Abstract
Background
Besides the lungs, coronavirus disease 2019 (COVID-19) can affect the cardiovascular, digestive, urinary, hepatic, and central nervous systems. Other than its short-term effects, COVID-19 may also cause long-term complications. In this study, we assessed long-term COVID-19 cardiovascular symptoms among patients in a cardiovascular clinic.
Method
A retrospective cohort was conducted between October 2020 to May 2021 on patients at an outpatient cardiovascular clinic in Shiraz, Iran. Patients with a history of COVID-19 at least one year before their referral were included. Baseline information was extracted from the clinic’s database. Data were collected regarding symptoms like dyspnea, chest pain, fatigue, and palpitations after a year of COVID-19. We also noted any major adverse cardiac events (MACE).
Results
Most common symptoms after a year of COVID-19 were exertional dyspnea (51.2%), dyspnea at rest (41.6%), fatigue (39%), and chest pain (27.1%). The symptoms were more prevalent in hospitalized patients than in non-hospitalized patients. The prevalence of MACE was about 6.1% during the 12-month follow-up, with this rate being higher in those with a history of hospitalization or comorbid diseases.
Conclusion
The prevalence of cardiovascular symptoms was fairly high in patients at our clinic a year after COVID-19, and the most common symptom was dyspnea. Hospitalized patients had more MACE. (Clinicaltrial.gov number: NCT05715879)(04/02/2023).
Similar content being viewed by others
Introduction
The coronavirus disease 2019 (COVID-19) pandemic has affected all aspects of life, including the health system and education [1,2,3,4,5]. The agent responsible, severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), is a single-stranded RNA β-coronavirus believed to invade human cells via the angiotensin-converting enzyme 2 (ACE2) receptor. Hence, despite primarily affecting the pulmonary system, COVID-19 can damage other organ systems that express ACE2 receptors [6, 7]. Importantly, COVID-19 causes the release of reactive oxygen species that contribute to cell death. Reactive oxygen species release factors such as nuclear factor kappa B (NF-κB) can trigger a cytokine storm. Moreover, COVID-19 also induces the cytokine storm via the release of inflammatory factors by immune and non-immune cells [8, 9]. Through multiple mechanisms, COVID-19 can affect the pulmonary, cardiovascular, digestive, urinary, hepatic, and central nervous systems [10].
Cardiovascular diseases (CVDs) are one of the most critical factors affecting the morbidity and mortality caused by COVID-19. Coronary artery disease (CAD), heart failure (HF), hypertension (HTN), and arrhythmias lead to a higher mortality rate in COVID-19 patients [11]. On the other hand, COVID-19 causes cardiovascular complications that can be fatal, including myocarditis, myocardial infarction, acute heart failure, arrhythmia, and venous thromboembolism [12]. Therefore, it seems there is a mutual relationship between COVID-19 and CVDs.
Recent studies have found that COVID-19 has long-term effects on general health besides its short-term complications. The long-term complications of COVID-19 involve the cardiovascular, pulmonary, central nervous, renal, and gastrointestinal systems [13]. A meta-analysis indicated that 80% of COVID-19 patients remained symptomatic after two weeks past the beginning of the infection. The most common symptoms were dyspnea, fatigue, headache, hair loss, and attention deficit disorders. The study found that COVID-19 had more than fifty long-term consequences on patients’ health [14]. Continuation of COVID-19 signs and symptoms for more than four weeks is known as ‘prolonged COVID-19’, including ‘ongoing symptomatic COVID-19’ (having symptoms for 4 to 12 weeks) and ‘post-COVID-19 syndrome’ (experiencing symptoms for more than 12 weeks) [15].
Prolonged COVID-19 can cause disorders in cardiac rhythm, including supraventricular and ventricular tachycardias, atrial fibrillation, and even complete heart block; similarly, cardiovascular complications were seen in the long-term follow-up of SARS patients [16, 17]. Even among those not experiencing acute coronary syndrome (ACS), myocardial injury is thought to not be uncommon, with possible long-term effects [18]. Davis and colleagues reported that cardiovascular symptoms such as palpitations (68%), chest pain (53%), and fainting (13%) were detected in up to 86% of patients with prolonged COVID-19 after seven months [19]. Cardiovascular risk factors may also be affected since the previous SARS outbreak was linked with hyperlipidemia and glucose intolerance in the long term [20].
