Abstract
Background
Exploring reliable prediction scoring systems is valuable for the poor prognosis of patients after coronary artery bypass grafting (CABG). Herein, we explored and compared the predictive performance of vasoactive-inotropic score (VIS), vasoactive-ventilation-renal (VVR) score, and modified VVR (M-VVR) score in the poor prognosis of patients undergoing CABG.
Methods
A retrospective cohort study was performed in Affiliated Hospital of Jining Medical University, and data of 537 patients were collected from January 2019 to May 2021. The independent variables were VIS, VVR, and M-VVR. Study endpoint of interest was the poor prognosis. Association between VIS, VVR, M-VVR and poor prognosis was assessed using logistic regression analysis, and odds ratios (OR) and 95% confidence intervals (CIs) were reported. The performance of VIS, VVR, and M-VVR to predict the poor prognosis was assessed by calculating the area under the curve (AUC), and differences of the AUC of the three scoring systems were compared using DeLong test.
Results
After adjusting gender, BMI, hypertension, diabetes, surgery methods, and left ventricular ejection fraction (LVEF), VIS (OR: 1.09, 95%CI: 1.05–1.13) and M-VVR (OR: 1.09, 95%CI: 1.06–1.12) were associated with the increased odds of poor prognosis. The AUC of M-VVR, VVR, and VIS was 0.720 (95%CI: 0.668–0.771), 0.621 (95%CI: 0.566–0.677), and 0.685 (95%CI: 0.631–0.739), respectively. DeLong test displayed that the performance of M-VVR was better than VVR (P = 0.004) and VIS (P = 0.003).
Conclusions
Our study found the good prediction performance of M-VVR for the poor prognosis of patients undergoing CABG, indicating that M-VVR may be a useful prediction index in the clinic.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Background
Coronary artery bypass grafting (CABG) is a common operation for revascularization through grafting bypass vessels, and has been the standard for the treatment of coronary artery disease (CAD) [1, 2]. It is estimated that 370,000 CABG are performed in the United States annually [3]. A continuously increasing trend of this operation is observed in China [4]. Despite a decrease in the operative complications, operative mortality, and in-hospital mortality due to improvements in surgery technology and nursing quality, in-hospital prognosis for patients undergoing CABG remains a common concern [5,6,7].
Some prediction scoring systems, such as Acute Physiology and Chronic Health Evaluation (APACHE), European System for Cardiac Operative Risk Evaluation (EuroSCORE), and vasoactive-inotropic score (VIS), have been developed to predict the poor prognosis of patients undergoing CABG [8, 9]. APACHE performs well in the prediction of renal complications, while the performance is not good in the prediction of cardiovascular and respiratory complications [8]. Evidence has showed that vasoactive-inotropic score (VIS) is a more important scoring system than EuroSCORE in predicting the prognosis of patients undergoing CABG [9]. VIS is a numerical scale demonstrating the amount of vasoactive and inotropic support, and has been reported as an effective predictor for mortality and morbidity of infants and adults in the cardiac surgery [10, 11]. Baysal et al. found that VIS can independently predict early postoperative morbidity and mortality in patients undergoing CABG [9]. Due to the heterogeneity of patients’ anatomy and pathophysiology, vasoactive-ventilation-renal (VVR) score is reported [12]. VVR score is a novel disease severity index based on VIS, and calculated as ventilation index (VI) + VIS + Δ creatinine (ΔCr) × 10 [13], which incorporates the markers of cardiovascular, pulmonary and renal function and has been reported to outperform VIS in predicting hospital-stay following congenital heart surgery [12,13,14]. In CABG, the predictive value of VVR has not been reported.
Dr Colombo points out that assessing renal dysfunction in VVR score by calculating ΔCr is inaccurate, and creatinine clearance (Ccr) is a more reliable marker [15]. Evidence has shown Ccr is an effective marker to predict renal dysfunction [16, 17]. Therefore, our study modifies the calculation of VVR, and uses Ccr to replace ΔCr in the former formula, which called as modified VVR (M-VVR).
In this study, we aimed to explore the predictive value of M-VVR, VVR, and VIS in the in-hospital prognosis after CABG, and to compare the prediction performance of these three scoring systems.
