Abstract
Background
Left ventricular (LV) geometry is closely associated with cardiovascular disease; however, few studies have evaluated the relationship between basal septal hypertrophy (BSH) and LV geometry. In this study, we examined the relationship between BSH and LV geometry in a Beijing community population.
Methods
The clinical and echocardiographic data of 1032 participants from a community in Beijing were analyzed. BSH was defined as a basal interventricular septal thickness ≥ 14 mm and a basal septal thickness/mid-septal thickness ≥ 1.3. On the basis of their echocardiographic characteristics, patients were described as having a normal geometry, concentric remodeling, concentric hypertrophy, or eccentric hypertrophy. Multivariable logistic regression was used to analyze the relationship between BSH, LV mass index (LVMI), and relative wall thickness (RWT).
Results
The prevalence of BSH was 7.4% (95% confidence interval [CI] 5.8–9.0%). Basal and middle interventricular septal thickness, LV posterior wall thickness, and RWT were greater, while LVMI and LV end-diastolic dimension were lower in the BSH group than in the non-BSH group (p < 0.05). The BSH group accounted for the highest proportion of patients with concentric remodeling. A multivariable regression analysis showed that BSH increased by 3.99-times (odds ratio [OR] 3.99, 95% CI 2.05–7.78, p < 0.01) when RWT was > 0.42, but not when LVMI increased (OR 0.16, 95% CI 0.02–1.19, p = 0.07). There were no interactions between BSH and age, body mass index, sex, diabetes mellitus, coronary heart disease, stroke, and smoking in relation to an RWT > 0.42.
Conclusion
BSH was independently associated with an RWT > 0.42.
Similar content being viewed by others
Background
The rate of basal septal hypertrophy (BSH) is approximately 10% in the general population [1]. However, the prevalence of BSH varies by definition, age group, and comorbidities. For example, a previous study reported that the rate of BSH is 18% in older individuals, while the rate of BSH in hypertensive cohorts is approximately 20% [2].
BSH is not independently associated with an adverse cardiovascular prognosis [2]. However, some studies have demonstrated that BSH is an early manifestation of hypertension. Therefore, self-measured blood pressure and ambulatory blood pressure monitoring should be performed in all patients to improve the detection of hypertension [3]. Significant BSH is associated with left ventricular (LV) outflow tract obstruction and heart failure with preserved ejection fraction [1, 4], as well as with impaired LV diastolic function [2]. Moreover, LV geometry is closely associated with cardiovascular disease, especially in patients with hypertension [5]. However, few studies have evaluated the relationship between BSH and LV geometry. Therefore, we aimed to explore the relationship between BSH and LV geometry in a Beijing community population.
Methods
Population
All residents who lived in the Shi Jing Shan District of Beijing and who were aged > 40 years were invited to participate. The investigation methods have been published previously [6]. Of 5593 subjects, 1069 volunteered to participate. The investigation started in 2004 and ended in 2005. Participants who underwent echocardiography were recruited, while participants who had regional wall movement abnormalities, moderate or severe aortic valve stenosis, rheumatic heart disease, or congenital heart disease were excluded. Finally, 1032 participants were included. The study was approved by the institutional review board of Peking University First Hospital, and informed consent was obtained from all participants.
Definition of cardiovascular risk factors and disease
The methods used to measure height, weight, blood pressure, heart rate, fasting blood glucose, oral glucose tolerance, and blood lipid concentrations have been described previously [7]. Current smokers and participants with a history of smoking were defined as smokers. Hypertension was defined as a systolic blood pressure of ≥ 140 mmHg and/or a diastolic blood pressure of ≥ 90 mmHg or a history or usage of antihypertensive drugs. Diabetes mellitus was diagnosed according to each participant’s history. Participants with a fasting blood glucose concentration of ≥ 7.0 mmol/L and a 2 h glucose concentration of ≥ 11.1 mmol/L were also defined as having diabetes mellitus. BMI ≥ 28 kg/m2 was defined as obesity. Stroke, including intracerebral hemorrhage, cerebral infarction, and transient ischemic attack, was defined by the patient’s history. A history of myocardial infarction, percutaneous coronary intervention, and coronary artery bypass grafting were all included in coronary heart disease (CHD).
