Abstract
Background
Insertable cardiac monitors (ICMs) are a clinically effective means of detecting atrial fibrillation (AF) in high-risk patients, and guiding the initiation of non-vitamin K oral anticoagulants (NOACs). Their cost-effectiveness from a US clinical payer perspective is not yet known. The objective of this study was to evaluate the cost-effectiveness of ICMs compared to standard of care (SoC) for detecting AF in patients at high risk of stroke (CHADS2 ≥ 2), in the US.
Methods
Using patient data from the REVEAL AF trial (n = 393, average CHADS2 score = 2.9), a Markov model estimated the lifetime costs and benefits of detecting AF with an ICM or with SoC (specifically intermittent use of electrocardiograms and 24-h Holter monitors). Ischemic and hemorrhagic strokes, intra- and extra-cranial hemorrhages, and minor bleeds were modelled. Diagnostic and device costs, costs of treating stroke and bleeding events and medical therapy—specifically costs of NOACs were included. Costs and health outcomes, measured as quality-adjusted life years (QALYs), were discounted at 3% per annum, in line with standard practice in the US setting. One-way deterministic and probabilistic sensitivity analyses (PSA) were undertaken.
Results
Lifetime per-patient cost for ICM was $31,116 versus $25,330 for SoC. ICMs generated a total of 7.75 QALYs versus 7.59 for SoC, with 34 fewer strokes projected per 1000 patients. The model estimates a number needed to treat of 29 per stroke avoided. The incremental cost-effectiveness ratio was $35,528 per QALY gained. ICMs were cost-effective in 75% of PSA simulations, using a $50,000 per QALY threshold, and a 100% probability of being cost-effective at a WTP threshold of $150,000 per QALY.
Conclusions
The use of ICMs to identify AF in a high-risk population is likely to be cost-effective in the US healthcare setting.
Similar content being viewed by others
Introduction
Atrial fibrillation (AF) is associated with an increased risk of stroke [1, 2]. Anticoagulation therapy particularly with non-vitamin-K oral anticoagulants (NOACs) is effective at reducing ischemic stroke (IS) risk in patients with AF [3]. This benefit only occurs in the presence of a clear electrocardiographic diagnosis of AF due to the increased risk of bleeding associated with NOACs: patients who do not have AF that receive face an increased risk of bleeding with no reduction in IS risk [4]. Because the symptoms associated with suspected AF can vary widely, and AF episodes themselves may be transient [5], there is a risk that diagnoses are missed by opportunistic and short-term monitoring solutions [such as intermittent 24-h Holter monitor or electrocardiogram (ECG) use].
Insertable cardiac monitors (ICMs) can provide continuous long-term cardiac monitoring in patients with potential arrhythmias. The REVEAL AF study [6] (ClinicalTrials.gov, Number: NCT01727297) monitored the heart rhythms of patients at high risk of AF and non-specific symptoms. Study participants had a CHADS2 score of at least 3 or a score of 2 with at least one additional stroke risk factor. All 393 patients had 24 h or more of external monitoring within 90 days prior to enrolment or ICM insertion, with any prior AF detection being an exclusion criterion. In 81 enrolled patients, the risk profile included a previous diagnosis of heart failure (HF). It is increasingly appreciated that AF and HF are co-morbidities, with a bidirectional adverse impact on patient outcomes [7].
While the REVEAL AF study demonstrated the ability of ICMs to detect previously undiagnosed AF, whether the use of ICMs for AF detection in a high-risk population is cost-effective is unknown. The objective of this analysis was to estimate the cost effectiveness of ICMs compared to standard of care in detecting AF, in a population at high risk for AF, from a US clinical payer perspective. A list of abbreviations used within this paper is presented in Table 1.
Methods
A model was adapted based on a previous analysis in the UK setting [8], to a US setting. The model was a Markov model, which, as a cohort state transition model, was designed to simulate costs and benefits of monitoring for AF with an ICM compared to standard of care (SoC), in patients with a high risk of stroke and no confirmed AF diagnosis. It was developed using Excel, Microsoft 365. Costs of monitoring and treatment represent a US payer perspective.
Health benefits were expressed as quality-adjusted life years (QALYs) and discounted, along with costs, at 3% annually. The Markov model used a lifetime horizon to capture all costs and health-related quality of life (QoL) benefits. Life-years gained and IS events avoided were also compared between ICM and SoC strategies. The model cohort was set up to have the same baseline characteristics as recorded in the REVEAL AF study [6] (Additional file 1: Table S1), and the duration of model cycles (3 months) also matched the observation intervals in REVEAL AF.
Model structure
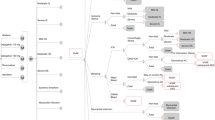
The model simulates the expected efficacy of ICM or SoC monitoring in detecting AF. If AF is detected, patients begin NOAC therapy, which reduces the risk of IS. The increased risk of major bleeds associated with NOACs is modelled, as is anticoagulant discontinuation. All relevant costs and health benefits are estimated separately for ICM and SoC. Movement of patients through the model is shown in Fig. 1.