Methods
Study design and study population
This retrospective cohort study was performed in the Affiliated Hospital of Jining Medical University from January 2019 to May 2021, and has been approved by the Ethics Committee of Affiliated Hospital of Jining Medical University (2021C165). All participants have provided the informed consent.
Participants who aged 18–80 years (male or female), met the indications for CABG [18] and underwent CABG; surviving over 48 h in the intensive care unit (ICU), and with complete medical records were included in our study. Those who met one of the following criteria were excluded: (1) undergoing other concomitant cardiac surgeries; (2) with acute myocardial infarction within 30 days before the surgery; (3) previously taking hormonotherapy and immunosuppressant therapy; (4) with malignant tumors or immune diseases; (5) with infectious diseases before the surgery; (6) with severe hepatic and renal insufficiency before the surgery; (7) with valvular heart disease and other heart diseases; (8) not the first time to undergo coronary artery intervention.
Independent variables
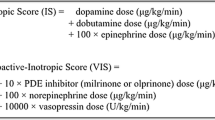
VIS, VVR and M-VVR were independent variables and data at postoperative 24 h were collected. The calculation formulas of VIS, VVR and M-VVR were shown in Supplementary file 1.
Study endpoint
The study endpoint was the poor prognosis, including at least one of the following: death, cardiopulmonary resuscitation, mechanical circulatory support, low cardiac output syndrome (LCOS; cardiac index < 2.5 L/min/m2), stroke, acute kidney injury (with need for renal replacement therapy), and nervous system damage.
Potential confounders
The following variables were potential confounders in this study: (1) physical characteristics [gender, age, body mass index (BMI)]; (2) living habits (smoking and drinking); (3) history of diseases (hypertension, diabetes, hyperlipidemia, cerebrovascular disease, chronic nephrosis, chronic obstructive pulmonary disease); (4) comorbidity (no/yes); (5) surgery methods (off-pump and on-pump); and (6) left ventricular ejection fraction (LVEF). LVEF was categorized into poor (< 30%), moderate (30-50%), normal (> 50%) groups according to previous study [19].
Sample size estimation
VVR ≥ 35 were taken as the predictor for mortality to calculate the sample size (OR: 4.95) [12]. α was equal to 0.05, and β was equal to 0.1. Exposure rate of control group was 0.02. The estimated sample size was 214 in each group, calculated by the power analysis software PASS V.15 (NCSS, Kaysville, Utah, USA). Considering a dropout rate of 20%, at least 535 participants were needed.
Statistical analysis
Continuous variables in normal distribution were expressed as mean ± standard deviation (Mean ± SD), and differences between two groups were compared using t test. Continuous variables in skew distribution were expressed as median and interquartile range [M (Q1, Q3)], and differences between two groups were compared using Mann-whitney U test. Counting data were expressed as number (n) and percentage (%), and differences between two groups were compared using chi-square test.
Univariate and multivariable logistic regression analysis was used to explore the association between VIS, VVR, M-VVR and poor prognosis, and odds ratios (OR) and 95% confidence intervals (CIs) were reported. In the multivariable logistic regression model, gender, BMI, hypertension, diabetes, surgery methods, and LVEF were adjusted. Receiver operating characteristics (ROC) curves of VIS, VVR, and M-VVR predicting the poor prognosis were generated using R version 4.0.3 (The R Foundation for Statistical Computing, Vienna, Austria). Prediction performance of VIS, VVR, M-VVR was assessed by calculating the area under the curve (AUC), with 95%CI. DeLong test was used to compare differences of the AUC of VIS, VVR, and M-VVR. The calibration of the scoring system was assessed using Hosmer-lemeshow goodness of fit test.
To further verify the predictive performance of M-VVR, we compared the prediction ability of M-VVR with that of Sino System for Coronary Operative Risk Evaluation (SinoSCORE), which was divided into three groups according to the risk scores (≤ 1, 2–5, ≥ 6) [20]. P value less than 0.05 was considered statistically significant. Statistical analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC, USA) and R (version 4.0.3).
