Abstract
Background
Diagnosis of aortic graft infections (AGI) is challenging. Here, we report a case of AGI with splenomegaly and splenic infarction.
Case presentation
A 46-year-old man who underwent total arch replacement for Stanford type A acute aortic dissection one year prior presented to our department with fever, night sweat, and a 20-kg weight loss over several months. Contrast-enhanced computed tomography (CT) revealed splenic infarction with splenomegaly, fluid collection, and thrombus around the stent graft. Positron emission tomography-CT (PET-CT) revealed abnormal 18F-fluorodeoxyglucose uptake in the stent graft and spleen. Transesophageal echocardiography revealed no vegetations. The patient was diagnosed with an AGI and underwent graft replacement. Blood and tissue cultures in the stent graft yielded Enterococcus faecalis. After the surgery, the patient was successfully treated with antibiotics.
Conclusions
Splenic infarction and splenomegaly are the clinical findings of endocarditis but are rare in graft infection. These findings could be helpful to diagnose graft infections, which is often challenging.
Similar content being viewed by others
Background
Aortic graft infection (AGI) is a rare but potentially fatal condition. The rate of AGI following aortic grafting has generally been reported to be between 1 and 3% [1]. Diagnosis of AGI is challenging. Computed tomography (CT) often reveals fluid collection more than three months after insertion and peri-graft gas more than seven weeks after insertion [2]. On the other hand, splenomegaly is a known finding in infective endocarditis [3], but there have been no case reports of splenomegaly with intravascular infection. In addition, emboli to the brain, lung, or spleen occur in 30% of patients as the presenting feature [4]. We report the first case of splenomegaly and splenic infarction in a patient with an AGI.
Case presentation
The patient was a 46-year-old man who had undergone vascular grafting of the ascending aorta and aortic arch for Stanford type A aortic dissection one year before coming to our department. He had experienced occasional fevers and night sweats for 6 months prior to his presentation to our department (4 months after surgery). He later presented weight loss of 10 kg and a fever of 39 °C, and visited the emergency room the day before coming to our department. At the time of his emergency visit, his CRP was only slightly elevated, with no obvious focus of fever, and his general condition was relatively good; therefore, blood cultures were taken, and follow-up was done as an outpatient. However, from blood cultures (4/4), gram-positive cocci were detected, and the patient was referred to our department for consultation and hospitalized the next day.
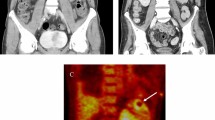
On admission, his vital signs were as follows: consciousness level clear, temperature 39.3 ℃, blood pressure 116/74 mmHg, pulse rate 96/min, respiratory rate 21/min, oxygen saturation 98% on room air. No abnormalities were found on head and neck examination. Heart and lung sounds were normal. Abdominal examination revealed no tenderness or hepatomegaly, but showed splenomegaly. There were no petechiae, Janeway lesions, Osler nodes, swollen or inflamed skin and joints, or lymphadenopathy. Laboratory data revealed 7, 700/μL (neutrophil 81.0%, lymphocyte 12.4%, monocyte 4.5%) of white blood cells; 7.9 mg/dL of C-reactive protein CRP; ferritin, 353 ng/mL; rheumatoid factor, 19 IU/mL; C3, 83 mg/dL; C4, 28 mg/dL; anti-nuclear antibody < 40 titers; PR3-ANCA, 2.7 IU/mL; MPO-ANCA, 1.4 IU/mL; and soluble Interleukin 2 receptor 1130 U/mL. T-SPOT.TB was negative. Urinary dip test revealed protein 3 + and blood 1 + . Contrast-enhanced computed tomography (CT) revealed fluid correction around the aortic graft, hepatomegaly (spleen index = 720), and splenomegaly with multiple thrombi (Fig. 1).
Because meeting the modified Duke criteria was possible, we suspected aortic graft infection with infective endocarditis, and intravenous ampicillin (2 g every 4 h) and ceftriaxone (2 g every 12 h) were initiated. Blood culture identified Enterococcus faecalis (E. faecalis) by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF Biotyper, Bruker Daltonics). Transthoracic and transesophageal echocardiography revealed no vegetation. Positron emission tomography (PET)-CT on the 10th day showed hyperaccumulation around the aortic graft and spleen (Fig. 2). We finally diagnosed aortic graft infection due to E. faecali, and the cardiac surgeon performed aortic arch replacement on the 20th day. After surgery, we de-escalated antibiotics to ampicillin monotherapy. The fever resolved on postoperative day 2 (the 22nd day from admission), and E. faecalis was identified in the tissue culture of the graft (Fig. 3). After six weeks of intravenous ampicillin monotherapy, his clinical condition improved, and he was discharged on postoperative day 44 (the 64th day from admission) and switched to oral amoxicillin for three months (Fig. 4). After a half-year follow-up, the size of the splenomegaly decreased (Fig. 5), and there was no relapse after antibiotic therapy was completed.