In the existing literature, there is little evidence about the long-term effects of COVID-19 on cardiovascular symptoms after a 12-month follow-up. Hence, this study assessed cardiovascular symptoms and complications 12 months after COVID-19 in patients at a cardiovascular clinic.
Methods
This retrospective cohort study was conducted between October 2020 to May 2021. The study population was patients referring to Professor Kojuri Cardiovascular Clinic in Shiraz, Iran (email: kojurij@yahoo.com, webpage: http://kojuriclinic.com). A database of patients' information is kept at the clinic, including data on underlying diseases, signs and symptoms, medications, laboratory tests, electrocardiography, and echocardiography. Expert cardiologists document the data on every visit. Most patients are healthy individuals who visit the clinic for check-ups, though some have a cardiovascular condition.
The inclusion criteria were having a history of COVID-19 confirmed by polymerase chain reaction (PCR) or suggested by High-Resolution Computed Tomography (HRCT) findings at least one year ago. The exclusion criteria were having a history of documented COVID-19 less than a year ago or having a probable history of COVID-19 not confirmed by PCR or HRCT. Patients with incomplete data before COVID-19 or with outdated data were also excluded. The data had a time interval of more than one month before COVID-19, or one month after one year of recovery from COVID-19, are considered outdated data.
We contacted the enrolled patients by telephone to get informed about their symptoms, such as dyspnea at rest, dyspnea on exertion (DOE), orthopnea, paroxysmal nocturnal dyspnea (PND) [21], chest pain (CP) [22], fatigue [23], and palpitations [24]. Patients were asked to rate their dyspnea at rest from 0 to 10, according to the 10-category ratio. A score of zero means no breathing discomfort, and ten indicates the most severe dyspnea. A score between 1 to 4 is considered mild, 5 to 6 moderate, and 7 to 10 severe. We also used functional classes 1 to 4 to assess dyspnea. Functional class 1 means no limitations in daily activities; class 2 means mild exertional dyspnea; class 3 indicates moderate dyspnea with daily activities; and class 4 denotes dyspnea at rest [25]. Chest pain was defined in line with the American Heart Association’s classification system as “non-cardiac,” “possible cardiac,” or “cardiac” [22].
A history of major adverse cardiovascular events (MACE) during the year after COVID-19 and admission due to COVID-19 were also noted. MACE is defined as myocardial infarction (MI), admission due to heart failure (HF), stroke, cardiac death, and revascularization procedures, including a coronary artery bypass graft (CABG) or percutaneous coronary intervention (PCI) [26].
Hypertension was defined as a clinical systolic blood pressure ≥ 140 mm Hg or diastolic blood pressure ≥ 90 mm Hg on repeated assessments [27]. Diabetes mellitus (DM) was diagnosed based on the 2020 American Diabetes Association (ADA) guidelines [28]. Dyslipidemia was defined as abnormalities in triglycerides (TG; > 150 mg/dL), low-density lipoprotein (LDL) cholesterol (> 100 mg/dL), or high-density lipoprotein (HDL) cholesterol (< 40 mg/dL) [29]. Current smokers were defined as those who had smoked ≥ 100 cigarettes and had smoked during the 30 days preceding the study. Former smokers had stopped smoking more than 30 days before the research [30].
We extracted data patients’ information before COVID-19 from the clinic’s database. This included baseline demographic data, COVID-19 vaccination history, HTN, dyslipidemia or hyperlipidemia (HLP), DM, smoking, CVD, and prescribed medications.
Statistical analysis was performed using SPSS for Windows ver. 26 (IBM Corp., Armonk, NY, USA). We described continuous variables by mean ± standard deviation. Categorical variables were described by frequency and percentage. We used repeated measure ANOVA and the paired-sample t-test for normal distribution variables and Wilcoxon’s signed-rank test for repeated categorical variables. Pearson's chi-squared and Kruskal–Wallis tests were applied to categorical data. We controlled the effects of confounding factors by using generalized linear models and repeating analyses in different subgroups. We estimated the minimum sample size by [n = (Z2 × P × (1-P))/e2], considering the 95% confidence interval and 50% prevalence of the symptoms. The minimum sample size was 386 patients. Statistical significance was indicated when P < 0.05.
