
Not applicable.
Abbreviations
- SCP:
-
stable chest pain
- CCS:
-
chronic coronary syndrome
- CAD:
-
coronary artery disease
- CCTA:
-
coronary computed tomography angiography
- CIT:
-
cardiac imaging testing
- NICE strategy:
-
2016 U.K. National Institute of Health and Care Excellence guideline-determined diagnostic strategy
- ESC strategy:
-
2019 European Society of Cardiology guideline-determined diagnostic strategy
- PTP:
-
pretest probability
- RCT:
-
randomized controlled trial
- MACE:
-
major adverse cardiac events
- ECG:
-
electrocardiogram
- NFT:
-
noninvasive functional testing
- ICA:
-
invasive coronary angiography
- OMT:
-
optimal medication treatment
- PMRT:
-
PROMISE minimal risk tool
- ITT:
-
intention-to-treat
- HRQOL:
-
health-related quality of life
- CACS-CL:
-
coronary artery calcium score-weighted clinical likelihood
- RF-CL:
-
risk factor-weighted clinical likelihood
- CR:
-
coronary revascularization
References
Hecht HS, Shaw L, Chandrashekhar YS, Bax JJ, Narula J. Should NICE guidelines be universally accepted for the evaluation of stable coronary disease? A debate. Eur Heart J. 2019;40(18):1440–53.
Gibbons RJ, Miller TD. Declining accuracy of the traditional Diamond-Forrester estimates of Pretest Probability of Coronary Artery Disease: Time for New Methods. JAMA Intern Med. 2021;181(5):579–80.
Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, Prescott E, Storey RF, Deaton C, Cuisset T, Agewall S, Dickstein K, Edvardsen T, Escaned J, Gersh BJ, Svitil P, Gilard M, Hasdai D, Hatala R, Mahfoud F, Masip J, Muneretto C, Valgimigli M, Achenbach S, Bax JJ. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41(3):407–77.
Gulati M, Levy PD, Mukherjee D, Amsterdam E, Bhatt DL, Birtcher KK, Blankstein R, Boyd J, Bullock-Palmer RP, Conejo T, Diercks DB, Gentile F, Greenwood JP, Hess EP, Hollenberg SM, Jaber WA, Jneid H, Joglar JA, Morrow DA, O’Connor RE, Ross MA, Shaw LJ, 2021 AHA/ACC/ASE/CHEST. Evaluation and diagnosis of chest Pain: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2021;78(22):e187–e285. /SAEM/SCCT/SCMR Guideline for the.
Timmis A, Roobottom CA. National Institute for Health and Care Excellence updates the stable chest pain guideline with radical changes to the diagnostic paradigm. Heart. 2017;103(13):982–6.
Gibbons RJ, Carryer D, Hodge D, Miller TD, Roger VL, Askew JW. Stress testing in the evaluation of stable chest Pain in a Community Population. Mayo Clin Proc. 2020;95(2):319–27.
Douglas PS, Hoffmann U, Patel MR, Mark DB, Al-Khalidi HR, Cavanaugh B, Cole J, Dolor RJ, Fordyce CB, Huang M, Khan MA, Kosinski AS, Krucoff MW, Malhotra V, Picard MH, Udelson JE, Velazquez EJ, Yow E, Cooper LS, Lee KL. Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med. 2015;372(14):1291–300.
Cheng VY, Berman DS, Rozanski A, Dunning AM, Achenbach S, Al-Mallah M, Budoff MJ, Cademartiri F, Callister TQ, Chang HJ, Chinnaiyan K, Chow BJ, Delago A, Gomez M, Hadamitzky M, Hausleiter J, Karlsberg RP, Kaufmann P, Lin FY, Maffei E, Raff GL, Villines TC, Shaw LJ, Min JK. Performance of the traditional age, sex, and angina typicality-based approach for estimating pretest probability of angiographically significant coronary artery disease in patients undergoing coronary computed tomographic angiography: results from the multinational coronary CT angiography evaluation for clinical outcomes: an international multicenter registry (CONFIRM). Circulation. 2011;124(22):2423–32.