Echocardiography
Echocardiography was performed using a 3 MHz transducer and an ultrasound system (Vivid-7; General Electric). According to previously published guidelines [8], standard images were collected and stored. One experienced clinician who was blinded to the clinical picture of the participants measured the echocardiography parameters at the central laboratory of Peking University First Hospital.
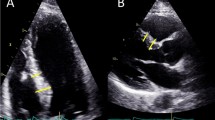
For the patients without BSH, LV end-diastolic dimension (LVEDD), LV end-systolic dimension and wall thicknesses (LVESD) including middle IVS thickness (MIVST), and LV posterior wall thickness (LVPWT) were measured at the mitral chordae level by parasternal long-axis view by 2 D method according to ASE guideline [8], basal interventricular septal thickness (BIVST) was measured simultaneously. For the patients with BSH, MIVST, LVPWT, LVEDD and LVESD were measured below the basal hypertrophy where the septal thickness was uniform, maximal BIVST thickness was measured simultaneously. LVEF was calculated by Teichholtz method. Left atrial diameter (LAD) was anteroposterior (AP) linear dimension obtained from the parasternal long-axis view in 2D image according to ASE guideline [8]. LV mass (LVM) was calculated as follows: LVM = 0.8 × 1.04 × ([PWTd + SWTd + LVIDd]3 − [LVIDd]3) + 0.6 g, where PWTd and SWTd are the posterior and middle septal wall thicknesses at LV end-diastole, respectively, and LVIDd is the LV dimension at end-diastole. LVM index (LVMI) was then calculated, as previously described [8].Relative wall thickness (RWT) was calculated using the following formula: (2 × LV PWT) ÷ LVEDD. An LVMI > 115 g/m2 (male) or > 95 g/m2 (female) was defined as increased LVMI, and RWT > 0.42 was defined as increased RWT as well. Normal geometry is defined as increased LVMI = 'NO' and increased RWT = 'NO' while concentric remodeling as increased LVMI = 'NO' and increased RWT = 'YES'. Concentric hypertrophy is defined as increased LVMI = = 'YES' and increased RWT = = 'YES' while eccentric hypertrophy as increased LVMI = 'YES' and increased RWT = 'NO' [8]
BSH was defined when all three of the following criteria were fulfilled [8]: (1) a basal IVS thickness ≥ 14 mm; (2) a basal IVS thickness/mid IVS thickness ≥ 1.3; and (3) no wall motion abnormalities or scarring in the middle septum that could result in isolated septal thickening.
Statistical analysis
Continuous data with normal distribution are presented as mean ± standard deviation, while presented as median plus quartile when the data are abnormal distribution. Count data are presented as percentages. Student t test was used to compare between the two groups when continuous data are normal distribution. Continuous data were compared between the two groups using non-parametric test (Median Test for k samples) when the data are abnormal distribution. Categorical data were compared between the two groups using the Chi square test or the Fisher’exact test if needed.
Multivariable logistic regression was used to analyze the relationship between BSH, the increase in LVMI, and the increase in RWT, adjusting for age, sex, obesity, hypertension, diabetes mellitus, and heart rate concerned about univariable logistic regression analysis results and clinical significance. Subgroup analyses and interaction tests were used to examine the relationship between BSH and the increase in RWT according to age (< 60 years and ≥ 60 years), sex (male and female), BMI (< 24 kg/m2 and ≥ 24 kg/m2), diabetes mellitus (yes or no), CHD (yes or no), stroke (yes or no), and smoking status (yes or no) by multivariable logistic regression. The intraclass correlation coefficient was used to evaluate intra-observer consistency. A two-sided p value of < 0.05 was considered statistically significant for all tests. All analyses were performed using statistical software (Empower (R) [www.empowerstats.com]; X&Y solutions, Inc., Boston, MA, USA; R [http://www.R-project. org] v3.4.3; SPSS v13.0).