All patients were informed about the details of this research and provided their informed consent.. For informed consent, we contacted the patients by telephone and described the detail of the study and the anonymity of their data. Patients who declined to participate were excluded. The study protocol was approved by the Ethics Committee of Shiraz University of Medical Sciences under code IR.SUMS.MED.REC.1401.465. All methods were performed in accordance with the Helsinki guidelines and regulations.
Results
Demographic
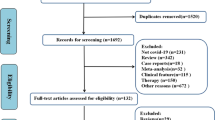
There were 879 eligible patients for this study. We excluded 36 patients because of incomplete data, inaccessibility, and lack of consent to answer the questions by telephone. Finally, the study was conducted on 843 patients; 449 women (53.4%) and 394 (46.7%) men. The mean age of the participants was 57.2 ± 13.6 years.
The number of patients with a previous revascularization history was 229 (≈27.2%), including 183 post-PCI patients (≈21.7%), 25 post-CABG patients (≈3%), and 21 patients with a history of both PCI and CABG (≈2.5%). The prevalence of underlying diseases was 62.2% for HTN, 27.2% for DM, 24.9% for dyslipidemia, and 5.6% for HF. In this study, 48 people (5.7%) were current cigarette smokers, and 47 (5.6%) were previous cigarette smokers.
At the beginning of the study, only 50 patients (5.9%) had received two doses of COVID-19 vaccination, and 38 people (4.5%) had been vaccinated with the first dose. Totally, 154 patients (18.3%) were admitted to a hospital due to COVID-19. The mean duration of the days after COVID-19 was about 383 ± 19 days.
Dyspnea
The prevalence of dyspnea a year after COVID-19 was about 41.6% (351 patients); the frequency of mild, moderate, and severe dyspnea was estimated at 254 (30.1%), 56 (6.6%), and 41 (4.9%), respectively. The prevalence of different functional classes was I (411, 48.8%), II (348, 41.3%), III (47, 5.6%), and IV (37, 4.4%). Patients with a history of admission due to COVID-19 had a higher functional class than outpatients (P = 0.001). Women had more activity limitations (considering their functional class) than men after a year of COVID-19 recovery (P = 0.003) (Table 1).
At the end of the study, 407 patients (48.3%) reported no differences in regard to their dyspnea before and after a year following COVID-19. On the other hand, 157 patients (18.6%) reported worsening of their dyspnea, and interestingly, dyspnea was alleviated in 279 patients (33.1%). There were no differences between dyspnea scores before and after a year of COVID-19 based on the 10-score scale (P = 0.408). There was also no significant difference between the severity of dyspnea before and after a year of COVID-19 (P = 0.494).
Among the admitted ones due to COVID-19, 76 patients (49.4%) reported no differences between their dyspnea before and after a year following COVID-19; however, 55 patients (35.7%) reported that their dyspnea worsened after a year following COVID-19, and 23 patients (14.9%) reported improvement of their symptoms (P < 0.001). On the other hand, among patients not admitted due to COVID-19, 331 participants (48%) reported no differences between their baseline and follow-up dyspnea. However, 256 patients (37.2%) reported alleviation of their dyspnea, and 102 patients (14.8%) reported worsening of their symptoms (P < 0.001) (Table 2).
In hypertensive patients, the prevalence of dyspnea development was significantly higher than in non-hypertensives (P = 0.013). Patients with CAD experienced more worsening dyspnea than patients without CAD (P = 0.042). There was no association between dyspnea differences and any other cardiovascular risk factors, including age (P = 0.130), DM (P = 0.975), smoking (P = 0.438), and HLP (P = 0.446) (Table 2).
Dyspnea on exertion, orthopnea, and paroxysmal nocturnal dyspnea
A year after COVID-19, 432 patients (51.2%) reported suffering from exertional dyspnea. Among patients admitted to the hospital, more cases (91 patients, 21.1%) reported DOE compared with those managed in outpatient settings (63 patients, 15.3%) (P = 0.033). Women reported more DOE than men (P = 0.032). There was also no association between DOE and any of the cardiovascular risk factors, including age (P = 0.197), HTN (P = 0.570), DM (P = 0.938), smoking (P = 0.876), HLP (P = 0.301), and CAD (P = 0.395) (Table 3).
After a year, 85 patients (10.1%) reported orthopnea. Among hospitalized patients, 26 patients (16.9%) had orthopnea, and among outpatient ones, 59 cases (8.6%) suffered from orthopnea after a year following COVID-19 (P = 0.004). The prevalence of orthopnea among women (56, 12.5%) was higher in comparison to men (29, 7.4%) (P = 0.016). There was no association between orthopnea and any of the cardiovascular risk factors, including age (P = 0.88), HTN (P = 0.639), DM (P = 0.609), smoking (P = 0.111), HLP (P = 0.793), and CAD (P = 0.156) (Table 3).