Newby DE, Adamson PD, Berry C, Boon NA, Dweck MR, Flather M, Forbes J, Hunter A, Lewis S, MacLean S, Mills NL, Norrie J, Roditi G, Shah ASV, Timmis AD, van Beek EJR, Williams MC. Coronary CT angiography and 5-Year risk of myocardial infarction. N Engl J Med. 2018;379(10):924–33.
Shan D, Yang J, Chen Y. Noninvasive cardiac imaging technologies in detecting coronary artery disease: from research to clinical practice. Cardiol Plus. 2020;5(1):13.
Winther S, Schmidt SE, Mayrhofer T, Botker HE, Hoffmann U, Douglas PS, Wijns W, Bax J, Nissen L, Lynggaard V, Christiansen JJ, Saraste A, Bottcher M, Knuuti J. Incorporating coronary calcification into Pre-Test Assessment of the Likelihood of Coronary Artery Disease. J Am Coll Cardiol. 2020;76(21):2421–32.
Adamson PD, Newby DE, Hill CL, Coles A, Douglas PS, Fordyce CB. Comparison of International Guidelines for Assessment of suspected stable angina: insights from the PROMISE and SCOT-HEART. J Am Coll Cardiol Img. 2018;11(9):1301–10.
Adamson PD, Hunter A, Williams MC, Shah ASV, McAllister DA, Pawade TA, Dweck MR, Mills NL, Berry C, Boon NA, Clark E, Flather M, Forbes J, McLean S, Roditi G, van Beek EJR, Timmis AD, Newby DE. Diagnostic and prognostic benefits of computed tomography coronary angiography using the 2016 National Institute for Health and Care Excellence guidance within a randomised trial. Heart. 2018;104(3):207–14.
Bing R, Singh T, Dweck MR, Mills NL, Williams MC, Adamson PD, Newby DE. Validation of European Society of Cardiology pre-test probabilities for obstructive coronary artery disease in suspected stable angina. Eur Heart J Qual Care Clin Outcomes. 2020;6(4):293–300.
Winther S, Schmidt SE, Rasmussen LD, Juárez Orozco LE, Steffensen FH, Bøtker HE, Knuuti J, Bøttcher M. Validation of the European Society of Cardiology pre-test probability model for obstructive coronary artery disease. Eur Heart J. 2021;42(14):1401–11.
Zhou J, Li C, Cong H, Duan L, Wang H, Wang C, Tan Y, Liu Y, Zhang Y, Zhou X, Zhang H, Wang X, Ma Y, Yang J, Chen Y, Guo Z. Comparison of different investigation strategies to Defer Cardiac Testing in patients with stable chest Pain. J Am Coll Cardiol Img. 2022;15(1):91–104.
Zhou J, Zhao J, Li Z, Cong H, Wang C, Zhang H, Wang X, Ma Y, Li C, Guo Z. Coronary calcification improves the estimation for clinical likelihood of obstructive coronary artery disease and avoids unnecessary testing in patients with borderline pretest probability. Eur J Prev Cardiol. 2021;zwab036.
Zhao J, Wang S, Zhao P, Huo Y, Li C, Zhou J. Comparison of Risk Assessment Strategies for Patients with Diabetes Mellitus and Stable Chest Pain: A Coronary Computed Tomography Angiography Study. Journal of Diabetes Research. 2022;2022.
Bjerking LH, Hansen KW, Biering-Sørensen T, Brønnum-Schou J, Engblom H, Erlinge D, Haahr-Pedersen SA, Heitmann M, Hove JD, Jensen MT. Cost-effectiveness of adding a non-invasive acoustic rule-out test in the evaluation of patients with symptoms suggestive of coronary artery disease: rationale and design of the prospective, randomised, controlled, parallel-group multicenter FILTER-SCAD trial. BMJ open. 2021;11(8):e049380.
Nanna MG, Vemulapalli S, Fordyce CB, Mark DB, Patel MR, Al-Khalidi HR, Kelsey M, Martinez B, Yow E, Mullen S. The prospective randomized trial of the optimal evaluation of cardiac symptoms and revascularization: Rationale and design of the PRECISE trial. Am Heart J. 2022;245:136–48.
