Abstract
Background
Evidence about safety and efficacy of transcatheter aortic valve replacement (TAVR) with the Venus A-Valve system (Venus Medtech, Hangzhou, China) remains limited for patients with pure native aortic regurgitation (PNAR).
Objectives
The single-center study sought to report the one-year clinical outcomes of the Venus A-Valve in the treatment of PNAR.
Methods
This study was a retrospective analysis of prospectively collected data. Data was from all consecutive patients who had PNAR and underwent TAVR with the Venus A-Valve system at our center from July 2020 and June 2021. Procedural and clinical outcomes up to one year were analyzed using Valve Academic Research Consortium-2 criteria.
Results
A total of 45 consecutive patients with PNAR underwent transfemoral TAVR with the Venus A-Valve system. The Mean age was 73.5 ± 5.5 years and 26.7% were female. All the TAVR procedures were performed via transfemoral access. Implantations were successful in 44 cases (97.8%). Only one patient was converted to surgical aortic valve replacement. No patient died intraoperatively. No second valve was implanted. In-hospital mortality rate was 2.3%. The one-year all-cause mortality rate was 4.7% without cardiovascular related death. No patient had moderate or severe paravalvular leakage during follow-up. At one year, the mean pressure gradient was 8.8 ± 0.9 mmHg, and left ventricular ejection fraction increased to 61.5 ± 3.6%.
Conclusions
This single-center study demonstrated the safety and efficacy of transfemoral TAVR with the Venus A-Valve in the treatment of patients with PNAR.
Similar content being viewed by others
Introduction
Aortic regurgitation (AR) affects about 13% of patients suffering from native valvular heart diseases [1, 2]. Pure native aortic regurgitation (PNAR) is usually characterized by leaflet degeneration, aortic root dilatation with aortic annulus enlargement, or both [3]. It is well known that surgical aortic valve replacement (SAVR) remains the standard treatment for patients with PNAR [4]. However, some patients have high surgical risks and postoperative mortality, resulting in many patients losing the chances of surgery. When left untreated, these patients face an annual mortality risk of 20% [5].
Transcatheter aortic valve replacement (TAVR) has been established as a treatment alternative for patients with symptomatic severe aortic stenosis (AS) who were at prohibitive or high-risk for SAVR [6]. Off-label uses of TAVR for treatment of PNAR has been reported with several devices. Overall outcomes of these studies were basically promising [7,8,9]. However, outcomes varied significantly between studies using different devices. The presence of large annular anatomy and the absence of valvular calcification have made the transcatheter treatment of PNAR challenging, mainly due to the risk of inadequate anchoring, prosthesis dislodgment, and residual paravalvular leak (PVL) after implantation [10, 11]. In the latest updated American College of Cardiology/American Heart Association guideline, it is stated that TAVR may be considered in experienced centers for selected patients with PNAR who are ineligible for SAVR [12].
The Venus A-Valve system (Venus Medtech, Hangzhou, China) is made of a self-expanding nitinol frame and tri-leaflet bovine pericardial valve [13]. TAVR with Venus A-Valve system to treat PNAR had encouraging results but the experience was still limited [8,9,10,11,12,13,14]. We now report the one-year outcomes of the single-center study with transfemoral implantation of the Venus A-Valve in patients with PNAR.
Methods
Study design
The study was a single-center retrospective analysis of prospectively collected data. Data was from all consecutive patients who had PNAR and underwent TAVR with the Venus A-Valve system at our center from July 2020 and June 2021. All patients finished one-year follow-up.
Every patient experienced clinical examination, laboratory test, transthoracic echocardiography and contrast-enhanced multidetector computed tomography (MDCT) at admission. The degree of AR is graded by measurement of the narrowest width of the proximal regurgitant jet (vena contracta) by Color Doppler [15]. A jet width < 0.3 cm indicates mild AR, while a width > 0.6 cm indicates severe AR.
All patients were evaluated by our heart team before operation and considered to be at prohibitive or high risk for SAVR. The inclusion criteria were: (1) age ≥ 60 with New York Heart Association (NYHA) functional class II-IV; (2) symptomatic PNAR with resting LVEF ≤ 50% or LVESD > 50 mm; (3) logistic EuroScore > 20%. The exclusion criteria were: (1) patients with failed bioprosthetic surgical heart valves; (2) dimension of aortic root > 50 mm; (3) diameter of aortic annulus > 29 mm or <18 mm; (4) AS with peak aortic valve pressure gradient > 20 mmHg or peak aortic velocity > 2.5 m/s; (5) serious comorbidities such as acute aortic dissection, severe coagulation disorder, and multiple organ failure.