The prevalence of PND was 14.2% (120 patients) after one year following the infection. There was no meaningful association between PND and hospitalization (P = 0.610), gender (P = 0.694), age (P = 0.305), HTN (P = 0.478), DM (P = 0.825), smoking (P = 0.117), HLP (P = 0.649), and CAD (P = 0.321) (Table 3).
Chest pain, fatigue, and palpitations
After one year, the prevalence of CP was 229 (27.1%). Among these, 66 patients (7.8%) reported cardiac CP, 163 patients (19.3%) had non-cardiac CP, and 614 cases (72.8%) reported no CP. The prevalence of cardiac and non-cardiac CP among hospitalized patients was 16.2% (25 patients) and 24.7% (38 patients), respectively. Among non-hospitalized patients, 41 (6%) reported cardiac CP, and 125 patients (18.1%) had non-cardiac CP (P < 0.001). There was no association between CP and sex (P = 0.875), age (P = 0.749), HTN (P = 0.176), DM (P = 0.702), smoking (P = 0.198), HLP (P = 0.865), and CAD (P = 0.233) (Table 4).
The prevalence of fatigue and palpitations was about 329 (39%) and 92 (10.9%) after a year of COVID-19. Patients with hyperlipidemia had more palpitations than others (P = 0.04). There was no association between fatigue or palpitations and hospitalization, sex, age, HTN, DM, smoking, and CAD (Table 4).
Major adverse cardiac events
After a one-year follow-up of the COVID-19 patients, 54 cases (6.4%) showed MACE, including 44 ACS (5.2%), five hospitalizations due to heart failure (0.6%), four cerebrovascular accidents (0.5%), and one cardiac death (0.1%). The prevalence of MACE was higher in hospitalized patients (20, 13%) than in outpatients (34, 4.9%) (P = 0.001). Hypertensive cases had more incidence of MACE after a year of COVID-19 than non-hypertensives (P < 0.001). In patients with hyperlipidemia, 23 people (11%) reported MACE, while the prevalence of MACE in non-hyperlipidemic patients was 31 (4.9%) (P = 0.003). Patients with a history of previous revascularization had more MACE (P = 0.002). The prevalence of MACE in current and former smokers was 7 (14.6%) and 5 (10.6%). Although, 42 non-smokers (5.6%) had MACE after a year of COVID-19 (P = 0.049) (Table 4).
Discussion
Dyspnea, as an important cardiovascular symptom, was reported by our study to be significantly common after COVID-19 (41.6%), even after one year. We found that in the subgroup of patients with more advanced diseases who needed hospitalization, worsening of dyspnea was more frequently reported compared to those not admitted to a hospital. The prevalence of worsening dyspnea was also higher in patients with CAD than in non-CAD patients. The frequency of DOE and orthopnea was higher in women than in men. Because dyspnea has different sources, we also used the NYHA classification to determine the severity of dyspnea, more specifically in cardiovascular settings. Women and hospitalized patients had more severe symptoms after a year of COVID-19 recovery, based on the NYHA. One study showed that about 43% of patients reported suffering from dyspnea two months after COVID-19 [31]. On the other hand, a meta-analysis by Alkodaymi et. Al showed that despite the heterogenicity of studies, the frequency of dyspnea was about 25% at three to six months but reached 31% when following patients for more than 12 months [32].
Mendola M et al. conducted a study on healthcare workers admitted due to COVID-19 to assess long-term symptoms. The most common symptom after recovery from COVID-19 was exertional and resting dyspnea. After ten months, the symptoms decreased significantly; however, mental problems continued [33]. Comelli et al. found that exertional dyspnea was the most common symptom after 12 months following COVID-19. Their study showed that comorbidities and being female were associated with more sequelae after 12 months from COVID-19 hospital admission [34]. Other studies also confirmed that comorbidities and the female gender have a significant role in experiencing more long-term symptoms. The differences may be due to various biologic responses or different patterns of receptors that the virus uses to enter the body in females and males [35,36,37]. Older patients and those with underlying lung disease or longer hospital courses are more prone to have fibrotic changes in their lungs after COVID-19. These changes lead to experiencing more pulmonary symptoms after a long-term follow-up [38].