Model flow. Notes: a, NOACs are administered in base-case analysis, warfarin is substituted in sensitivity analysis. Abbreviations: AF: atrial fibrillation; CRNM: clinically relevant non-major; ECH: extracranial hemorrhage; HS: hemorrhagic stroke; ICH: intracranial hemorrhage; IS: ischemic stroke; NOAC: non-vitamin K oral anticoagulants
The hypothetical cohort is assumed to enter the model without an established diagnosis of AF. In each cycle, their AF status can remain the same or change and be detected or go undetected. Prior to AF detection, patients receive either aspirin (73%) or no treatment (27%) [6]. When AF is detected, the patient is assumed to start NOAC therapy, which is associated with a reduction in the risk of IS but an increase in the risk of hemorrhagic strokes (HS) and other bleeding events. Following a stroke, patients move to one of three post-stroke health states where they have a reduced QoL and higher costs, corresponding to the severity of the stroke experienced. Stroke events can also be fatal. Detection of AF and initiation of NOAC therapy can still occur among patients in post-stroke health states.
SoC and ICM clinical practice
SoC was assumed to involve intermittent 24-h Holter and ECG use (see Additional file 1: Sect. 8.2). ICM was assumed to involve the insertion and regular monitoring of an ICM, followed by device explantation after battery expiry at three years and a return to SoC for the remaining years.
Population
The baseline characteristics of the patient cohort match the REVEAL AF trial population. Mean age was 71.3 years, 73% were on aspirin, and 20% had one or more strokes in the year prior to recruitment. The mean virtual CHADS2 score was 2.94 (Additional file 1: Sect. 1). CHADS2 is a clinical prediction scoring system to determine stroke risk in patients with atrial fibrillation. It accounts for history of congestive heart failure, hypertension, age (> 75 years), diabetes mellitus and stroke/transient ischemia attack/ thromboembolism [3].
AF detection, frequency and management post-detection
AF detection rates for ICM were based on those seen in the REVEAL AF study [6]. Our base case analysis used the study primary endpoint definition of AF as an episode lasting ≥ 6 min. Given the lack of consensus around the duration of AF to both diagnose the condition and for the risk of stroke to increase, device recordings were re-analyzed to also create a scenario in which AF was only diagnosed if episodes lasted for ≥ 5.5 h [9]. The REVEAL AF trial data informed the rate of AF detection for the first 30 months in the model (Fig. 2). After 30 months, the AF risk was extrapolated by fitting a logarithmic curve (excluding the first 3 months). To reflect the potential for some AF episodes to go undetected by ICMs, the model assumed a device sensitivity of 96.1% [10].
AF detection rates in ICM and SoC—all patients. Notes: Both ICM and SoC detection curves are modelled based on analysis of the REVEAL AF trial dataset (5). The AF burden is extrapolated from the end of the 30-month study and the end of the battery life at 36 months, using a linear extrapolation from month 3 to month 30 (3.9% per 3-month cycle). Abbreviations: AF: atrial fibrillation; ICM: insertable cardiac monitor; SoC, standard of care
For SoC, the rate of detection was sourced from a simulated comparison of AF monitoring strategies using trial data from REVEAL AF [11]. Using data from this comparison, the relative diagnostic yield of ICM compared to SoC, expressed as a hazard ratio, was 33.9 (95% CI 13.2—NE). Further details are provided in the Additional file 1: Table S4.
Modelled patients were given NOAC therapy in the same cycle they received an AF diagnosis. A scenario analysis explored the impact of a lower NOAC prescription rate (66.35%) post-AF diagnosis; this scenario was informed by prescription rate in the REVEAL AF study [6].
Ischemic stroke risk and severity
Per 3-month cycle, the risk of ischemic stroke (IS) was based on AF status, virtual CHADS2 score, anticoagulation treatment and patient age (see Table 2).It was necessary to combine evidence from a number of publications to allow this risk stratification [1, 12,13,14,15]. The increased IS risk associated with device-detected AF was taken from Mahajan et al. [15]. IS severity was modelled using estimates from previous cost-effectiveness publications [16, 17]. The IS severity distribution was independent of patients’ current anticoagulation treatment (see Additional file 1: Table S12). An IS was assumed to have a permanent impact on a patient’s health management costs, QoL and mortality risk, with the level of impact corresponding to stroke severity (Additional file 1: Tables S15 and S17).