Comprehensive clinical and echocardiographic assessments were scheduled before discharge, at 30 days, and at 6 and 12 months. Clinical follow-up was performed by direct or telephone interview according to each center’s practice. Data on baseline characteristics, operative details, postoperative outcomes, and follow-up information were collected prospectively and entered electronically in a dedicated Microsoft Access database.
TAVR procedures
Pre-procedure aorta-iliac-femoral computed tomography was performed to evaluate the size of vessel caliber and feasibility of transfemoral approach. MDCT was used to assess the morphology of the aortic root (Fig. 1).
Aortic root measurements. (A) AA plane cross section; (B) LVOT plane cross section; (C) STJ plane cross section; (D) AAO plane cross section; (E) LCA height; (F) RCA height. AA, aortic annulus. LVOT, left ventricular outflow tract; STJ, sinotubular junction; AAO, ascending aorta; LCA, left coronary artery;RCA, right coronary artery
All procedures were performed under general anesthesia in the hybrid catheterization laboratory. The aortic Sinuses of Valsalva was positioned by angiography (Fig. 2A). The Venus A-Valve was carefully advanced, highly positioned (Fig. 2B), and slowly deployed under rapid ventricular pacing (180–200 beats/min) (Fig. 2C). The intended implantation depth is ranging from 3 to 5 mm below the virtual annular plane (Fig. 2D). After final deployment, rapid ventricular pacing was kept at 120–140 beats/min until delivery system removal. The final contrast injection showed proper prosthesis expansion, no central or paravalvular leak, and coronary arteries with adequate flow (Fig. 2E).
Fluoroscopic demonstration of transcatheter aortic valve replacement (TAVR) intraprocedural steps with the Venus A-Valve system. (A) the aortic Sinuses of Valsalva was positioned by angiography; (B) Transcatheter heart valve initial deployment position; (C) The valve was slowly deployed under rapid ventricular pacing; (D) implant height (3–5 mm depth); (E) A final deployment position
Study endpoints
Procedural and clinical outcomes up to one year were analyzed using Valve Academic Research Consortium-2 (VARC-2) criteria [16]. The primary endpoint was the composite endpoint of device success, defined as: absence of procedural mortality, successful vascular access, delivery and deployment of the device, successful retrieval of the delivery system, correct final position of the device, proper functioning of the prosthetic heart valve (mean gradient < 20 mm Hg, peak velocity < 3 m/s, absence of moderate or severe AR), and no need for valve-in valve implantation or surgical conversion. Secondary endpoints were the other echocardiographic assessment of the valve and cardiac function.
Statistical analysis
Normally distributed continuous variables were presented as mean ± standard deviation (SD), non-normally distributed variables as median and range, categorical variables as raw counts and percentages. Assessment of normality was performed using the Shapiro-Wilk test. Statistical analyses were performed using statistical analysis software (SPSS version 22.0, IBM, New York, NY).
Results
Baseline characteristics
Between July 2020 and June 2021, 133 consecutive patients who underwent transfemoral TAVR with the Venus A-Valve system at our center, of which 23 patients who had combined AS and AR. Among the remaining 110 patients, 65 had only AS; rest 45 had PNAR which was our study population. The mean age was 73.5 ± 5.5 years and 26.7% were female. One patient had congenital bicuspid aortic valve, while the others had tricuspid aortic valves. The mean risk score according to the logistic EuroSCORE (European System for Cardiac Operative Risk Evaluation) was 28.5 ± 7.5%, and 95.6% of the patients were in NYHA functional class ≥ III. The detailed description of patient characteristics was listed in Table 1.
Procedural details and in-hospital outcomes
All the TAVR procedures were performed via transfemoral access. The procedure was successful in 97.8% (44/45). One patient was converted to SAVR because of valve embolism into aortic arch, and recovered well after the aortic valve and hemi-arch replacement. In our study, the 26-mm, 29-mm, and 32-mm valve was implanted in 3 patients (6.8%), 23 patients (52.2%), and 18 patients (40.9%), respectively. The average procedural time were 71.1 ± 16.6 min. No patient died intraoperatively. One patient with a bicuspid aortic valve had moderate PVL, and died of low cardiac output syndrome after refusing further treatment on postoperative day 5. The other patients developed PVL no more than mild degree. Additionally, no balloon post-dilation was performed in any patient. No coronary obstruction, prosthesis malposition, annular rupture or new cerebrovascular accident occurred. No second valve or new permanent pacemaker was implanted. Two patients needed blood transfusion due to preprocedural anemia. According to the Acute Kidney Injury (AKI) Network classification [14], five patients with prior chronic renal dysfunction developed stage 1 AKI, while the renal function gradually recovered before discharge. In-hospital mortality rate was 2.3%. The mean duration of intensive care unit (ICU) stay and postoperative hospital stay was 2.5 ± 0.5 and 4.0 ± 1.5 days, respectively. Procedural details and in-hospital outcomes were listed in Table 2.