Chest pain (CP) is the cardinal manifestation of cardiac and respiratory diseases. It also has various non-cardiopulmonary sources, such as chest wall syndrome, psychogenic etiologies, esophageal conditions, and other gastrointestinal disorders [39]. Although not negligible, when compared to dyspnea, CP was less frequently observed (27.7%) among patients in our study. Notably, the relative frequency of cardiac CP was much higher (P < 0.001) in hospitalized patients than in those not needing admission due to COVID-19. A previous study estimated the prevalence of CP at 20% in patients two months after COVID-19 [40]. Davis et al. showed that 53% of 3,762 patients experience CP after a 7-month follow-up from the infection [19]. Huang and colleagues demonstrated that CP continued in 7% of patients after a year following COVID-19 [41]. Furthermore, along with dyspnea and fatigue, CP is another component of the so-called “long COVID-19”, which may occur in 4.7–80% of cases, according to a systematic review of 25 observational studies [42]. In another review of 69 studies about post-COVID-19 complications, cardiac symptoms such as CP and palpitations were reported as common post-cure complications [43].
Long-term cardiovascular symptoms such as CP result from increased myocardial demand and inflammatory reactions, as seen in severe cases [44, 45]. Dani and colleagues figured that long-term symptoms, including CP, result from instability in the autonomic nervous system due to destruction by the virus or immune response [46]. In one study, approximately 78% of patients suffering from long-term symptoms had an abnormal cardiac MRI [47]. Hence, cardiovascular symptoms in prolonged COVID-19 should not be underestimated.
We figured that fatigue is among the most common symptoms that persist after 12 months of COVID-19. A cross-sectional study illustrated that about 93% of patients had fatigue three months after COVID-19. They also found that fatigue had no association with the severity of the disease [48]. Ansey et al. showed that approximately 60% of patients suffered from fatigue during a 12-month follow-up [49]. Studies revealed no associations between the severity of COVID-19, inflammatory markers, and fatigue. Although, women and patients with mental diseases more frequently experience fatigue [50]. Finally, it seems that different mechanisms and factors have roles in the presence of fatigue in the long-term [51].
Shechter et al. showed that the prevalence of MACE was zero after a 12-month follow-up [52]. The incidence of MACE in patients following COVID-19 may be predictable by biomarkers such as troponin [53]. Considerably, we found that about 6% of our patients developed MACE after COVID-19 in a one-year follow-up. Although cardiovascular complications of COVID-19 are well described [54], data about late cardiac events are lacking. Presumed pathogenesis consists of direct and indirect injury (immune and thromboembolism-mediated) [55]. We showed that patients with comorbid diseases, including HTN, smoking, HLP, and CAD, had a greater risk of developing MACE. Moreover, hospitalized patients were more prone to developing MACE than those not hospitalized. As a result, it’s necessary to follow COVID-19 patients with a history of hospital admission and comorbidities more precisely for evaluating cardiovascular complications.
Our study demonstrated that cardiovascular symptoms, including dyspnea, orthopnea, DOE, PND, and CP, are not uncommon among patients even after a long period following COVID-19. As noted previously, COVID-19 has considerable effects on the cardiovascular system besides the well-known respiratory problems. As a presumed mechanism, TGF-β1, angiotensin II, and other cytokines, which are elevated because of the severe inflammation evoked by COVID-19, are thought to affect cardiac myofibroblast differentiation, leading to fibrosis [56]. Moreover, vascular endothelium may be a target for the virus itself. Small and large vessels are damaged through several mechanisms leading to various presentations, including ACS, stroke, venous thromboembolism, and even limb ischemia. Altogether, regular follow-up of patients with COVID-19, considering the long-term consequences, is of great importance. Rehabilitation could help patients with prolonged COVID-19 relieve their symptoms, including fatigue and dyspnea, and improve their quality of life [57].
Study limitations
The limitations of this study included its reliance on retrospective data collected via telephone interviews, which may lead to recall bias. However, the effects of such bias were minimized by the large sample size and the use of the clinic database. Another limitation was the fact that patients were selected from those regularly attending the clinic (mostly for check-ups), and there was no control group for the COVID-19 patients. Hence, the findings must be interpreted with caution. Further studies should assess long-term COVID-19 cardiovascular complications in different populations, considering the documented effects of this viral disease on cardiac functions.