Chan AW, Tetzlaff JM, Altman DG, Laupacis A, Gøtzsche PC, Krleža-Jerić K, Hróbjartsson A, Mann H, Dickersin K, Berlin JA, Doré CJ, Parulekar WR, Summerskill WS, Groves T, Schulz KF, Sox HC, Rockhold FW, Rennie D, Moher D. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. 2013;158(3):200–7.
Zhou J, Chen Y, Zhang Y, Wang H, Tan Y, Liu Y, Huang L, Zhang H, Ma Y, Cong H. Epicardial Fat volume improves the prediction of obstructive coronary artery disease above traditional risk factors and coronary calcium score. Circ Cardiovasc Imaging. 2019;12(1):e008002.
Diamond GA. A clinically relevant classification of chest discomfort. J Am Coll Cardiol. 1983;1(2 Pt 1):574–5.
Visseren FLJ, Mach F, Smulders YM, Carballo D, Koskinas KC, Bäck M, Benetos A, Biffi A, Boavida JM, Capodanno D, Cosyns B, Crawford C, Davos CH, Desormais I, Di Angelantonio E, Franco OH, Halvorsen S, Hobbs FDR, Hollander M, Jankowska EA, Michal M, Sacco S, Sattar N, Tokgozoglu L, Tonstad S, Tsioufis KP, van Dis I, van Gelder IC, Wanner C, Williams B. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. 2021;42(34):3227–337.
Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd-Jones D, McEvoy JW, Michos ED, Miedema MD, Muñoz D, Smith SC Jr, Virani SS, Williams KA, Sr., Yeboah J, Ziaeian B. 2019 ACC/AHA Guideline on the primary Prevention of Cardiovascular Disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e596–e646.
Lawton JS, Tamis-Holland JE, Bangalore S, Bates ER, Beckie TM, Bischoff JM, Bittl JA, Cohen MG, DiMaio JM, Don CW, Fremes SE, Gaudino MF, Goldberger ZD, Grant MC, Jaswal JB, Kurlansky PA, Mehran R, Metkus TS Jr, Nnacheta LC, Rao SV, Sellke FW, Sharma G, Yong CM, Zwischenberger BA. 2021 ACC/AHA/SCAI Guideline for Coronary Artery revascularization: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145(3):e18–14.
Narula J, Chandrashekhar Y, Ahmadi A, Abbara S, Berman DS, Blankstein R, Leipsic J, Newby D, Nicol ED, Nieman K, Shaw L, Villines TC, Williams M, Hecht HS. SCCT 2021 Expert Consensus Document on Coronary computed Tomographic Angiography: a report of the Society of Cardiovascular computed Tomography. J Cardiovasc Comput Tomogr. 2021;15(3):192–217.
Winchester DE, Maron DJ, Blankstein R, Chang IC, Kirtane AJ, Kwong RY, Pellikka PA, Prutkin JM, Russell R, Sandhu AT, ACC/AHA/ASE/ASNC/ASPC/HFSA/HRS/SCAI/SCCT/SCMR/. STS 2023 Multimodality Appropriate Use Criteria for the Detection and Risk Assessment of Chronic Coronary Disease. J Am Coll Cardiol. 2023.
Cury RC, Leipsic J, Abbara S, Achenbach S, Berman D, Bittencourt M, Budoff M, Chinnaiyan K, Choi AD, Ghoshhajra B, Jacobs J, Koweek L, Lesser J, Maroules C, Rubin GD, Rybicki FJ, Shaw LJ, Williams MC, Williamson E, White CS, Villines TC, Blankstein R. Tomography (SCCT), the American College of Cardiology (ACC), the American College of Radiology (ACR), and the North America Society of Cardiovascular Imaging (NASCI). J Cardiovasc Comput Tomogr. 2022;16(6):536–57. CAD-RADS™ 2.0–2022 Coronary Artery Disease-Reporting and Data System: An Expert ConsensusDocument of the Society of Cardiovascular Computed.