Positron-emission tomography-computed tomography. Hyperaccumulation (SUVmax: 11.8) in the fluid collection around the graft (left). Hyperaccumulation was observed throughout the spleen (SUVmax: 4.1), and localized hyperaccumulation was consistent with the area of splenic infarction (SUVmax:5.5) (right)
Clinical picture of the infected aortic graft removed in surgery. Pathological examination revealed inflammatory cell infiltration with neutrophils, foam cells, and foreign body giant cells with fibrosis, including fibroblasts, in the aortic graft. Debris and hemosiderin deposits were also observed in the hematoma. The tissue in the red circle was positive for Enterococcus faecalis
Spleen size followed up with computed tomography. Computed tomography revealed gradual enlargement of the spleen but improvement in spleen size after aortic graft replacement. A admission due to aortic dissection; B 7 months after surgery; C 13 months after surgery; D admission due to aortic graft infection 14 months after surgery; E 5 months after aortic graft replacement
Discussion and conclusion
We experienced a case of splenomegaly with splenic infarction caused by AGI due to E. faecalis. In this case, the main differential diagnosis was a foreign body reaction. Although the J-graft used in the patient potentially have the possibility, it has been improved and is believed to have fewer the reaction [5]. In addition, if the coating material induces an inflammatory response, the inflammatory response is thought to disappear when the coating material as an antigen is degraded, absorbed, and removed, although this varies depending on the material. The absorption of the coating material is 2 to 4 weeks for gelatin and 6 weeks to 3 months for collagen [6]. In this case, the same type of stent as the first surgery was used, but no clinical findings to date indicate a foreign body reaction. These clinical findings not only before but after the radical surgery supported our diagnosis of aortic graft infection.
AGI is rare, and the incidence reported in the literature is between 0.5% and 6%, although it depends on procedure types [7]. In addition, a search using the keywords “splenomegaly” and “bacteremia” in the electronic databases PubMed, Embase, and Ichushi by March 31, 2022 (Additinal file 1) revealed no reports of splenomegaly due to intravascular graft infection. Thus, we found that this is the first reported case of splenomegaly with splenic infarction due to AGI. In general, the causes of splenomegaly include hepatic, hematologic, infectious, autoimmune, vascular, and malignant diseases [8]. In this case, there were no causes of splenomegaly such as liver, autoimmune, or hematological diseases other than graft infection. In addition, this was also supported by the improvement in splenomegaly after treatment of the infection.
The mechanism of splenomegaly in AGI is not clear yet, but that in infectious endocarditis might be a clue. In infective endocarditis, the association between embolism, thrombus, splenic infarction, and splenomegaly has been previously reported [2,3,4, 9]. Several case reports of infective endocarditis and pacemaker infection combined with splenomegaly have been reported [10, 11]. Splenic infarction and splenomegaly are associated with septic embolic inflammation in the former and hyperplasia of lymphoid follicles and increased reticuloendothelial cells in the latter [12]. In the present case, although different from infective endocarditis, there were splenic findings suggesting bacteremia and septic embolic inflammation. A similar condition could have led to splenic infarction and splenomegaly.