Results
The intra-observer values for BIVST and MIVST were 0.86 (95% confidence interval [CI] 0.65–0.96, p < 0.01) and 0.81 (95% CI 0.52–0.93, p < 0.01), respectively. The general characteristics of the participants are shown in Table 1. The median age of the participants was 65 years, and 51.8% of the participants were male. The prevalence of BSH was 7.4% (95% CI 5.8–9.0%). Participants in the BSH group were older. The prevalence of diabetes mellitus and obesity were also higher in the BSH group than in the non-BSH group (p < 0.05).
The echocardiographic parameters of the participants are shown in Table 2. Compared with levels in the non-BSH group, basal and middle IVS thickness, LVPW thickness, and RWT were greater, while LVMI and LVEDD were lower in the BSH group (p < 0.01). The BSH group accounted for the highest proportion of participants with concentric remodeling, with approximately 84.2% of participants being from the BSH group and 48.7% of participants being from the non-BSH group (p < 0.01).
The multivariate regression analysis showed that BSH increased by 3.99-times (odds ratio [OR] 3.99, 95% CI 2.05–7.78, p < 0.01) when RWT was > 0.42, but not when LVMI increased (OR 0.16, 95% CI 0.02–1.19, p = 0.07). Detailed information is presented in Table 3. The results of the subgroup analysis showed that there were no interactions between BSH and the covariates of age, BMI, sex, diabetes mellitus, CHD, stroke, and smoking in relation to the increase in RWT. The details are shown in Fig. 1.
Discussion
BSH detected by routine echocardiography is prominent in older individuals. In this study, BSH was independently associated with an increase in RWT, but not with an increase in LVMI. The subgroup analysis showed no interactions between BSH and the covariates of age, sex, BMI, diabetes mellitus, CHD, stroke, and smoking status in relation to the increase in RWT.
The mechanism leading to BSH is unclear. BSH is recognized early in patients with essential hypertension [9]. This localized thickening decreases in response to antihypertensive treatment [10]. Thus, BSH might be valuable to detect masked hypertension in the general population [3]. Central blood pressure correlates with basal IVS thickness, but not with mid-IVS thickness [11]. Thus, stricter blood pressure management and hypertension screening should be conducted in patients with BSH. A previously published cohort study showed that hypertensive subjects with BSH were older than non-BSH subjects and had a higher BMI and systolic blood pressure [12]. In our study, patients in the BSH group were older than those in the non-BSH group, while the systolic blood pressure tended to be higher in patients with BSH than in those without, although the difference was not significant. There was no difference in the prevalence of hypertension between the BSH group and the non-BSH group, which may be because the overall population was older and the prevalence of hypertension was higher in our study. In the present study, the BSH group had a higher incidence of diabetes mellitus, which is in contrast to the study of Loncaric et al., who observed no difference in the incidence of diabetes mellitus between the BSH group and the non-BSH group [12], which might be due to the younger age and lower prevalence of diabetes in this group.
A previous cohort study showed that hypertensive patients with BSH had a higher LV ejection fraction and lower LV end-diastolic and end-systolic volumes, while no significant differences were observed in left atrial size between the two groups [12]. In the present study, we found that LVEDD was lower in patients with BSH (p < 0.05). Unlike previous studies [12], the LVMI in the BSH group was lower than in the non-BSH group in our study, which may be related to the baseline level of the included populations. Early clinical studies suggested that BSH is related to LV diastolic function [2]. Another study showed that BSH is less likely to cause increased LV stiffness without LV hypertrophy [13], but that it can affect LV diastolic function during stress [14]. BSH is related to cardiac function in patients with hypertension with well-controlled blood pressure. Basal and mid-posterior wall systolic deformation, LV diastolic function, and left atrial function are decreased in these patients [12]. Thus, such patients are prone to heart failure with preserved ejection fraction.