Zhou J, Li C, Zhang H, Liu C, Yang J, Zhao J, Hou Y, Tan Y, Wang H, Li Y, Xie C, Wang M, Wang C, Zhang E, Wang S, Zhao P, Shan D, Liang S, Gao Y, Huo Y, Cong H, Guo Z, Chen Y. Association between Coronary Artery Disease Reporting and Data System–recommended post–coronary CT Angiography Management and Clinical Outcomes in patients with stable chest Pain from a chinese Registry. Radiology. 2023;307(5):e222965.
Maron DJ, Hochman JS, Reynolds HR, Bangalore S, O’Brien SM, Boden WE, Chaitman BR, Senior R, Lopez-Sendon J, Alexander KP, Lopes RD, Shaw LJ, Berger JS, Newman JD, Sidhu MS, Goodman SG, Ruzyllo W, Gosselin G, Maggioni AP, White HD, Bhargava B, Min JK, Mancini GBJ, Berman DS, Picard MH, Kwong RY, Ali ZA, Mark DB, Spertus JA, Krishnan MN, Elghamaz A, Moorthy N, Hueb WA, Demkow M, Mavromatis K, Bockeria O, Peteiro J, Miller TD, Szwed H, Doerr R, Keltai M, Selvanayagam JB, Steg PG, Held C, Kohsaka S, Mavromichalis S, Kirby R, Jeffries NO, Harrell FE Jr, Rockhold FW, Broderick S, Ferguson TB Jr, Williams DO, Harrington RA, Stone GW, Rosenberg Y, Group IR. Initial invasive or conservative strategy for stable coronary disease. N Engl J Med. 2020;382(15):1395–407.
Yang J, Shan D, Dong M, Wang Z, Ma X, Hu X, Zeng H, Chen Y. The effect of on-site CT-derived fractional flow reserve on the management of decision making for patients with stable chest pain (TARGET trial): objective, rationale, and design. Trials. 2020;21(1):728.
Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD. Fourth Universal Definition of myocardial infarction (2018). J Am Coll Cardiol. 2018;72(18):2231–64.
Douglas PS, Hoffmann U, Lee KL, Mark DB, Al-Khalidi HR, Anstrom K, Dolor RJ, Kosinski A, Krucoff MW, Mudrick DW, Patel MR, Picard MH, Udelson JE, Velazquez EJ, Cooper L. PROspective Multicenter Imaging Study for evaluation of chest pain: rationale and design of the PROMISE trial. Am Heart J. 2014;167(6):796–803e1.
Pocock SJ, Ariti CA, Collier TJ, Wang D. The win ratio: a new approach to the analysis of composite endpoints in clinical trials based on clinical priorities. Eur Heart J. 2012;33(2):176–82.
Schafer JL. Multiple imputation: a primer. Stat Methods Med Res. 1999;8(1):3–15.
Knuuti J, Ballo H, Juarez-Orozco LE, Saraste A, Kolh P, Rutjes AWS, Juni P, Windecker S, Bax JJ, Wijns W. The performance of non-invasive tests to rule-in and rule-out significant coronary artery stenosis in patients with stable angina: a meta-analysis focused on post-test disease probability. Eur Heart J. 2018;39(35):3322–30.
Juarez-Orozco LE, Saraste A, Capodanno D, Prescott E, Ballo H, Bax JJ, Wijns W, Knuuti J. Impact of a decreasing pre-test probability on the performance of diagnostic tests for coronary artery disease. Eur Heart J Cardiovasc Imaging. 2019;20(11):1198–207.