Results
Patient characteristics
A total of 537 patients undergoing CABG were finally included in our study according to the inclusion and exclusion criteria, and the poor prognosis occurred in 133 of the patients. The number and percentage of each complication were shown in Supplementary table S1. There were 66.48% of male (n = 357) and 33.52% of female (n = 180). The mean age was 65.39 ± 7.66 years and mean BMI was 24.97 ± 3.22 kg/m2. Gender, BMI, hypertension, diabetes, surgery methods, LVEF, VIS, VVR, and M-VVR were statistically different between the two groups (all P < 0.05) (Table 1).
Association of VIS, VVR, and M-VVR with the poor prognosis
In the unadjusted model, VIS (OR: 1.10, 95%CI: 1.07–1.13), VVR (OR: 1.01, 95%CI: 1.00-1.02), and M-VVR (OR: 1.10, 95%CI: 1.07–1.12) were associated with the increased odds of poor prognosis in patients undergoing CABG. After adjusting gender, BMI, hypertension, diabetes, surgery methods, and LVEF, VIS (OR: 1.09, 95%CI: 1.05–1.13) and M-VVR (OR: 1.09, 95%CI: 1.06–1.12) were found to be associated with the poor prognosis. There was no statistical significance between VVR and the poor prognosis, with OR of 1.00 (95%CI: 0.99–1.01) and P value of 0.270. The results were shown in Table 2.
Comparing the performance of VIS, VVR, and M-VVR
Figure 1 shows that ROC curves of VIS, VVR, and M-VVR predicting the poor prognosis of patients undergoing CABG. The AUC of M-VVR, VVR, and VIS was 0.720 (95%CI: 0.668–0.771), 0.621 (95%CI: 0.566–0.677), and 0.685 (95%CI: 0.631–0.739), respectively. Results of DeLong test displayed that the prediction performance of M-VVR was superior to VVR (P = 0.004) and VIS (P = 0.003) for the poor prognosis (Table 3). The calibration plot showed that M-VVR had a good calibration for the prediction of poor prognosis in patients undergoing CABG (Fig. 2).
Comparing the performance of M-VVR with SinoSCORE
Supplementary table S2 shows that there was no association between SinoSCORE and the poor prognosis in patients undergoing CABG (OR: 1.20, 95%CI: 0.59–2.45; OR: 0.93, 95%CI: 0.49–1.78). Supplementary Fig. 1 shows the ROC curve of SinoSCORE predicting the poor prognosis of patients undergoing CABG. The AUC of SinoSCORE was 0.561 (95%CI: 0.508–0.613). DeLong test displayed that the prediction performance of M-VVR was superior to that of SinoSCORE (P < 0.001) (Supplementary table S3).
Discussion
With the increasing trend of CABG in China, the prognosis of patients undergoing CABG becomes a common concern [4]. Many patients undergoing CABG suffer from poor prognosis, such as death, acute kidney injury, and heart failure [21, 22]. VIS has been reported as an independent factor for the mortality of patients after CABG [9]. VVR is an index based on VIS; although VVR has been reported in the congenital disease [13], its role remains unclear in the prognosis of patients undergoing CABG. M-VVR is a modified score for VVR, which is also not reported in CABG. In this study, we explored the performance of VIS, VVR, and M-VVR in predicting the poor prognosis of patients undergoing CABG. The results showed that higher VIS and M-VVR were associated with the increased odds of poor prognosis. The performance of M-VVR was superior to VIS and VVR in the prediction of poor prognosis.
Previous studies have confirmed that developing reliable scoring systems can be useful to predict the risk of poor prognosis [23, 24]. Ju et al. reported that age, creatinine, ejection fraction (ACEF) I score, and ACEF II score could be useful tools for prognostication after CABG [25]. In a Spanish cohort study, Leicester score (LS) was proven to be a valid score to identify the risk of acute kidney injury following cardiac surgery (CSA-AKI) [26]. Although these scores showed a good performance, they either ignored the pulmonary dysfunction or only focused on single outcome. M-VVR, an improvement on VVR, is a scoring system compositing VI, VIS, and Ccr, which used to assess cardiovascular, pulmonary, and renal functions [15]. In our study, M-VVR was confirmed to be positively associated with the poor prognosis of patients undergoing CABG. Previous studies have reported the positive association between VIS and poor prognosis in cardiac surgery [9, 27]. Kwon et al. manifested that increased postoperative VIS was independently correlated with the risk of 1-year mortality after CABG in adults [27]. Similarly, in this study, higher VIS was found to be associated with an increased odds of poor prognosis in patients undergoing CABG. Of the poor prognosis, the incidence of LCOS was near to 20% (97/537). Evidence has shown that the low LVEF was associated the high odds of LCOS [28]. In this study, LVEF of 29.8% of the patients was below the normal range.