In the present study, concentric remodeling was the most frequent LV geometry since the higher prevalence of hypertension in our cohort. CR was the most frequent LV geometry in patients with BSH as well which imply that CR was the main LV geometry type in BSH people. A retrospective analysis of a large population (n = 35,602) showed an abnormal LV geometry in 46% of patients, with concentric remodeling present in 35% of patients and LV hypertrophy present in 11% of patients [5]. Patients with hypertension had race-related differences in LV geometry and RWT. A descriptive study previously reported that Africans exhibited a greater IVS thickness and RWT than Caucasians [15]. A study with a mean follow-up period of 2.5 years assessed the effect of potential changes in cardiac structure and found that 1610 patients (45%) demonstrated no change in LV geometry and maintained a pattern of concentric remodeling, 439 patients (12%) progressed to LV hypertrophy, and 1567 patients (43%) converted to a normal LV geometry. There was a strong relationship between an abnormal LV geometry and all-cause mortality. Patients with concentric remodeling and LV hypertrophy exhibited considerably higher mortality than patients with a normal LV geometry [5]. An American population-based case–control study showed that concentric remodeling is associated with stroke risk [16]. A prospective study showed that all-cause mortality was significantly more likely in patients with concentric remodeling (hazard ratio 1.417, 95% CI 1.045–1.920) [17]. Therefore, follow-up and risk factor control of patients with concentric remodeling should be strengthened to reduce the occurrence of cardiovascular events, but we did not pay sufficient attention to LV geometry in a real-world setting.
In this study, BSH independently correlated with an increase in RWT. A previous study showed that an increase in RWT is a strong independent predictor of mortality [5]. RWT significantly increases stroke risk, but no interactions have been detected between RWT and LVM [16]. A prospective study showed that RWT is an independent predictor of all-cause and cardiovascular mortality in patients who experience ischemic stroke, whereas the association between LVMI and all-cause death is not significant [17]. In the present study, hypertensive patients with BSH demonstrated a greater RWT and accounted for the highest proportion of patients with concentric remodeling. Therefore, if LV geometry is routinely measured in clinical practice, cardiovascular risk in patients with BSH may be increased.
Our study has several limitations that should be noted. First, because of the cross-sectional study design, a causal relationship between BSH and LV geometry could not be determined. Prospective studies examining whether BSH is predictive of LV geometry and cardiovascular events are required. Second, the majority of patients were aged > 40 years; thus, our findings may not reflect the characteristics of BSH in a younger population. Finally, according to our inclusion criteria, some patients with hypertrophic cardiomyopathy may have been included in this study. And all patients were volunteer, which might affect the result in general population.
Conclusion
In this study, we showed that patients with BSH accounted for the highest proportion of patients with concentric remodeling. BSH independently correlated with an increase in RWT. Subgroup analysis showed that there were no interactions between BSH and the covariates of in relation to the increase in RWT.
Availability of data and materials
The datasets generated and analyzed during the current study are not publicly available owing to data security issues, but are available from the corresponding author on reasonable request.
Abbreviations
- BSH:
-
Basal septal hypertrophy
- BMI:
-
Body mass index
- CHD:
-
Coronary heart disease
- CI:
-
Confidence interval
- CR:
-
Concentric remodeling
- CH:
-
Concentric hypertrophy
- EH:
-
Eccentric hypertrophy
- IVS:
-
Interventricular septal
- LV:
-
Left ventricular
- LVM:
-
Left ventricular mass
- LVMI:
-
Left ventricular mass index
- LVEDD:
-
Left ventricular end-diastolic dimension
- LVESD:
-
Left ventricular end-systolic dimension
- NG:
-
Normal geometry
- OR:
-
Odds ratio
- PWT:
-
Posterior wall thickness
- PWTd:
-
Posterior wall thicknesses at LV end-diastole
- RWT:
-
Relative wall thickness
- SWTd:
-
Septal wall thicknesses at LV end-diastole
References
Kelshiker MA, Mayet J, Unsworth B, Okonko DO. Basal septal hypertrophy. Curr Cardiol Rev. 2013;9:325–30.
Diaz T, Pencina MJ, Benjamin EJ, Aragam J, Fuller DL, Pencina KM, et al. Prevalence, clinical correlates, and prognosis of discrete upper septal thickening on echocardiography: the Framingham Heart Study. Echocardiogr (Mount Kisco, NY). 2009;26:247–53.
Gaudron PD, Liu D, Scholz F, Hu K, Florescu C, Herrmann S, et al. The septal bulge–an early echocardiographic sign in hypertensive heart disease. J Am Soc Hypertens JASH. 2016;10:70–80.