One-year outcomes
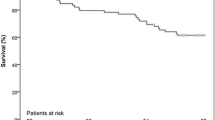
All patients were followed up postoperatively up to one year by telephone or direct interview (100% completed / no lost at follow-up). The one-year all-cause mortality rate was 4.7% without cardiovascular related death. One patient died of severe acute pancreatitis 9 months after discharge, and the other patient died of a car accident 11 months after discharge. All of the remaining 41 patients fortunately survived. Only one patient was in NYHA functional class III, who developed a third-degree atrioventricular block 10 months after discharge and received permanent pacemaker implantation. No patient experienced new myocardial infarction, new cerebrovascular accident, valve thrombosis, or valve-related reintervention. Detailed outcomes at one-year follow-up were listed in Table 3.
Echocardiography assessments
The degree of AR at baseline and the PVL during follow-up were listed in Fig. 3. No patient had moderate or severe PVL at one month, six months and twelve months. The mean pressure gradient was 8.1 ± 1.2 mmHg at one month, 8.8 ± 0.9 mmHg at one year (Fig. 4). The aortic valve peak velocity remained stable at one month, six months and twelve months. The LVEF significantly increased from 41.5 ± 4.6% at baseline to 61.5 ± 3.6% at one year (Fig. 5). Additionally, significant decreases were observed in LVEDD (from 62.1 ± 4.9 mm at baseline to 46.0 ± 3.6 mm at one year) and LVESD (from 51.2 ± 4.8 mm at baseline to 36.9 ± 3.7 mm at one year).
Discussion
This single-center study confirmed the feasibility of transfemoral TAVR with the Venus A-Valve in patients with PNAR. The procedure was successful in 97.8%. No patient died intraoperatively. No second valve was implanted in any case. Echocardiographic measurements showed adequate hemodynamic function without PVL > moderate degree, significant improvement in LVEF and decreased mean pressure gradient during one-year follow-up.
The Venus A-Valve is a self-expanding frame, porcine pericardial valve with supra-annular leaflets [17]. The prosthesis is available in 23-mm, 26-mm, 29-mm and 32-mm sizes and has three radiopaque markers 6 mm from the inflow to aid in precise positioning. The implantation depth is ranging from 3 to 5 mm below the virtual annular plane. At 3 to 5 mm, the outward radial forces of the aortic valve stent frame and the annular coverage of the conforming frame are optimal and should provide excellent results.
The Venus A-Valve system has some unique advantages over other self-expanding valves [18]. First, it can be fully retrieved if there is significant residual PVL or the prosthesis position is not proper; second, it can correct the deployment position in real time; third, it can check the stability of the prosthesis during operation. Despite these advantages, in our early procedures, one patient was converted to SAVR due to valve embolism into the aortic arch. Of note, the size, positioning, and anchoring of the prosthetic valve may be important factors in this valve embolization event. The increased stroke volume caused by significant AR and the low implantation height due to the absence of fluoroscopic calcific landmarks may also be the important factors. To prevent this complication, we updated our protocol in subsequent cases, such as a prolonged observation time without removing the wires to avoid valve inversion in case of embolization and to allow subsequent balloon recapture maneuvers. Since then, valve embolization never happened again.
The diameter of aortic annulus for sizing the prosthesis was calculated by the perimeter and area of the native aortic annulus [19]. It was necessary to have a prosthesis/annulus oversizing of 15–25% to minimize the risk of insufficient prosthesis anchoring and PVL [20]. Oversizing beyond 25% was not recommended due to the risk of annular rupture and conduction system abnormality. In our experience, 10–20% oversizing of the native aortic annulus was recommended for the Venus A system. Anchoring of transcatheter heart valve in PNAR relied on aortic annulus, left ventricular outflow tract (LVOT), sinotubular junction (STJ), and thickening leaflet [21]. Aortic annulus and LVOT in general crucial for stable anchoring of a transcatheter heart valve. STJ may provide an anchoring for the “crown” of the prosthetic valve and avoid it slipping down. Moreover, the thickening leaflets provides the much greater friction between the native valve and prosthetic valve frame.