The prediction performance of M-VVR was found to be superior to VIS and VVR in this study. M-VVR addresses three organ systems dysfunctions that most commonly affected by cardiac bypass surgery: cardiovascular, pulmonary, and renal, while VIS primarily measures the integrity of the cardiovascular system [11, 15]. The increased precision of M-VVR score compared to VIS may be explained by that M-VVR can capture the patients who have preserved hemodynamic integrity but have severe disease burden from postoperative lung or kidney damage. Miletic et al. has reported that adding measures of respiratory and renal dysfunction to the VIS is better to predict outcomes in cardiac surgery [29]. Both VVR and M-VVR were developed to measure cardiovascular, pulmonary, and renal functions, but the difference between them was the index used to assess renal function, which used ΔCr in VVR and Ccr in M-VVR. The reason for the superiority of M-VVR to VVR may be that Ccr is a better marker than ΔCr to estimate renal function [15]. ΔCr shows the change in postoperative serum creatinine from baseline [13]. Ge et al. have clarified that serum creatinine can be affected by age, diet, and change of muscle volume [30]. Compared to serum creatinine, Ccr decreases the impact of weight and age on outcomes, and is a quantitative indicator to measure renal damage due to it can reflect glomerular filtration rate and roughly evaluate the number of effective nephrons [30]. SinoSCORE is a scoring system developed by Chinese researchers and has been generally recognized as being able to predict the adverse prognosis after cardiac surgery [20, 31]. Compared to SinoSCORE, M-VVR showed the superior performance in the prediction of poor prognosis in this study, indicating that M-VVR may be a convincing tool to be used in the clinic for patients undergoing CABG. More studies are needed to further verify our findings.
There are some advantages in our study. First, we modify the VVR score. The serum creatinine needs to be tested only once (after the surgery) in M-VVR, while it needs to be tested twice (before and after the surgery) in VVR. Compared to VVR, M-VVR is a simpler and more convenient tool to be used in the clinic. Second, we exclude patients who is not the first time to undergo coronary artery intervention, which eliminates the impact caused by history of coronary artery intervention on the prognosis. Also, there are some limitations in our study. First, due to the limited sample size in death, mechanical circulatory support, stroke, and acute kidney injury, the prediction value of M-VVR for the single outcome cannot be explored. Second, we mainly explore the short-term (in-hospital) outcomes. Future study should concern on the prediction performance of M-VVR for the long-term outcomes of patients. Third, acute postoperative blood loss is also an important risk factor for the development of complications after heart surgery; however, due to serious data missing, we cannot include hemostasis-relevant variables in our analysis. Future studies should collect indicators of the hemostasis system and violations of this system to further verify our findings.
Conclusion
In conclusion, we found that M-VVR score had a good performance in predicting the poor prognosis of patients undergoing CABG. Our findings indicated that a robust and easily calculated disease severity score may be developed in our study to predict the outcomes of patients undergoing CABG.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CABG:
-
Coronary artery bypass grafting
- CAD:
-
Coronary artery disease
- VIS:
-
Vasoactive-inotropic score
- VVR:
-
Vasoactive-ventilation-renal
- Ccr:
-
Creatinine clearance
- VI:
-
Ventilation index
- LCOS:
-
Low cardiac output syndrome
- BMI:
-
Body mass index
- LVEF:
-
Left ventricular ejection fraction
- SinoSCORE:
-
Sino System for Coronary Operative Risk Evaluation
- Mean ± SD:
-
Mean ± standard deviation
- M (Q1, Q3):
-
Median and interquartile range
- OR:
-
Odds ratios
- CIs:
-
Confidence intervals
- ROC:
-
Receiver operating characteristics
- AUC:
-
Area under the ROC curve
References
Khan MR, Kayani WT, Pelton J, Ansari A, Paniagua D, Khalid U, et al. Coronary artery bypass grafting Versus Percutaneous Coronary intervention in patients with left ventricular systolic dysfunction. Cardiovasc Drugs Ther. 2021;35:575–85.