Conclusions
We showed that the prevalence of cardiovascular symptoms in COVID-19 patients was significantly high, even after a long-term follow-up. The most common symptoms include exertional dyspnea, dyspnea on rest, and fatigue. However, it should be noted that these symptoms may have other origins. The symptoms were more prevalent in hospitalized patients than in non-hospitalized. The prevalence of cardiovascular symptoms was higher in patients with comorbid diseases. Also, cardiovascular symptoms and major adverse cardiac events were more prevalent in hospitalized patients than in non-hospitalized ones.
Availability of data and materials
All data are available in professor Kojuri cardiology clinic, registry and available with reasonable request.
Abbreviations
- ACE2:
-
Angiotensin-converting enzyme 2
- ACS:
-
Acute coronary syndrome
- SARS-CoV-2:
-
Severe Acute Respiratory Syndrome Corona Virus 2
- CABG:
-
Coronary artery bypass graft
- CAD:
-
Coronary artery disease
- CP:
-
Chest pain
- CVD:
-
Cardiovascular diseases
- DM:
-
Diabetes mellitus
- DOE:
-
Dyspnea on exertion
- HF:
-
Heart failure
- HLP:
-
Hyperlipidemia
- HRCT:
-
High-Resolution Computed Tomography
- HTN:
-
Hypertension
- MACE:
-
Major adverse cardiovascular events
- MI:
-
Myocardial infarction
- PCR:
-
Polymerase chain reaction
- PCI:
-
Percutaneous coronary intervention
- PND:
-
Paroxysmal nocturnal dyspnea
- WHO:
-
World Health Organization
References
Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20:533–4.
Huang X, Wei F, Hu L, Wen L, Chen K. Epidemiology and clinical characteristics of COVID-19. Arch Iran Med. 2020;23:268–71.
Wang W, Tang J, Wei F. Updated understanding of the outbreak of 2019 novel coronavirus (2019-nCoV) in Wuhan, China. J Med Virol. 2020;92:441–7.
Cucinotta D, Vanelli M. WHO declares COVID-19 a pandemic. Acta bio-medica: Atenei Parmensis. 2020;91:157–60.
Hayat AA, Keshavarzi MH, Zare S, et al. Challenges and opportunities from the COVID-19 pandemic in medical education: a qualitative study. BMC Med Educ. 2021;21:1–13.
Tsang HF, Chan LWC, Cho WCS, et al. An update on COVID-19 pandemic: the epidemiology, pathogenesis, prevention and treatment strategies. Expert Rev anti-infective therapy. 2021;19:877–88.
Ni W, Yang X, Yang D, et al. Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Crit Care (London England). 2020;24:422.
Bhaskar S, Sinha A, Banach M, et al. Cytokine storm in COVID-19-Immunopathological mechanisms, clinical considerations, and therapeutic approaches: the REPROGRAM Consortium position paper. Front Immunol. 2020;11:1648.
Hojyo S, Uchida M, Tanaka K, et al. How COVID-19 induces cytokine storm with high mortality. Inflamm regeneration. 2020;40:37.
Kordzadeh-Kermani E, Khalili H, Karimzadeh I. Pathogenesis, clinical manifestations and complications of coronavirus disease 2019 (COVID-19). Future Microbiol. 2020;15:1287–305.
Hessami A, Shamshirian A, Heydari K, et al. Cardiovascular diseases burden in COVID-19: systematic review and meta-analysis. Am J Emerg Med. 2021;46:382–91.
Farshidfar F, Koleini N, Ardehali H. Cardiovascular complications of COVID-19. JCI insight. 2021;6(13).
Higgins V, Sohaei D, Diamandis EP, Prassas I. COVID-19: from an acute to chronic disease? Potential long-term health consequences. Crit Rev Clin Lab Sci. 2021;58:297–310.
Lopez-Leon S, Wegman-Ostrosky T, Perelman C, et al. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep. 2021;11:16144.
Mahase E. Long covid could be four different syndromes, review suggests. BMJ (Clinical research ed). 2020;371:m3981.
Desai AD, Boursiquot BC, Melki L, Wan EY. Management of arrhythmias associated with COVID-19. Curr Cardiol Rep. 2021;23:1–9.
Yu C, Wong RS, Wu E, et al. Cardiovascular complications of severe acute respiratory syndrome. Postgrad Med J. 2006;82:140–4.