There is no gold standard for the diagnosis of AGI; therefore, splenomegaly might be helpful in future diagnosis. The principles for diagnosis of AGI include the following: (1) index of suspicion; (2) recognition of the differences in clinical presentations of VGI; (3) time of onset postoperatively; (4) physical findings; (5) laboratory test results, including cultures of blood, purulent material from a draining sinus, or aspirates of perigraft fluid and surgical specimens; and (6) imaging [13]. Accordingly, Lyons et al. proposed dividing the criteria into clinical/surgical, radiologic, and laboratory categories, with major/minor criteria in each category [14]. The diagnosis of AGI is proposed when at least one major/minor criterion is met in addition to one major criterion in another category. The major and minor criteria in radiology include CT and PET-CT findings, respectively, and it has recently been reported that PET-CT may be more sensitive than CT [15]. The study reported that PET/CT had a sensitivity of 93% and specificity of 91% [16]. Technetium-99 m/indium-111-labeled leukocyte imaging is also reported to be helpful for diagnosis [17], with a sensitivity approaching 100% [12]. However, these tests have issues of false positivity [17, 18] and are sometimes difficult to access. Gallium 67 (Ga) scintigraphy has been previously used to detect VGI; however, the sensitivity was not significantly different between CT-scan and 67-Ga imaging [19]. The radiological tests are helpful, but the diagnosis is still made in combination with findings in other categories. On the other hand, the signs and symptoms of AGI are not specific, including fever and chills. However, if AGI involves the aortic root, they can be similar to those of infective endocarditis (IE), such as heart failure and murmur, but not in all cases of AGI. Moreover, a microbiological investigation that consists of laboratory categories is often difficult because of the anatomical location of the vascular graft. Therefore, splenomegaly, which can be examined at the bedside, may facilitate the diagnosis, even though it cannot confirm it.
Finally, the candidates for the treatment of graft infections include surgical drainage and antibiotics [2]. One small case series proposed that splenectomy for endocarditis was complicated by splenic abscess and persistent bacteremia [20]. However, surgery is more invasive, with a reported mortality rate of 18%–30% [2]. Moreover, splenectomy itself poses a higher risk of subsequent overwhelming post-splenectomy infection due to encapsulated bacteria, such as Streptococcus pneumoniae and Haemophilus influenzae [21]. In our case, the patient failed to respond to the antibiotic therapy alone, and surgical intervention without splenectomy was performed to control infection. The size of his spleen decreased after graft replacement and antibiotic therapy, and he completely recovered without splenectomy.
Splenomegaly and splenic infarction are rare, but they could be complicated by AGI. While the diagnosis of graft infections is challenging, these findings could be helpful for its diagnosis.
Availability of data and materials
Not applicable.
Abbreviations
- CT:
-
Computed tomography
- CRP:
-
C-reactive protein
- PET:
-
Positron emission tomography
References
Young MH, Upchurch GR Jr, Malani PN. Vascular graft infections. Infect Dis Clin North Am. 2012;26:41–56.
Lyons OT, Baguneid M, Barwick TD, Bell RE, Foster N, Homer-Vanniasinkam S, Hopkins S, Hussain A, Katsanos K, Modarai B, Sandoe JA, Thomas S, Price NM. Diagnosis of aortic graft infection: a case definition by the Management of Aortic Graft Infection Collaboration (MAGIC). Eur J Vasc Endovasc Surg. 2016;52:758–63.
Nakatani S, Ohara T, Ashihara K, Izumi C, Iwanaga S, Eishi K, Okita Y, Daimon M, Kimura T, Toyoda K, Nakase H, Nakano K, Higashi M, Mitsutake K, Murakami T, Yasukochi S, Okazaki S, Sakamoto H, Tanaka H, Nakagawa I, Nomura R, Fujiu K, Miura T, Morizane T, Japanese Circulation Society Joint Working Group. JCS 2017 guideline on prevention and treatment of infective endocarditis. Circ J. 2019;83:1767–809.
Thuny F, Di Salvo G, Belliard O, Avierinos JF, Pergola V, Rosenberg V, Casalta JP, Gouvernet J, Derumeaux G, Iarussi D, Ambrosi P, Calabró R, Riberi A, Collart F, Metras D, Lepidi H, Raoult D, Harle JR, Weiller PJ, Cohen A, Habib G. Risk of embolism and death in infective endocarditis: prognostic value of echocardiography: a prospective multicenter study. Circulation. 2005;112:69–75.
Moriyama S, Kunitomo R, Sakaguchi H, Okamoto K, Yoshinaga T, Tazume H, Kawasuji M. Postoperative Inflammatory Reactions of J Graft SHIELD NEO® for Surgery of Abdominal Aortic Aneurysm. Jpn J Vasc Surg. 2014;23:1–6. published in Japanese.
Higami T, Kawaharada N, Ito T. Jinko Kekkann (artificial blood vessels). Jinko Zoki Artificial organs. 2009;38:134–9. published in Japanese.
Berger P, Vaartjes I, Moll FL, De Borst GJ, Blankensteijn JD, Bots ML. Cumulative incidence of graft infection after primary prosthetic aortic reconstruction in the endovascular era. Eur J Vasc Endovasc Surg. 2015;49(58):1–5.