Sousa-Uva M, Neumann FJ, Ahlsson A, Alfonso F, Banning AP, Benedetto U, et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur J cardio-thoracic surgery: official J Eur Association Cardio-thoracic Surg. 2019;55:4–90.
Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, et al. Heart Disease and Stroke Statistics-2022 update: a Report from the American Heart Association. Circulation. 2022;145:e153–e639.
Ma L, Wu Y, Chen W. China cardiovascular disease report 2018: an updated summary. Chin J Hypertens. 2019;27:3–134.
Elbadawi A, Hamed M, Elgendy IY, Omer MA, Ogunbayo GO, Megaly M, et al. Outcomes of reoperative coronary artery bypass graft surgery in the United States. J Am Heart Association. 2020;9:e016282.
Worrall N, Brevig J, Jin R, Gluckman T, Hunter R, Ducsik M, et al. Reduction in coronary artery bypass grafting surgery mortality and morbidity during a 3-year multicenter quality improvement project. J Thorac Cardiovasc Surg. 2020;159:1779–91.
Santarpino G, Ruggieri VG, Mariscalco G, Bounader K, Beghi C, Fischlein T, et al. Outcome in patients having salvage coronary artery bypass grafting. Am J Cardiol. 2015;116:1193–8.
Franzotti S, Sloboda D, Silva J, Souza E, Reboreda J, Ferretti-Rebustini R, et al. Performance of severity indices to Estimate Postoperative Complications of myocardial revascularization. Arquivos brasileiros de cardiologia. 2020;11:452–9.
Baysal PK, Güzelmeriç F, Kahraman E, Gürcü ME, Erkılınç A, Orki T. Is vasoactive-inotropic score a predictor for mortality and morbidity in patients undergoing coronary artery bypass surgery? Brazilian J Cardiovasc Surg. 2021;36:802–6.
Gaies MG, Gurney JG, Yen AH, Napoli ML, Gajarski RJ, Ohye RG et al. Vasoactive-inotropic score as a predictor of morbidity and mortality in infants after cardiopulmonary bypass. Pediatric critical care medicine: a journal of the society of critical Care Medicine and the World Federation of Pediatric Intensive and critical Care Societies. 2010; 11: 234–8.
Yamazaki Y, Oba K, Matsui Y, Morimoto Y. Vasoactive-inotropic score as a predictor of morbidity and mortality in adults after cardiac surgery with cardiopulmonary bypass. J Anesth. 2018;32:167–73.
Cashen K, Costello JM, Grimaldi LM, Narayana Gowda KM, Moser EAS, Piggott KD et al. Multicenter Validation of the vasoactive-ventilation-renal score as a predictor of prolonged mechanical Ventilation after neonatal cardiac surgery. Pediatric critical care medicine: a journal of the society of critical Care Medicine and the World Federation of Pediatric Intensive and critical Care Societies. 2018; 19: 1015–23.
Scherer B, Moser EA, Brown JW, Rodefeld MD, Turrentine MW, Mastropietro CW. Vasoactive-ventilation-renal score reliably predicts hospital length of stay after surgery for congenital heart disease. J Thorac Cardiovasc Surg. 2016;152:1423–9e1.
Torpoco Rivera DM, Garcia RU, Aggarwal S. Vasoactive-ventilation-renal score: a reliable prognostic index for perioperative outcomes following congenital heart surgery in adults. Cardiol Young. 2021;31:762–8.
Colombo J. Predictors of Outcomes after Pediatric Cardiac surgery: a proposal to improve the vasoactive-ventilation-renal score. Ann Thorac Surg. 2016;102:1413.
Schini M, Peel N, Toronjo-Urquiza L, Thomas E, Salam S, Khwaja A et al. Evaluation of estimated glomerular function (eGFR) versus creatinine clearance (CrCl) to predict acute kidney injury when using zoledronate for the treatment of osteoporosis. Osteoporosis international: a journal established as result of cooperation between the European Foundation for osteoporosis and the national osteoporosis Foundation of the USA. 2022; 33: 737–44.