Bleeding events: NOAC cessation and QoL impact
NOAC treatment carries a bleeding risk, which was weighted in the model by age as per a previous cost-effectiveness publication [17]. Bleed severity (see Additional file 1: Sect. 5.2 and Table S13) was independent of the particular anticoagulation treatment given [18]. Non-fatal extracranial hemorrhage (ECH) was assumed to cause cessation of NOAC therapy in the patients who were receiving NOAC at the time of the bleed. For 75% of patients with ECH, NOAC discontinuation was for 6 weeks only; for the remaining 25% the discontinuation was permanent. Patients who experienced the ECH while receiving aspirin or no treatment were barred from future NOAC use in 25% of cases [19, 20]. Similarly, intracranial hemorrhages (ICH) that were not HS, triggered temporary (6 week) NOAC discontinuation in 44% of patients on NOAC and permanent discontinuation in 56%. Patients on aspirin or no treatment at the time of an ICH were precluded from future NOAC use in 56% of cases [19, 20]. NOAC was not discontinued in the event of a clinically relevant non-major (CRNM) bleed. HS led to permanent NOAC discontinuation in 100% of patients on NOAC, and exclusion from any future NOAC use in all categories of patient, regardless of their AF status, AF diagnosis or therapy received. Additionally, there was an annual NOAC discontinuation rate of 14.8% for reasons unrelated to bleeding events [17].
A HS, like an IS, was assumed to have a permanent impact on a patient’s healthcare costs, QoL and mortality risk (Additional file 1: Sect. 7 and Table S17). The effects of a non-fatal ECH, other ICH (i.e. non-hemorrhagic stroke) and CRNM bleed were temporary, applied only during the cycle when the bleed occurred.
Mortality for causes other than cerebrovascular events
All patients had an age-related all-cause mortality risk in the background, based on US life tables but adjusted to remove risk attributable to cerebrovascular events (which the model already covers) [21, 22]. Following a non-fatal IS or HS, this mortality risk was assumed to increase depending on stroke severity and the anticoagulation treatment currently received (Additional file 1: Sect. 6).
Health-related QoL
Patient’s mean QoL, expressed as a utility value between 0 and 1, was sourced from EQ-5D QoL questionnaires collected at baseline in the REVEAL AF study. The OXVASC study and Gage 1996 were then used to estimate the expected QoL decrement (or disutility value) associated with strokes and their aftermath. Previously published health economic analyses were used to obtain estimates of disutility or utility reduction associated with various bleeds [16, 17, 23, 24]. The mean utilities used, and further details on their calculation are provided in Table 3.
Resource use and costs
Device and monitoring costs
Costs of purchasing, inserting and later removing the ICM following battery expiry (after 3 years) were informed by U.S. Medicare 2020 national average payer paid amounts [28]. There were also per-cycle costs for in-person and remote clinician visits. The number of visits was informed by informed by the LINQ Registry study [29] and the costs were informed by U.S. Medicare 2020 national average payer paid amounts [28]. Patients who did not have AF detected continued to receive SoC after ICM removal. Unplanned ICM removal before battery expiry due to a range of clinical, technical, or personal factors occurred at a rate of 2.9% per year based on REVEAL AF (Medtronic data on file).
Patients receiving SoC in the model received 0.17 24-h Holter monitors and 3.40 ECGs annually on average. This was informed by real-world claims data for a cohort with similar characteristics [30]. The unit costs of this monitoring were estimated from U.S. Medicare 2020 national average payer paid amounts [28].
The base case used public payer costs taken from Medicare [28], and a scenario analysis, in which device and monitoring costs were increased by 25%, was performed to model a hypothetical cohort of 100% private payer patients.
Treatment and event-related costs
The costs of oral anticoagulation were estimated using data from the national average payer paid amounts (Additional file 1: Table S19).
Strokes and bleeds had high one-time costs associated with managing the event, and where they caused permanent health consequences, additional per-cycle costs were applied in a set of “post-stroke” health states. Health state and event costs were derived from published literature [26] and adjusted where necessary to 2020 levels using the “Medical Care component of the consumer price index [32] (Table 3).
Model output and sensitivity analysis
The incremental cost-effectiveness ratio (ICER) for ICM versus SoC was calculated using total QALYs and healthcare costs over the cohort lifetime. Results were also generated for a hypothetical cohort of 1000 patients. Deterministic sensitivity analyses were undertaken to assess the impact of assumptions about the method and accuracy of AF detection, OAC treatment uptake and discontinuation, the type of OAC therapy that is given following AF detection (warfarin versus NOACs) and the costs of detection and medication. Probabilistic sensitivity analysis, varying all model parameters simultaneously within appropriate distributions, was performed storing the results of 1000 samples. Cost-effectiveness was tested at the $50,000 and $150,000 thresholds, in keeping with US guidelines [33].
Subgroup analysis
Subgroup analyses by CHADS2 score and by history of heart failure (Fig. 3) were performed, using AF detection and patient demographic data from REVEAL AF. In addition to using the REVEAL subgroup data, the modelling of patients with a history of heart failure required an updated set of bleed risks associated with NOACs, derived from relevant subgroup analyses from published studies (Additional file 1: Table S11).
AF detection rates in ICM and SoC—heart failure subgroup. Notes: Both ICM and SoC detection curves are modelled based on analysis of the REVEAL AF trial dataset (5). The AF burden is extrapolated from the 24 months of study data (after which it becomes underpowered) and the end of the battery life at 36 months, using a linear extrapolation from month 3 to month 30 (3.9% per 3-month cycle). Abbreviations: AF: atrial fibrillation; ICM: insertable cardiac monitor; SoC, standard of care
Results
Base case
The base-case analysis (Table 4) found that across patients’ lifetimes, the ICM strategy provided an additional 0.16 QALYs compared to SoC. The incremental cost was $5786, giving an ICER of $35,528 per QALY gained. There were 34 fewer ischemic strokes per 1000 patients in the ICM group compared to SoC, and an overall life-year gain of 0.17. These results suggest that ICM is a cost-effective monitoring strategy compared to SoC in high-risk AF patients. The model estimates a number needed to treat (NNT) of 29 per stroke avoided.