Haase R, Schlattmann P, Gueret P, Andreini D, Pontone G, Alkadhi H, Hausleiter J, Garcia MJ, Leschka S, Meijboom WB, Zimmermann E, Gerber B, Schoepf UJ, Shabestari AA, Norgaard BL, Meijs MFL, Sato A, Ovrehus KA, Diederichsen ACP, Jenkins SMM, Knuuti J, Hamdan A, Halvorsen BA, Mendoza-Rodriguez V, Rochitte CE, Rixe J, Wan YL, Langer C, Bettencourt N, Martuscelli E, Ghostine S, Buechel RR, Nikolaou K, Mickley H, Yang L, Zhang Z, Chen MY, Halon DA, Rief M, Sun K, Hirt-Moch B, Niinuma H, Marcus RP, Muraglia S, Jakamy R, Chow BJ, Kaufmann PA, Tardif JC, Nomura C, Kofoed KF, Laissy JP, Arbab-Zadeh A, Kitagawa K, Laham R, Jinzaki M, Hoe J, Rybicki FJ, Scholte A, Paul N, Tan SY, Yoshioka K, Rohle R, Schuetz GM, Schueler S, Coenen MH, Wieske V, Achenbach S, Budoff MJ, Laule M, Newby DE, Dewey M. Diagnosis of obstructive coronary artery disease using computed tomography angiography in patients with stable chest pain depending on clinical probability and in clinically important subgroups: meta-analysis of individual patient data. BMJ. 2019;365:l1945.
Bularga A, Saraste A, Fontes-Carvalho R, Holte E, Cameli M, Michalski B, Williams MC, Podlesnikar T, D’Andrea A, Stankovic I, Mills NL, Manka R, Newby DE, Schultz-Menger J, Haugaa KH, Dweck MR. EACVI survey on investigations and imaging modalities in chronic coronary syndromes. Eur Heart J Cardiovasc Imaging. 2021;22(1):1–7.
Liu K, Hsieh C, Zhuang N, Gao Y, Li Z, Ren X, Yang L, Zhang J, Budoff MJ, Lu B. Current utilization of cardiac computed tomography in mainland China: a national survey. J Cardiovasc Comput Tomogr. 2016;10(1):76–81.
Hachamovitch R. Updating Algorithms for Predicting Pre-Test Likelihood of Coronary Artery Disease: a cure for Inappropriate Testing? J Am Coll Cardiol Img. 2018;11(3):447–9.
Mesnier J, Ducrocq G, Danchin N, Ferrari R, Ford I, Tardif JC, Tendera M, Fox KM, Steg PG. International Observational analysis of evolution and outcomes of chronic stable angina: the multinational CLARIFY study. Circulation. 2021;144(7):512–23.
Adamson PD, Fordyce CB, McAllister DA, Udelson JE, Douglas PS, Newby DE. Identification of patients with stable chest pain deriving minimal value from coronary computed tomography angiography: an external validation of the PROMISE minimal-risk tool. Int J Cardiol. 2018;252:31–4.
Rasmussen LD, Nissen L, Westra J, Knudsen LL, Madsen LH, Holm NR, Christiansen EH, Bøtker HE, Bøttcher M, Winther S. Validation and update of the minimal risk tool in patients suspected of chronic coronary syndrome. Int J Cardiovasc Imaging. 2021;37(2):699–706.
Rasmussen LD, Fordyce CB, Nissen L, Hill CL Jr, Alhanti B, Hoffmann U, Udelson J, Bøttcher M, Douglas PS, Winther S. The PROMISE minimal risk score improves risk classification of symptomatic patients with suspected CAD. J Am Coll Cardiol Img. 2022;15(8):1442–54.
Fordyce CB, Douglas PS, Roberts RS, Hoffmann U, Al-Khalidi HR, Patel MR, Granger CB, Kostis J, Mark DB, Lee KL, Udelson JE. Prospective Multicenter Imaging Study for evaluation of chest Pain I. Identification of patients with stable chest Pain Deriving Minimal Value from Noninvasive Testing: the PROMISE minimal-risk Tool, a secondary analysis of a Randomized Clinical Trial. JAMA Cardiol. 2017;2(4):400–8.
Mahmoudi M, Nicholas Z, Nuttall J, Bresser M, Maishman T, Berry C, Hlatky MA, Douglas P, Rajani R, Fox K, Curzen N. Fractional Flow Reserve Derived from Computed Tomography Coronary Angiography in the Assessment and Management of stable chest Pain: Rationale and Design of the FORECAST trial. Cardiovasc Revasc Med. 2020;21(7):890–6.
Group DT. CT or invasive coronary angiography in stable chest Pain. New England Journal of Medicine; 2022.