Robertson F, Leander P, Ekberg O. Radiology of the spleen. Eur Radiol. 2001;11:80–95.
Monteiro TS, Correia MG, Golebiovski WF, Barbosa GIF, Weksler C, Lamas CC. Asymptomatic and symptomatic embolic events in infective endocarditis: associated factors and clinical impact. Braz J Infect Dis. 2017;21:240–7.
Thipphavong S, Duigenan S, Schindera ST, Gee MS, Philips S. Nonneoplastic, benign, and malignant splenic diseases: cross-sectional imaging findings and rare disease entities. AJR Am J Roentgenol. 2014;203:315–22.
Arber N, Pras E, Copperman Y, Schapiro JM, Meiner V, Lossos IS, Militianu A, Hassin D, Pras E, Shai A, et al. Pacemaker endocarditis. Report of 44 cases and review of the literature. Medicine (Baltimore). 1994;73:299–305.
Bennett JE, Dolin R, Blaser MJ. Douglas, & Bennett’s principles & practice of infectious diseases, 9th ed. in 2 vols.
Wilson WR, Bower TC, Creager MA, Amin-Hanjani S, O’Gara PT, Lockhart PB, Darouiche RO, Ramlawi B, Derdeyn CP, Bolger AF, Levison ME, Taubert KA, Baltimore RS, Baddour LM, American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Surgery and Anesthesia; Council on Peripheral Vascular Disease; and Stroke Council. vascular graft infections, mycotic aneurysms, and endovascular infections: a Scientific Statement From the American Heart Association. Circulation. 2016;134:e412–60.
Lyons OTA, Baguneid M, Barwick TD, Bell RE, Foster N, Homervanniasinkam S, Hopkins S, Hussain A, Katsanos K, Modarai B, Sandoe JAT, Thomas S, Price NM. Diagnosis of aortic graft infection: a case definition by the Management of Aortic Graft Infection Collaboration (MAGIC). Eur J Vasc Endovasc Surg. 2016;52:758e763.
Kim S-J, Lee S-W, Jeong SY, Pak K, Kim K. A systematic review and meta-analysis of 18F-fluorodeoxyglucose positron emission tomography or positron emission tomography/computed tomography for detection of infected prosthetic vascular grafts. J Vasc Surg. 2019;70:307–13.
Keidar Z, Engel A, Hoffman A, Israel O, Nitecki S. Prosthetic vascular graft infection: the role of 18F-FDG PET/CT. J Nucl Med. 2007;48:1230–6.
Puges M, Bérard X, Ruiz JB, Debordeaux F, Desclaux A, Stecken L, Pereyre S, Hocquelet A, Bordenave L, Pinaquy JB, Cazanave C. Retrospective Study Comparing WBC scan and 18F-FDG PET/CT in Patients with Suspected Prosthetic Vascular Graft Infection. Eur J Vasc Endovasc Surg. 2019;57:876–84.
Shim H, Sung K, Kim WS, Lee YT, Park PW, Jeong DS. Diagnosis of graft infection using FDG PET-CT. Korean J Thorac Cardiovasc Surg. 2012;45:189–91.
Johnson KK, Russ PD, Bair JH, Friefeld GD. Diagnosis of synthetic vascular graft infection: comparison of CT and gallium scans. Am J Roentgenol. 1990;154:405–9.
Magilligan DJ Jr, Quinn EL. The role of splenectomy in endocarditis. Henry Ford Hosp Med J. 1983;31:43–6.
Di Sabatino A, Carsetti R, Corazza GR. Post-splenectomy and hyposplenic states. Lancet. 2011;378:86–97.
Acknowledgements
Not applicable
Funding
This study did not receive any funding.
Author information
Authors and Affiliations
Contributions
The manuscript was reviewed and approved by all authors and is not under consideration for publication elsewhere. All authors contributed to the work in this report. K.I. and Y.K. collected clinical data and wrote the initial draft of the manuscript. FK performed the literature review. NM supervised and edited the manuscript. The authors (s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
The patient has given written consent for his personal and clinical details. The authors certify that they have obtained all appropriate patient consent forms. In the form, the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kim, Y., Ishikawa, K., Kawai, F. et al. First case report of splenomegaly with splenic infarction due to aortic graft infection. BMC Cardiovasc Disord 23, 237 (2023). https://doi.org/10.1186/s12872-023-03259-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-023-03259-y