Knight DS, Kotecha T, Razvi Y, et al. COVID-19: myocardial injury in survivors. Circulation. 2020;142:1120–2.
Davis HE, Assaf GS, McCorkell L, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;38:101019.
Wu Q, Zhou L, Sun X, et al. Altered lipid metabolism in recovered SARS patients twelve years after infection. Sci Rep. 2017;7:1–12.
Mann D, Zipes D, Libby P, Bonow R. Braunwalds heart disease: a textbook of cardiovascular medicine, single volume. Philadelphia: Elsevier/Saunders; 2014.
Members WC, Gulati M, Levy PD, et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR guideline for the evaluation and diagnosis of chest pain: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2021;78:e187–e285.
Phillips RO. A review of definitions of fatigue–and a step towards a whole definition. Transp Res part F: traffic Psychol Behav. 2015;29:48–56.
Zimetbaum P, Josephson ME. Evaluation of patients with palpitations. N Engl J Med. 1998;338:1369–73.
Crisafulli E, Clini EM. Measures of dyspnea in pulmonary rehabilitation. Multidisciplinary respiratory medicine. 2010;5:1–9.
Choi BG, Rha SW, Yoon SG, Choi CU, Lee MW, Kim SW. Association of major adverse cardiac events up to 5 years in patients with chest pain without significant coronary artery disease in the korean population. J Am Heart Association. 2019;8:e010541.
Unger T, Borghi C, Charchar F, et al. 2020 International Society of Hypertension global hypertension practice guidelines. Hypertension. 2020;75:1334–57.
Association AD. 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes—2020. Diabetes Care. 2020;43:14–S31.
Fletcher B, Berra K, Ades P, et al. Managing abnormal blood lipids: a collaborative approach: cosponsored by the councils on cardiovascular nursing; arteriosclerosis, thrombosis, and vascular biology; basic cardiovascular sciences; cardiovascular disease in the young; clinical cardiology; epidemiology and prevention; nutrition, physical activity, and metabolism; and stroke; and the preventive cardiovascular nurses association. Circulation. 2005;112:3184–209.
Ryan H, Trosclair A, Gfroerer J. Adult current smoking: differences in definitions and prevalence estimates—NHIS and NSDUH, 2008. J Environ Public Health. 2012;2012:918368. https://doi.org/10.1155/2012/918368. Epub 2012 May 9.
Carfì A, Bernabei R, Landi F. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324:603–5.
Alkodaymi MS, Omrani OA, Fawzy NA et al. Prevalence of post-acute COVID-19 syndrome symptoms at different follow-up periods: a systematic review and meta-analysis. Clin Microbiol Infect. 2022;28(5):657-66. https://doi.org/10.1016/j.cmi.2022.01.014. Epub 2022 Feb 3.
Mendola M, Leoni M, Cozzi Y, et al. Long-term COVID symptoms, work ability and fitness to work in healthcare workers hospitalized for Sars-CoV-2 infection. La Medicina del Lavoro. 2022;113:e2022040.
Comelli A, Viero G, Bettini G, et al. Patient-reported symptoms and sequelae 12 months after COVID-19 in hospitalized adults: a Multicenter Long-Term Follow-Up study. Front Med. 2022;9:834354.
Seeßle J, Waterboer T, Hippchen T, et al. Persistent symptoms in adult patients 1 year after coronavirus disease 2019 (COVID-19): a prospective cohort study. Clin Infect Dis. 2022;74:1191–8.
Boscolo-Rizzo P, Guida F, Polesel J, et al. Sequelae in adults at 12 months after mild-to-moderate coronavirus disease 2019 (COVID-19). 2021.
Bai F, Tomasoni D, Falcinella C, et al. Female gender is associated with long COVID syndrome: a prospective cohort study. Clin Microbiol Infect. 2022;28:611. e619-611. e616.
Han X, Fan Y, Alwalid O, et al. Six-month follow-up chest CT findings after severe COVID-19 pneumonia. Radiol. 2021;299(1):E177-86. https://doi.org/10.1148/radiol.2021203153. Epub 2021 Jan 26.
Haasenritter J, Biroga T, Keunecke C, et al. Causes of chest pain in primary care–a systematic review and meta-analysis. Croatian Med J. 2015;56:422–30.
Carfì A, Bernabei R, Landi F. Group ftGAC-P-ACS. Persistent symptoms in patients after Acute COVID-19. JAMA. 2020;324:603–5.