Greenwood JP, Ripley DP, Berry C, McCann GP, Plein S, Bucciarelli-Ducci C, Dall’Armellina E, Prasad A, Bijsterveld P, Foley JR, Mangion K, Sculpher M, Walker S, Everett CC, Cairns DA, Sharples LD, Brown JM. Effect of Care guided by Cardiovascular magnetic resonance, myocardial perfusion Scintigraphy, or NICE guidelines on subsequent unnecessary angiography rates: the CE-MARC 2 Randomized Clinical Trial. JAMA. 2016;316(10):1051–60.
Newby DE, Williams MC, Dweck MR. Forget Ischemia: it’s all about the Plaque. In: Am Heart Assoc; 2021, 1039–41.
Shaw LJ, Blankstein R, Bax JJ, Ferencik M, Bittencourt MS, Min JK, Berman DS, Leipsic J, Villines TC, Dey D, Al’Aref S, Williams MC, Lin F, Baskaran L, Litt H, Litmanovich D, Cury R, Gianni U, van den Hoogen I, Budoff M, Chang HJ, H EH, Feuchtner G, Ahmadi A, Ghoshajra BB, Newby D, Chandrashekhar YS, Narula J. Society of Cardiovascular Computed Tomography / North American Society of Cardiovascular Imaging - Expert Consensus Document on coronary CT imaging of atherosclerotic plaque. J Cardiovasc Comput Tomogr. 2021;15(2):93–109.
Bittencourt MS, Hulten EA, Murthy VL, Cheezum M, Rochitte CE, Di Carli MF, Blankstein R. Clinical outcomes after evaluation of stable chest Pain by Coronary computed Tomographic Angiography Versus Usual Care: a Meta-analysis. Circ Cardiovasc Imaging. 2016;9(4):e004419.
Adamson PD, Williams MC, Dweck MR, Mills NL, Boon NA, Daghem M, Bing R, Moss AJ, Mangion K, Flather M, Forbes J, Hunter A, Norrie J, Shah ASV, Timmis AD, van Beek EJR, Ahmadi AA, Leipsic J, Narula J, Newby DE, Roditi G, McAllister DA, Berry C. Guiding therapy by coronary CT angiography improves outcomes in patients with stable chest Pain. J Am Coll Cardiol. 2019;74(16):2058–70.
Acknowledgements
None.
Funding
The trial was funded by National Natural Science Foundation of China (62206197 and 62106160), Applied and Basic Research by Multi-input Foundation of Tianjin (21JCYBJC00820), Tianjin Health Research Project (TJWJ2022QN067), Youth Innovative Talents Training Program of Tianjin First Central Hospital Young Talents, Tianjin Key Laboratory of Cardiovascular Emergency and Critical Care certified by Tianjin Municipal Science and Technology Bureau, Tianjin Medical Discipline Construction Project and Tianjin Key Research Program of Traditional Chinese Medicine (2023006) and Tianjin Health Industry High-level Talent Selection and Training Project (TJSQNYXXR-D2-134). All sponsors have no role in the protocol development, data collection, analysis and interpretation and have no role in writing the manuscript.
Author information
Authors and Affiliations
Contributions
JZho are coordinating investigator and designed the study, drafted the manuscript and gained ethical approval. TX, YT, JP and TC drafted the manuscript and gained ethical approval. CLi, HW, CLiu, JZhao, CX, MW, CW, YuL, JZhang, YaL and SC drafted the manuscript. HC participated in the design of the study, provided supervision support and substantive revisions to the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The experimental protocol for this study has undergone independent peer-review from Tianjin Health Research Project (TJWJ2022QN067), was approved by the ethics committees of Tianjin Chest Hospital, and was registered in ClinicalTrials.gov (NCT05640752). All subjects are requested to provide written informed consent before study entry. All experiments are performed in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhou, J., Xin, T., Tan, Y. et al. Comparison of two diagnostic strategies for patients with stable chest pain suggestive of chronic coronary syndrome: rationale and design of the double-blind, pragmatic, randomized and controlled OPERATE Trial. BMC Cardiovasc Disord 23, 416 (2023). https://doi.org/10.1186/s12872-023-03424-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-023-03424-3