ICM had higher monitoring costs compared to SoC at initiation and during the 3 years of regular follow-up. Furthermore, bleed-related costs for the ICM strategy ($6803) were slightly higher than for SoC ($6302) because more patients on ICM had AF detected and went on to receive anticoagulation. However, costs related to IS were reduced in the ICM group compared to SoC ($7204 vs. $8225 respectively), since the improved AF detection in the ICM group ultimately led to the prevention of more stroke events (and their related costs). Other health state costs were also lower for ICM than for SoC ($8246 vs. $8609), due to lower post-stroke health costs. Overall, purchasing an ICM reduced costs in other areas.
Probabilistic sensitivity analysis found ICM had a 75% probability of being highly cost-effective at a willingness-to-pay (WTP) threshold of $50,000 per QALY, and a 100% probability of being cost-effective at a WTP threshold of $150,000 per QALY (Figs. 4, 5).
Subgroup analyses
The base case assumed a distribution of patient CHADS2 scores matching baseline characteristics in the REVEAL AF study. The analysis was repeated, restricting to each CHADS2 subgroup (see Table 5). The ICER in lower-risk patients (CHADS2 score = 2) was higher than the base case ($55,059 per QALY). The ICER in the group with CHADS2 score = 3 was similar to the base case (the base-case population had a mean CHADS2 score of 2.94). ICERs for patients with higher-risk CHADS2 scores (4, 5 or 6) were slightly higher than the base case, with smaller incremental costs and smaller QALY gains.
A subgroup analysis was performed in the 81 REVEAL AF study patients who had a history of heart failure. The ICER for this subgroup was $33,273, which is lower than in the base case, driven by a higher rate of AF detection (43.8% at 3 years) and higher average CHADS2 score.
Sensitivity and scenario analyses
Table 5 presents the main parameters and scenarios tested, alongside their impact on model results. The tornado diagram (Fig. 6) visually summarizes the results of key single-parameter sensitivity analyses and scenario analyses.
The primary efficacy parameter for this model was the rate of AF detection with ICM, in relation to SoC. In the base case, ICM was modelled to detect AF at a rate 33.9 times that of SoC. The ICER increased slightly (to $38,723 per QALY) when this ratio was set to its lower confidence limit of 13. The recent LOOP study by Svendsen et al. estimated a ratio of 3.17 between ICM and SoC for AF detection efficacy [34]. When this substantially lower ratio was substituted into the model, the ICER increased to $62,662 per QALY.
Using an alternative definition of AF (i.e. requiring a 5.5-h episode captured by ICM to trigger AF diagnosis) produced a higher ICER ($86,013).
In the base case, IS risk was assumed to increase 2.4-fold following a subclinical, device-detected AF diagnosis (based on Mahajan et al. [15]). When this relative risk figure was instead taken from a study (Gage et al. [1, 12]) that used more acute clinical, ECG-detected AF (RR = 4.8), the ICER fell to $20,717per QALY.
Costs of the ICM device and its insertion procedure impacted the ICER substantially, with a 30% cost increase driving the ICER up to $47,320 per QALY, and a 30% cost decrease reducing it to $23,735 per QALY. In the base case it was assumed that all patients received Medicare funded healthcare. Assuming 100% of patients receive private payer healthcare, resulted in an ICER of $44,479. Also linked to ICM costs but affecting a wider range of outcomes, we tested scenarios where the ICM battery lasted 50% longer (4.5 years vs. 3 years in the base case). Under these longer battery-life scenarios, the ICER decreased to $33,229, with 37 fewer strokes per 1000 patients in the ICM arm compared to SoC. This “4.5 years battery life” scenario was further explored using alternative methods of extrapolating AF detection data beyond the 30 months’ follow-up from the REVEAL AF trial. Linear extrapolation yielded an ICER of $27,986, whereas assuming no further AF detection beyond 30 months yielded an ICER of $37,594.
Uptake, efficacy and safety of NOAC therapy after AF detection have substantial effects on the ICER, as does the discontinuation rate for NOAC. Where NOAC uptake was assumed to be lower (66.35% vs. 100% in the base case), the ICER rose to $54,368 per QALY. If NOAC efficacy in reducing IS risk is assumed to be better (RR = 0.28 NOAC vs. aspirin, compared to 0.39 in the base case) the ICER improves to $30,128 per QALY. If the less-expensive warfarin is administered instead of NOACs, the ICER rises to $56,916 due to the increased hemorrhage risk, which causes QALY losses and net cost gains from treating bleeding events. Varying the assumed cost of NOACs, whilst holding their efficacy constant, resulted in an ICER of between $33,239 and $36,360. The base case allowed a range of reasons for NOAC discontinuation; however, the ICER improved to $15,843 per QALY if discontinuation was only allowed in the event of bleeds—this alternative discontinuation rule meant more patients remained on NOAC therapy and thus experienced reduced IS risk.