Huang L, Yao Q, Gu X, et al. 1-year outcomes in hospital survivors with COVID-19: a longitudinal cohort study. The lancet. 2021;398:747–58.
Cabrera Martimbianco AL, Pacheco RL, Bagattini ÂM, Riera R. Frequency, signs and symptoms, and criteria adopted for long COVID-19: a systematic review. Int J Clin Pract. 2021;75:e14357.
Elhiny R, Al-Jumaili AA, Yawuz MJ. What might COVID-19 patients experience after recovery? A comprehensive review. Int J Pharm Pract. 2022;30:404–13.
Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27:601–15.
Madjid M, Safavi-Naeini P, Solomon SD, Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020;5:831–40.
Dani M, Dirksen A, Taraborrelli P, et al. Autonomic dysfunction in ‘long COVID’: rationale, physiology and management strategies. Clin Med. 2021;21:e63.
Puntmann VO, Carerj ML, Wieters I, et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;5:1265–73.
Goërtz YM, Van Herck M, Delbressine JM et al. Persistent symptoms 3 months after a SARS-CoV-2 infection: the post-COVID-19 syndrome? ERJ Open Res. 2020;6(4):00542-2020. https://doi.org/10.1183/23120541.00542-2020.
Tansey CM, Louie M, Loeb M, et al. One-year outcomes and health care utilization in survivors of severe acute respiratory syndrome. Arch Intern Med. 2007;167:1312–20.
Townsend L, Dyer AH, Jones K, et al. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PLoS ONE. 2020;15:e0240784.
Crook H, Raza S, Nowell J, Young M, Edison P. Long covid-mechanisms, risk factors, and management. BMJ (Clinical research ed). 2021;374:n1648.
Shechter A, Yelin D, Margalit I, et al. Assessment of adult patients with long COVID manifestations suspected as Cardiovascular: a single-center experience. J Clin Med. 2022;75(10):1688-97. https://doi.org/10.1093/cid/ciac283.
Fiedler L, Motloch LJ, Jirak P, et al. Investigation of hs-TnI and sST-2 as potential predictors of Long-Term Cardiovascular Risk in patients with survived hospitalization for COVID-19 pneumonia. Biomedicines. 2022;10(11):2889. https://doi.org/10.3390/biomedicines10112889.
Long B, Brady WJ, Koyfman A, Gottlieb M. Cardiovascular complications in COVID-19. Am J Emerg Med. 2020;38:1504–7.
Siripanthong B, Asatryan B, Hanff TC, et al. The pathogenesis and long-term consequences of COVID-19 cardiac injury. Basic to Translational Science. 2022;7:294–308.
Katwa LC, Mendoza C, Clements M. CVD and COVID-19: emerging roles of cardiac fibroblasts and myofibroblasts. Cells. 2022;11:1316.
Nopp S, Moik F, Klok FA, et al. Outpatient Pulmonary Rehabilitation in patients with long COVID improves Exercise Capacity, Functional Status, Dyspnea, fatigue, and Quality of Life. Respir Int Rev Thorac Dis. 2022;101:593–601.
Acknowledgements
We thank Shiraz University of Medical Sciences for providing the ethical review and approval of our protocol.
Funding
None.
Author information
Authors and Affiliations
Contributions
Study conduction: RGV, MHR, JK Gathering data: RGV, RH, MHR, AT,SKM, BZ, FA, AA,AKH, NH, MMRandomization: RGV, RH,MHR, AT, SKM, BZ, FA, AA, AKH, MM, NHStatistical analysis: RGV, JKManuscript drafting: RGV, SAH, JKRevision: RGV,SAH, JK. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocol was approved by the Ethics Committee of Shiraz University of Medical Sciences under code IR.SUMS.MED.REC.1401.465. All methods were performed in accordance with the Helsinki guidelines and regulations. All participants were informed and filled the written informed consent. Informed consent from legally authorized representatives/guardians for study participation under age of 18 were taken.
Consent for publication
All authors are agree to publish the manuscript in BMC cardiovascular journal.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Golchin Vafa, R., Heydarzadeh, R., Rahmani, M. et al. The long-term effects of the Covid-19 infection on cardiac symptoms. BMC Cardiovasc Disord 23, 286 (2023). https://doi.org/10.1186/s12872-023-03322-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-023-03322-8