Fortenberry JD, Paden ML, Goldstein SL. Acute kidney injury in children: an update on diagnosis and treatment. Pediatr Clin North Am. 2013;60:669–88.
Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. 2019;40:87–165.
Bai Y, Wang L, Guo Z, Chen Q, Jiang N, Dai J, et al. Performance of EuroSCORE II and SinoSCORE in Chinese patients undergoing coronary artery bypass grafting. Interact Cardiovasc Thorac Surg. 2016;23:733–9.
Zheng Z, Zhang L, Li X, Hu S. SinoSCORE: a logistically derived additive prediction model for post-coronary artery bypass grafting in-hospital mortality in a chinese population. Front Med. 2013;7:477–85.
Butt JH, Sørensen R, Bäck C, Olsen PS, Thorsteinsson K, Torp-Pedersen C, et al. Short- and long-term cause of death in patients undergoing isolated coronary artery bypass grafting: a nationwide cohort study. J Thorac Cardiovasc Surg. 2018;156:54–60e4.
Olsson D, Sartipy U, Braunschweig F, Holzmann MJ. Acute kidney injury following coronary artery bypass surgery and long-term risk of heart failure. Circulation Heart failure. 2013;6:83–90.
Wang L, Yang F, Wang X, Xie H, Fan E, Ogino M et al. Predicting mortality in patients undergoing VA-ECMO after coronary artery bypass grafting: the REMEMBER score. Critical care (London, England). 2019; 23: 11.
Abacioglu OO, Yildirim A, Koyunsever NY, Ucak HA, Abacioglu S. Relationship between CANLPH score and in-hospital mortality in patients undergoing coronary artery bypass grafting. Biomark Med. 2021;15:1659–67.
Ju JW, Nam K, Hong H, Cheun H, Bae J, Lee S, et al. Performance of the ACEF and ACEF II risk scores in predicting mortality after off-pump coronary artery bypass grafting. J Clin Anesth. 2022;79:110693.
Molina Andújar A, Lucas A, Escudero VJ, Rovira I, Matute P, Ibañez C et al. Risk factors for acute kidney Injury following cardiac surgery and performance of Leicester score in a spanish cohort. J Clin Med. 2022; 11.
Kwon JH, Yoo SY, Kim S, Won H, Kim W, Her S, et al. Vasoactive inotropic score as a predictor of long-term mortality in patients after off-pump coronary artery bypass grafting. Sci Rep. 2022;12:12863.
Algarni KD, Maganti M, Yau TM. Predictors of low cardiac output syndrome after isolated coronary artery bypass surgery: trends over 20 years. Ann Thorac Surg. 2011;92:1678–84.
Miletic KG, Delius RE, Walters HL 3rd, Mastropietro CW. Prospective validation of a Novel Vasoactive-Ventilation-Renal score as a predictor of Outcomes after Pediatric Cardiac surgery. Ann Thorac Surg. 2016;101:1558–63.
Ge J, Jin Z, Feng X, Pan W, Liu L, Wu M, et al. Creatinine clearance rate predicts prognosis of patients with systemic lupus erythematosus: a large retrospective cohort study. Clin Rheumatol. 2021;40:2221–31.
Ma X, Wang Y, Shan L, Cang Z, Gu C, Qu N, et al. Validation of SinoSCORE for isolated CABG operation in East China. Sci Rep. 2017;7:16806.
Acknowledgements
Not applicable.
Funding
This study was funded by Jining Key Research and Development Project (2020JKNS007).
Author information
Authors and Affiliations
Contributions
YD and LT designed the study. YD wrote the manuscript. YD, WL, QC, HS, QL, CZ, YZ, and JL collected and analyzed the data. LT critically reviewed, and edited the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study has been approved by the Ethics Committee of Affiliated Hospital of Jining Medical University (2021C165). Each participant has provided the informed consent. All methods were carried out in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Du, Y., Li, W., Chen, Q. et al. Comparison of vasoactive-inotropic score, vasoactive-ventilation-renal score, and modified vasoactive-ventilation-renal score for predicting the poor prognosis after coronary artery bypass grafting. BMC Cardiovasc Disord 23, 274 (2023). https://doi.org/10.1186/s12872-023-03313-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-023-03313-9