Several of these key scenarios were repeated for the higher-risk heart failure subgroup (Table 5). The direction of change was always consistent with that observed in the all-patient population. For example, when ICM battery life was increased to 4.5 years in the heart failure subgroup, the ICER fell to $30,777. When this battery-life scenario was subdivided by AF data extrapolation method, the linear extrapolation method yielded an ICER of $20,967, whereas no extrapolation (i.e. no AF beyond 30 months) yielded an ICER of $35,332.
Discussion
This study modelled the indirect relationship between ICM use (a diagnostic strategy) and stroke reduction (event avoidance) via appropriate oral anticoagulation (improved treatment). Consistent with previous models in this area, the cost-effectiveness of ICM is driven by its diagnostic advantage over SoC, and the extent to which this influences subsequent health events and their costs. While implanted, ICM has been estimated to detect AF at a rate over 33 times higher than SoC on average. This allows a far greater proportion of patients to be prescribed NOACs, which in the model led to fewer strokes, improved life expectancy and higher quality of life. There were slightly greater costs related to bleeds in the ICM group, but these were offset by long-term savings associated with reduced IS. In the base case analysis, ICM was a cost-effective way to monitor for AF in a high-risk population within the US healthcare setting, with a mean ICER below the commonly-accepted lower threshold of $50,000 per QALY gained [35].
This is the first model of its kind developed for the US. It was adapted from a previous model developed in the UK [8] but with several key modifications. The UK model calculated IS risk based on a longitudinal study of the association of clinical, ECG-detected AF with IS [1]; thus each AF event substantially increased IS risk (RR for AF = 4.8). By contrast, this US model referenced a more relevant study into the association of subclinical, device-detected AF and IS [15]. Using this source, each AF event is associated with a lower, 2.4-fold increase in IS risk. An additional difference is that the SoC monitoring frequency in this model was informed by real-world data collected in a US setting [30]. Compared to a previous US-based model involving ICM in patients with cryptogenic stroke, the ICER from this analysis was slightly higher [36].
The main sensitivities explored for the model related to ICM diagnostic efficacy, longevity and cost, IS risk associated with AF, NOAC treatment and its various dimensions, as well as subgroup analyses.
Diagnostic accuracy of AF diagnosis through ICM versus SoC had only a modest effect on cost-effectiveness when varied based on confidence intervals from the publication source used. Assuming no change in other parameters, we determined that ICM only has to detect AF at about 4.5 times the rate of SoC to be considered cost-effective at the most stringent threshold of $50,000 USD per QALY considered ‘highly cost-effective.’ The recent LOOP study in the Danish healthcare setting [34] found a lower relative difference in AF detection rates between ICM and SoC. When this rate was applied in the model, it gave an ICER of $62,662. However, the LOOP study estimated a uniquely high rate of AF detection in its SoC arm which may not reflect the detection that could be expected in clinical practice. The particularly high levels of cardiac monitoring in the SoC arm may have been impacted by the unblinded nature of the study, and additionally may not be generalizable outside of the Danish setting. Moreover, the study conclusions regarding the general effectiveness of ICM in preventing strokes remain uncertain due to the ongoing unresolved equipoise around the stroke reduction of OAC therapy administered after device-detected AF, compared with ECG-detected AF. This issue may be resolved once the stroke outcome-based RCTs of ICM, ARTESIA and NOAH [37, 38], are completed.
Newer ICMs carry an extended battery life of 4.5 years. To reflect this greater battery longevity, our model extrapolated the extent of additional AF detection after the study duration of REVEAL AF. Changing assumed rates of AF detection at timepoints beyond the 30 months of observation in the REVEAL AF trial had a moderate impact on the ICER, although all tested assumptions produced an ICER which remained below the $50,000 cost-effectiveness threshold. Additionally, since ICMs represent a potential lifetime strategy for monitoring AF, the assumptions made in this model around monitoring durations and explantation could be programmed in a more sophisticated fashion if additional data become available. If new trials are conducted with longer time horizons of monitoring and managing AF, this would likely yield improved cost-effectiveness estimates for ICM use.
In our model, the diagnostic benefit of ICM is translated into cost and QALY benefits via the use of NOACs, which reduce IS risk. Real-world cost-effectiveness will be determined by how closely US clinical anticoagulation practices (and their results) reflect the model. For example, we assumed that all patients receive NOACs immediately upon AF detection, and that 14.8% of them discontinue treatment annually for reasons other than bleeding. It is possible that in practice, rates of anticoagulation uptake and patient compliance are lower, thus leading to poorer protection against strokes than we have assumed. Real-world estimates for NOAC uptake following AF diagnosis vary substantially, from 76 to 100% [39,40,41]. The model remained relatively robust to less favourable assumptions; annual discontinuation for reasons other than bleeds would need to exceed 26% for the ICER to exceed the $50,000 WTP threshold.