The choice of prosthesis can help avoid the most common complications of TAVR in the treatment of patients with PNAR, such as significant PVL and valve migration or embolization. In absence of significant aortic valve calcification for anchoring, newer generation prostheses performed better than old generation valves [22]. In addition, the repositionability and aortic stabilization of self-expanding valves (SEV) may be an attractive option, while balloon-expandable valves (BEV)balloon-expandable valves (BEV) designs with prominent outer skirts and the ability to oversize significantly may also make them reasonable alternatives. Of course, dedicated prostheses for PNAR have been developed with native leaflet anchoring design, including the J-Valve and Jena Valve, and the success rate of the procedure has increased to over 90% [23, 24]. In our study transfemoral TAVR with the Venus-A system could achieve a similar procedural success rate.
Based on our experience, there are several technical points that should be noted. First, accurately determining the size of the prosthetic valve is critical. Second, two pigtail catheters should be positioned in the aortic sinuses and transesophageal echocardiography is essential to guide the valve implantation. Third, rapid ventricular pacing is necessary to reduce stroke volume, stabilize the annulus, and limit prosthesis motion. Last but not least, cardiopulmonary bypass should be prepared in some special cases.
Study limitations
This study included a relatively small number of patients. The longest follow-up period was limited to one year. Further research with a larger patient population and longer follow-up duration are scheduled.
Conclusion
This single-center study demonstrated the feasibility of transfemoral TAVR with the Venus A-Valve in patients with PNAR. Procedural and one-year follow-up results were promising. Continued observation is now warranted to confirm persistent valve function during long-term follow-up. We also need to further develop devices specifically for the treatment of PNAR and gain surgical experience to provide better clinical outcomes in the future.
Data Availability
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
References
Iung B, Baron G, Butchart EG, Delahaye F, Gohlke-Barwolf C, Levang OW, et al. A prospective survey of patients with valvular heart disease in Europe: the Euro Heart Survey on Valvular Heart Disease. Eur Heart J. 2003;24(13):1231–43.
Aluru JS, Barsouk A, Saginala K, Rawla P, Barsouk A. Valvular Heart Disease Epidemiology Med Sci (Basel). 2022;10(2):32.
Tagliari AP, Petersen SR, Keller SE. Transcatheter aortic valve implantation for pure native aortic regurgitation: the last Frontier. J Clin Med. 2022;11(17):5181.
Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JR, Fleisher LA, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC Guideline for the management of patients with Valvular Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2017;70(2):252–89.
Stachon P, Kaier K, Heidt T, Bothe W, Zirlik A, Zehender M, et al. Nationwide outcomes of aortic valve replacement for pure aortic regurgitation in Germany 2008–2015. Catheter Cardiovasc Interv. 2020;95(4):810–16.
Avvedimento M, Tang G. Transcatheter aortic valve replacement (TAVR): recent updates. Prog Cardiovasc Dis. 2021;69:73–83.
Schofer J, Nietlispach F, Bijuklic K, Colombo A, Gatto F, De Marco F, et al. Transfemoral Implantation of a fully repositionable and retrievable transcatheter valve for noncalcified pure aortic regurgitation. JACC Cardiovasc Interv. 2015;8(14):1842–49.
Chen S, Zheng F, Li M, Hou S, Zhang W, Zhang L, et al. A study on correlation between preprocedural CT indexes and procedural success rate of transfemoral transcatheter aortic valve replacement with different self-expanding valves (VitaFlow or VenusA-Valve) in patients with pure native aortic regurgitation. Ann Transl Med. 2022;10(11):643.
Frerker C, Schewel J, Schewel D, Wohlmuth P, Schmidt T, Kreidel F, et al. Expansion of the indication of transcatheter aortic valve implantation–feasibility and outcome in “off-label” patients compared with “on-label” patients. J Invasive Cardiol. 2015;27(5):229–36.
Takagi H, Hari Y, Kawai N, Ando T. Meta-analysis and Meta-regression of transcatheter aortic valve implantation for pure native aortic regurgitation. Heart Lung Circ. 2020;29(5):729–41.
Madrazo-Shiordia JA, Martinez-Vazquez E, Zajarias-Kurschansky A, Zajarias A, Damas-De LSF. Transcatheter aortic valve replacement in pure native aortic regurgitation: when off-label indications match the patient’s requirements. Arch Cardiol Mex. 2022;92(4):438–45.
Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JR, Gentile F, et al. 2020 ACC/AHA Guideline for the management of patients with Valvular Heart Disease: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;143(5):e35–71.
Liao YB, Zhao ZG, Wei X, Xu YN, Zuo ZL, Li YJ, et al. Transcatheter aortic valve implantation with the self-expandable venus A-Valve and CoreValve devices: preliminary Experiences in China. Catheter Cardiovasc Interv. 2017;89(S1):528–33.
Wang Y, Yu S, Qian D, Li J, Fang Z, Cheng W, et al. Anatomic predictor of severe prosthesis malposition following transcatheter aortic valve replacement with self- expandable Venus-A Valve among pure aortic regurgitation: a multicenter retrospective study. Front Cardiovasc Med. 2022;9:1002071.
Zoghbi WA, Enriquez-Sarano M, Foster E, Grayburn PA, Kraft CD, Levine RA, et al. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and doppler echocardiography. J Am Soc Echocardiogr. 2003;16(7):777–802.
Kappetein AP, Head SJ, Genereux P, Piazza N, van Mieghem NM, Blackstone EH, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document (VARC-2). Eur J Cardiothorac Surg. 2012;42(5):45–60.
Wang R, Kawashima H, Mylotte D, Rosseel L, Gao C, Aben JP, et al. Quantitative Angiographic Assessment of aortic regurgitation after transcatheter implantation of the Venus A-valve: comparison with other Self-Expanding Valves and Impact of a learning curve in a single chinese Center. Glob Heart. 2021;16(1):54.
Liang Y, Wang W, Wang X, Hei F, Guan Y. A single-center analysis of outcomes, risk factors, and new valves in asian patients treated with early transcatheter aortic valve implantation. Cardiovasc Diagn Ther. 2021;11(4):967–79.
Orzalkiewicz M, Bruno AG, Taglieri N, Ghetti G, Marrozzini C, Galie N, et al. Transcatheter aortic valve replacement for pure aortic regurgitation in a large and noncalcified Annulus. JACC Cardiovasc Interv. 2021;14(19):e271–73.
Urena M, Himbert D, Ohlmann P, Capretti G, Goublaire C, Kindo M, et al. Transcatheter aortic valve replacement to treat pure aortic regurgitation on Noncalcified native valves. J Am Coll Cardiol. 2016;68(15):1705–706.
Yao J, Lu ZN, Modine T, Jilaihawi H, Piazza N, Tang YD, et al. Evaluation of the safety and efficacy of a novel anatomical classification and dUal anchoRing theory to optimize the tavR strategy for pure severe aortic regurgitation (AURORA): a prospective cohort study. BMC Cardiovasc Disord. 2022;22(1):445.
Wernly B, Eder S, Navarese EP, Kretzschmar D, Franz M, Alushi B, et al. Transcatheter aortic valve replacement for pure aortic valve regurgitation: “on-label” versus “off-label” use of TAVR devices. Clin Res Cardiol. 2019;108(8):921–30.
Shi J, Wei L, Chen Y, Wang X, Ye J, Qin C, et al. Transcatheter aortic valve implantation with J-Valve: 2-Year outcomes from a Multicenter Study. Ann Thorac Surg. 2021;111(5):1530–36.
Urena M, Himbert D, Ohlmann P, Capretti G, Goublaire C, Kindo M, et al. Transcatheter aortic valve replacement to treat pure aortic regurgitation on Noncalcified native valves. J Am Coll Cardiol. 2016;68(15):1705–6.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
JL, PH and WC were responsible for the study concept and design. HJZ, YBC, CJY, DQL, SJY, JL, PH and WC were responsible for the acquisition and analysis of data. All authors contributed to the interpretation of the data. HJZ and YBC drafted the manuscript. The corresponding author attests that all listed authors meet authorship criteria. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Ethical approval and consent to participate
This study was approved by the Institutional Review Board of Southwest Hospital of Third Military Medical University (Army Medical University) KY2022156 and conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Institutional Review Board of Southwest Hospital of Third Military Medical University (Army Medical University) waived the need for informed consent.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zheng, HJ., Cheng, YB., Yan, CJ. et al. Transfemoral transcatheter aortic valve replacement for pure native aortic regurgitation: one-year outcomes of a single-center study. BMC Cardiovasc Disord 23, 330 (2023). https://doi.org/10.1186/s12872-023-03329-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-023-03329-1