Pearson AC. The evolution of basal septal hypertrophy: from benign and age-related normal variant to potentially obstructive and symptomatic cardiomyopathy. Echocardiogr (Mount Kisco, NY). 2017;34:1062–72.
Milani RV, Lavie CJ, Mehra MR, Ventura HO, Kurtz JD, Messerli FH. Left ventricular geometry and survival in patients with normal left ventricular ejection fraction. Am J Cardiol. 2006;97:959–63.
Zhang L, Zuo L, Wang F, Wang M, Wang S, Lv J, et al. Cardiovascular disease in early stages of chronic kidney disease in a Chinese population. J Am Soc Nephrol. 2006;17:2617–21.
Liu L, Zhao F, Yang Y, Qi LT, Zhang BW, Chen F, et al. The clinical significance of carotid intima-media thickness in cardiovascular diseases: a survey in Beijing. J Hum Hypertens. 2008;22:259–65.
Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr Off Publ Am Soc Echocardiog. 2005;18:1440–63.
Verdecchia P, Porcellati C, Zampi I, Schillaci G, Gatteschi C, Battistelli M, et al. Asymmetric left ventricular remodeling due to isolated septal thickening in patients with systemic hypertension and normal left ventricular masses. Am J Cardiol. 1994;73:247–52.
Sakurai S, Ashida T, Takahashi N, Fujii J. Effects of antihypertensive treatment on the thickening of the basal portion of the interventricular septum in essential hypertension. J Cardiol. 1998;31:151–8.
Olafiranye O, Ibrahim M, Kamran H, Venner-Jones K, McFarlane SI, Salciccioli L, et al. Narrowed aortoseptal angle is related to increased central blood pressure and aortic pressure wave reflection. Cardiorenal Med. 2012;2:177–83.
Loncaric F, Nunno L, Mimbrero M, Marciniak M, Fernandes JF, Tirapu L, et al. Basal ventricular septal hypertrophy in systemic hypertension. Am J Cardiol. 2020;125:1339–46.
Sengupta PP, Khandheria BK, Narula J. Twist and untwist mechanics of the left ventricle. Heart Fail Clin. 2008;4:315–24.
Henein MY, O’Sullivan C, Sutton GC, Gibson DG, Coats AJ. Stress-induced left ventricular outflow tract obstruction: a potential cause of dyspnea in the elderly. J Am Coll Cardiol. 1997;30:1301–7.
Ejiofor L, Di Nora C, Cervesato E, Cosei I, Ravasel A, Popescu BA, et al. Differences in left ventricular geometry in hypertensive African-Europeans and Caucasian patients. Eur J Intern Med. 2019;62:43–7.
Di Tullio MR, Zwas DR, Sacco RL, Sciacca RR, Homma S. Left ventricular mass and geometry and the risk of ischemic stroke. Stroke. 2003;34:2380–4.
Park CS, Park JB, Kim Y, Yoon YE, Lee SP, Kim HK, et al. Left ventricular geometry determines prognosis and reverse J-shaped relation between blood pressure and mortality in ischemic stroke patients. JACC Cardiovasc Imaging. 2018;11:373–82.
Acknowledgements
None.
Funding
This study was financially supported by the National Key Research and Development Program of China (Subject No. 2021YFC2501106, Project No. 2021YFC2501100).
Author information
Authors and Affiliations
Contributions
Manuscript drafting: LG & WM; conception: WM & LG; study design: WM & BZ; interpretation of data: WM, ML, & YY; analysis: WM & ML; data acquisition: WM, CW, LQ, LG, & YY; supervision: WM, YZ, & YH. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The project has been approved by The Committees of Peking University First Hospital (Approval No.: 2020Y328). Informed consent from participants was waived by the Peking University First Hospital Human Research Ethics Committee. All the study procedures were conducted following the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Gao, L., Ma, W., Li, M. et al. Association between basal septal hypertrophy and left ventricular geometry in a community population. BMC Cardiovasc Disord 22, 579 (2022). https://doi.org/10.1186/s12872-022-03004-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-022-03004-x