The assumed efficacy of anticoagulation therapy also impacted cost-effectiveness, but the ICER withstood modest changes in the hazard ratio for NOAC efficacy. Furthermore, the relative clinical benefit of NOACs in stroke prevention is well established [14]. When the higher-risk warfarin was substituted as anticoagulation in scenario analysis, the ICER worsened but remained below the accepted WTP threshold.
Following a major bleed, clinicians must decide whether it is worth the risk of allowing patients to continue NOAC therapy, or if it is safer to stop. The exact approach taken can vary in the US clinical setting. The model took a conservative approach where hemorrhagic strokes led to immediate and permanent discontinuation of NOACs with no exceptions. Future analyses could explore alternative approaches here, for example allowing cautious use of NOACs in limited circumstances despite the occurrence of hemorrhagic strokes (either in the patient’s past history or during the modelled NOAC treatment phase).
Cost-effectiveness of ICM would normally be expected to improve in groups with higher underlying AF risk, because there should be more potential AF episodes to detect, which would then lead to more AF diagnoses and initiations of NOAC treatment, resulting in more IS events avoided. This pattern was partly consistent when we restricted our model by CHADS2 subgroups (values 2, 3 and 4–6). ICM was least cost effective in the lowest-risk group (CHADS2 = 2), and similar to base-case for CHADS2 = 3. However, the ICER was not improved for patients in the highest-risk CHADS2 groups (scores 4–6), indeed it was slightly higher than base-case. This counterintuitive result is explained by the REVEAL AF trial study data, wherein fewer patients in CHADS2 groups 4–6 received a confirmed AF diagnosis compared to the whole population. A larger real-world study would be expected to clarify this uncertainty, which may in part be due to the lower sample sizes collected in these subgroups.
In the heart failure subgroup, ICMs were found to be more cost-effective. This was mainly due to the higher rate of AF detection within the subgroup along with their higher average CHADS2 score [37, 38].
In a zero-cost scenario where SoC was assumed to comprise a pulse check and identified 1/24th of the AF detected by SoC in the base case, the ICER for ICM rose to $40,992 per QALY. In a scenario where SoC was assumed to comprise only a one-off 24-h Holter monitor, the ICER for ICM rose to $40,323. In practice, SoC can vary substantially, with patients receiving different monitor lengths with varying regularity; the cost-effectiveness of ICMs is robust to variations in clinical practice [39,40,41].
Limitations
First, the benefit of anticoagulation in reducing stroke risk among patients with clinical AF is well established. This paper modelled the benefits of anticoagulation in patients with device-detected AF, which is a less well-studied risk factor for IS. Both patients’ stroke risk (i.e., their CHADS2 score), and the efficacy of NOACs in reducing stroke risk, were varied in sensitivity analysis. Cost-effectiveness estimates were robust to changes in these parameters. Several ongoing studies will provide additional estimates of the absolute stroke reduction benefit of screening patients for subclinical AF using device detection, reducing uncertainty in this area [37, 38].
Second, this study modelled whether patients had AF diagnosed by an ICM, but not the extent of their AF burden. Increasing the threshold for AF diagnosis from 6 min to 5.5 h had limited impact on cost effectiveness, providing evidence that results were robust. The link between AF burden and IS risk remains uncertain. It is worth noting that elevated stroke risks have been observed in patients with short AF episodes of ≥ 6 min (irrespective of clinical diagnosis) [42] as well as in patients with ≥ 5.5 h AF episodes (detected via a cardiovascular implanted electronic device) [43]. The REVEAL study patient population had a wide distribution of AF episode lengths triggering diagnosis. Of the 128 patients with AF lasting 6 or more minutes, 113 (88.3%) had 30 or more minutes of AF, 97 (75.8%) had 1 or more hours of AF, and 53 (41.4%) had 6 or more hours of AF in a day at some point. Thirteen patients (10.2%) had at least one episode lasting 24 h or longer. Modelling a more sophisticated relationship between this extended range of AF burdens and stroke risk could give a clearer understanding of which patients may benefit most from anticoagulation. The real-world outcomes associated with more systematic screening for AF have been reinforced by new research [44], which could also form a useful validation reference for any such new models.
This study did not estimate the population health impact of increasing the use of ICMs among patients at high risk of atrial fibrillation. A future study focusing specifically on population health impact could help to further inform payer decision making regarding ICMs.
Further research
An updated version of CHADS2, called CHA2DS2-VASc, has recently been introduced [17]. This score was not captured in the REVEAL AF trial, but future models could potentially make use of a mapped version of this new risk score.
Extending the model with real-world observations from longer-term monitoring or building a model with greater flexibility to simulate alternative assumptions around AF burden and detection over time, could help identify which factors are the most important determinants of cost-effectiveness in ICM monitoring.
Conclusions
This analysis found ICM to be a cost-effective method for detecting AF in high-risk populations in the US healthcare setting, because of the benefit it can provide through timely diagnosis and anticoagulation to prevent strokes. ICM use is particularly more cost effective in patients who also have a history of heart failure. The model was developed for the US, but findings align with models developed for other countries (for example the UK [8] and for the related population of cryptogenic stroke patients [45]).
Long-term monitoring for AF and associated stroke prevention form part of an evolving field. There are regular updates to risk stratification methods, available anticoagulation medicines, and AF detection technology. The sensitivity analyses conducted suggest that most therapeutic and diagnostic advancements will further increase the cost-effectiveness of ICMs.
Availability of data and materials
Data are available upon request by contacting the corresponding author: lsawyer@symmetron.net.
References
Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22(8):983–8.
Kamel H, et al. Atrial fibrillation and mechanisms of stroke: time for a new model. Stroke J Cereb Circ. 2016;47(3):895–900.
January Craig T, et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration with the Society of Thoracic Surgeons. Circulation. 2019;140(2):e125–51.
Witte KK, et al. Burden of oral anticoagulation in embolic stroke of undetermined source without atrial fibrillation. BMC Cardiovasc Disord. 2021;21(1):160.
Strickberger SA, et al. Relationship between atrial tachyarrhythmias and symptoms. Heart Rhythm. 2005;2:125–31.
Reiffel JA, et al. Incidence of previously undiagnosed atrial fibrillation using insertable cardiac monitors in a high-risk population: the REVEAL AF study. JAMA Cardiol. 2017;2(10):1120–7.
Ling LH, et al. Comorbidity of atrial fibrillation and heart failure. Nat Rev Cardiol. 2016;13(3):131–47.
Rinciog CI, et al. Cost-effectiveness of an insertable cardiac monitor in a high-risk population in the UK. Open Heart. 2019;6(1):e001037.
Noseworthy PA, et al. Subclinical and device-detected atrial fibrillation: pondering the knowledge gap: a scientific statement from the American Heart Association. Circulation. 2019;140(25):e944–63.
Hindricks G, et al. Performance of a new leadless implantable cardiac monitor in detecting and quantifying atrial fibrillation: results of the XPECT trial. Circ Arrhythm Electrophysiol. 2010;3(2):141–7.
Reiffel JA, Verma A, Kowey P, Halperin J, Gersh B, Elkind MSV, et al. A comparison of atrial fibrillation monitoring strategies in patients at high risk for atrial fibrillation and stroke: results from the REVEAL AF study. J Am Coll Cardiol. 2018;71(11 Supplement):A274.
Gage BF, et al. Selecting patients with atrial fibrillation for anticoagulation: stroke risk stratification in patients taking aspirin. Circulation. 2004;110(16):2287–92.
Pisters R, et al. Stroke and thromboembolism in atrial fibrillation. Circ J. 2012;76(10):2289–304.
Tawfik A, et al. Systematic review and network meta-analysis of stroke prevention treatments in patients with atrial fibrillation. Clin Pharmacol. 2016;8:93–107.
Mahajan R, et al. Subclinical device-detected atrial fibrillation and stroke risk: a systematic review and meta-analysis. Eur Heart J. 2018;39:1407–15.
Dorian P, et al. Cost-effectiveness of apixaban vs. current standard of care for stroke prevention in patients with atrial fibrillation. Eur Heart J. 2014;35(28):1897–906.
Lip GY, et al. Cost-effectiveness of apixaban versus other new oral anticoagulants for stroke prevention in atrial fibrillation. Clin Ther. 2014;36(2):192–210.
Diamantopoulos A, et al. Cost-effectiveness of an insertable cardiac monitor to detect atrial fibrillation in patients with cryptogenic stroke. Int J Stroke. 2016;11(3):302–12.
Claassen DO, et al. Restarting anticoagulation therapy after warfarin-associated intracerebral hemorrhage. Arch Neurol. 2008;65(10):1313–8.
Sorensen SV, et al. Cost-effectiveness of warfarin: trial versus “real-world” stroke prevention in atrial fibrillation. Am Heart J. 2009;157(6):1064–73.
United States Mortality Database. United States Mortality Database. 2019.
Office for National Statistics. Mortality statistics: deaths registered in England and Wales (Series DR), 2012. 2013.
Luengo-Fernandez R, et al. Population-based study of disability and institutionalization after transient ischemic attack and stroke: 10-year results of the Oxford Vascular Study. Stroke. 2013;44(10):2854–61.
Luengo-Fernandez R, et al. Quality of life after TIA and stroke: ten-year results of the Oxford Vascular Study. Neurology. 2013;81(18):1588–95.
Gage BF, Cardinalli AB, Owens DK. The effect of stroke and stroke prophylaxis with aspirin or warfarin on quality of life. Arch Intern Med. 1996;156(16):1829–36.
Miller JD, et al. Cost-effectiveness of edoxaban versus rivaroxaban for stroke prevention in patients with nonvalvular atrial fibrillation (NVAF) in the US. ClinicoEcon Outcomes Res: CEOR. 2016;8:215.
Sullivan PW, Lawrence WF, Ghushchyan V. A national catalog of preference-based scores for chronic conditions in the United States. Med Care. 2005;43(7):736–49.
Centers for Medicare & Medicaid Services. Physician fee schedule. 2021. www.cms.gov/apps/physician-fee-schedule/license-agreement.aspx.
Medtronic Cardiac Rhythm and Heart Failure. Reveal LINQ Registry. 2019. https://clinicaltrials.gov/ct2/show/NCT02746471.
Optum. Electronic Health Record de-identified database 2007–2017.
Drugs.com. Warfarin prices, coupons and patient assistance programs. 2020. https://www.drugs.com/price-guide/warfarin.
Federal Reserve Bank of St. Louis. Consumer price index for all urban consumers: medical care in U.S. City average, percent change, annual, seasonally adjusted. 2020. https://fred.stlouisfed.org/series/CPIMEDSL#0.
Anderson Jeffrey L, et al. ACC/AHA statement on cost/value methodology in clinical practice guidelines and performance measures. Circulation. 2014;129(22):2329–45.
Svendsen JH, et al. Implantable loop recorder detection of atrial fibrillation to prevent stroke (The LOOP Study): a randomised controlled trial. The Lancet. 2021;398:1507–16.
Pearson SD. The ICER value framework: integrating cost effectiveness and affordability in the assessment of health care value. Value Health. 2018;21:258–65.
Sawyer LM, et al. Cost-effectiveness of insertable cardiac monitors to identify atrial fibrillation after cryptogenic stroke in the United States. In: HRS 2020. Online conference. 2020.
American College of Cardiology. mSToPS: active screening for AFib using ECG patch associated with significant improvement in outcomes at three years. 2020. https://www.acc.org/latest-in-cardiology/articles/2020/11/12/20/28/mon-1021am-mstops-aha-2020.
ClinicalTrials.gov. Apixaban for the reduction of thrombo-embolism in patients with device-detected sub-clinical atrial fibrillation (ARTESiA). 2020. https://clinicaltrials.gov/ct2/show/NCT01938248.
Nasir JM, et al. Predicting determinants of atrial fibrillation or flutter for therapy elucidation in patients at risk for thromboembolic events (PREDATE AF) study. Heart Rhythm. 2017;14:955–61.
Healey Jeff S, et al. Subclinical atrial fibrillation in older patients. Circulation. 2017;136(14):1276–83.
Philippsen TJ, et al. Detection of subclinical atrial fibrillation in high-risk patients using an insertable cardiac monitor. JACC Clin Electrophysiol. 2017;3(13):1557.
Kaplan RM, et al. Stroke risk as a function of atrial fibrillation duration and CHA(2)DS(2)-VASc score. Circulation. 2019;140:1639–46.
Singer DE, et al. Temporal association between episodes of atrial fibrillation and risk of ischemic stroke. JAMA Cardiol. 2021;6:1364–9.
Svennberg E, et al. Clinical outcomes in systematic screening for atrial fibrillation (STROKESTOP): a multicentre, parallel group, unmasked, randomised controlled trial. The Lancet. 2021;398:1498–506.
Chew D, et al. Cost-Effectiveness of extended ECG monitoring for detection of occult atrial fibrillation in patients with cryptogenic stroke. J Am Coll Cardiol. 2020;75(11 Supplement 1):315.
Acknowledgements
The authors would like to thank Marta Viola for her review of selected model inputs and Jana Tillotson for editorial support.
Funding
This cost-effectiveness analysis was funded by Medtronic.
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception and design of the study, collection and analysis of data and interpretation of results. LS, FGJ, CR, SR, PZ and DL were responsible for data collection; LS, FGJ and CR carried out the health economic analysis; ME, KW, SK and MR, provided advice on clinical validation of assumptions and data sources. All authors reviewed, edited and approved the manuscript and are responsible for its content.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
No ethics approval or consent to participate was required.
Consent for publication
Not applicable.
Competing interests
CR, LS and FG are employed by Symmetron Ltd, which received funding from Medtronic for this analysis. KW has received unconditional research funding from Medtronic to the University of Leeds for a Ph.D. Fellowship program and consultancy or speaker fees from Medtronic, Novartis, AstraZeneca, Pfizer, Napp, Abbott, Cardiac Dimensions, Microport. MR has received consultancy fees from Medtronic. SK has received consultancy fees from Medtronic and Bristol-Myers Squibb. ME’s institution received funding for his participation in these analyses, and ME receives study drug in kind but no personal compensation from the BMS-Pfizer Alliance for Eliquis and ancillary research funding from Roche, both for a National Institutes of Health-funded trial of stroke prevention in patients with atrial cardiopathy. SR, PZ, DL, and ST are Medtronic employees. The REVEAL AF study and this cost-effectiveness analysis were funded by Medtronic.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Supplementary information.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Elkind, M.S.V., Witte, K.K., Kasner, S.E. et al. Cost-effectiveness of an insertable cardiac monitor in a high-risk population in the US. BMC Cardiovasc Disord 23, 45 (2023). https://doi.org/10.1186/s12872-023-03073-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-023-03073-6