Abstract
Background
To investigate the relationship between the clinical features and progression of non-culprit lesions in patients with ST-elevation myocardial infarction (STEMI) after primary percutaneous coronary intervention (PPCI).
Methods
A total of 480 patients (57.1 ± 9.2 y) with STEMI who underwent PPCI between January 2016 and December 2017 in Beijing Anzhen Hospital were enrolled in this study. All patients underwent PPCI as a treatment for culprit lesions. Clinical and angiographic follow-up were performed for 12 months. All patients were divided into a non-culprit lesions (NCL) progression group (205 cases) and a control group (275 cases) based on angiographic follow-up outcomes at 12 months. The clinical and angiographic features were analyzed.
Results
Body mass index (BMI), serum creatinine (Scr), fasting blood glucose (FBG), glycated serum albumin, glycated hemoglobin and homocysteine levels in the NCL progression group were significantly higher than those in the control group (P < 0.05). A logistic regression analysis showed that FBG (odds ratio 1.274, 95% confidence interval 1.077–1.505, P = 0.005) and Scr (odds ratio 1.020, 95% confidence interval 1.002–1.038, P = 0.027) were independent predictors of NCL progression. A partial correlation analysis showed that FBG was positively correlated with NCL progression (r = 0.231, P = 0.001). A receiver operating characteristic curve showed that the boundary point of FBG to predict NCL progression was 5.715 mmol/L, and the sensitivity was 74.4% and the specificity was 46.4%.
Conclusion
FBG is a valuable predictor for NCL progression in patients with STEMI after PPCI.
Similar content being viewed by others
Background
Primary percutaneous coronary intervention (PPCI) can salvage dying myocardium, reduce cardiovascular events, and improve prognosis in patients with ST-elevation myocardial infarction (STEMI). However, recent clinical studies have shown that non-culprit lesions (NCLs) may progress after PPCI and the progression of NCLs could be the most significant factor affecting prognosis after PPCI [1, 2]. However, the clinical correlation factors for NCL progression are not clear. In the current study, we investigate the relationship between the clinical features and the progression of NCL in patients with STEMI after PPCI.
Methods
All participants or their family members were informed about the potential publication of their identities and images, and all of them completed consent forms. All procedures and protocols were approved by the ethics committee of Beijing Anzhen Hospital, Capital Medical University, and the experiments were conducted in accordance with the Declaration of Helsinki (1975 and subsequent revisions).
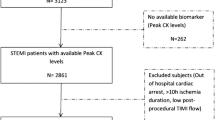
Between January 2016 and December 2017, 480 patients (400 men and 80 women) with acute STEMI who underwent PPCI treatment in Beijing Anzhen Hospital were enrolled in this retrospective study. Clinical and angiographic follow-up was performed in all patients for 12 months. The inclusion criteria were as follows. (1) Acute myocardial infarction lasting for < 12 h and only one NCL found in the setting of STEMI. Acute myocardial infarction was defined as follows: evidence of ischemic chest pain lasting for > 30 min, and new ST segment elevation of ≥ 2 mm in two or more contiguous electrocardiographic leads; a de novo lesion; single vessel treatment in a native vessel ≥ 2.5 mm in diameter and occluded, thrombus containing; thrombolysis in myocardial infarction (TIMI) flow grade of 0 to 2 in the culprit artery, and the grade of stenosis of NCLs was < 70%. (2) There was no contraindication for anticoagulation and antiplatelet therapy.
The main exclusion criteria included the following: previous percutaneous coronary intervention (PCI) in an infarction-related artery (IRA) (n = 7), Killip class ≥ 3 (n = 8), left or right bundle branch block (n = 10), IRA with excessive proximal tortuosity or severe calcification (n = 13), left ventricular ejection fraction < 35% (n = 14,), lack of clinical and angiographic follow-up (n = 25), in-hospital death after PPCI (n = 10,included 6 patients with cardiac shock ), myocardial infarction within 2 w of PPCI to exclude potential subacute stent thrombosis of the intervened arterial segment (n = 8), and repeated PCI of culprit coronary lesions for restenosis or progression (n = 41).
Coronary angiography was performed using the Judkins method, and coronary artery lesion classification was based on the American College of Cardiology/American Heart Association guidelines [3]. Thrombus aspiration catheters (DIVER CE, Invatec, Brescia, Italy) were used for thrombotic burden lesions. Stents were implanted using a routine method, and the procedure succeeded with residual stenosis < 20%, TIMI flow grade of 3 and no acute complications (death, myocardial infarction, emergency coronary artery bypass grafting (CABG)), and no major adverse cardiac events (cardiac death, myocardial infarction, target vessel revascularization). Clinical and angiography follow-up was performed for 12 months.
The culprit coronary lesions were clearly identified through a combination of electrocardiography and coronary angiography. NCLs were defined as those with a diameter of stenosis < 70%. All patients underwent PPCI for the culprit lesions.
Quantitative coronary angiography was performed during the first angiography. Follow-up angiography was performed by two independent investigators who were blinded to the results. We categorized the lesions in accordance with the American College of Cardiology/American Heart Association. Classification was performed on the basis of the morphological characteristics of lesions that cause significant stenosis of the coronary arteries [3]. These include two categories of simple lesions (A or B1 lesions) and complex lesions (B2 or C).
The collected data included demographic information, medical history, coronary artery disease risk factor status, detailed coronary angiographic information, biomarkers associated with coronary atherosclerosis at the time of baseline PCI, and coronary angiographic information at the time of angiographic follow-up.
All clinical, laboratory, and coronary angiographic data were evaluated by two independent investigators who were not involved in the angiographic procedures.
Definition of NCL progression [3]: (1) The stenosis degree of the NCL was ≥ 50% at the time of baseline PCI, and the degree of NCL progression was ≥ 10% at the time of angiographic follow-up. (2) The stenosis degree of the NCL was < 50% at the time of baseline PCI, and the degree of NCL progression was ≥ 30% at the time of angiographic follow-up. (3) The degree of NCL progression ≥ 30%, while there were no NCLs at the time of baseline PCI. (4) NCL progression to total occlusion.
Hypertension was defined as systolic blood pressure > 140 mmHg (1 mmHg = 0.133 kPa)/and/or diastolic blood pressure > 90 mmHg, or patients were taking antihypertensive drugs, in accordance with the 2010 Chinese Hypertension Prevention Guide revised edition [4].
Diabetes was defined as the typical symptoms of diabetes (drinking more, polyphagia, polyuria, weight loss) and fasting plasma glucose > 7.0mmol/L or blood sugar > 11.1mmol/L 2 h after an oral glucose tolerance test, in accordance with the China Guideline for the Prevention of Type 2 Diabetes (2017 Edition) [5].
SPSS20.0 software was used for all statistical analyses. Count data are expressed as cases and percentages, and the χ2 test was used for analysis. Numerical data are expressed as mean ± SD and were compared using the Student’s t test. Non-normally distributed numerical data are expressed as the median and 25th–75th interquartile range and were compared using a rank-sum test. A partial correlation analysis was used to evaluate the correlations between fasting blood glucose (FBG), and progression of NCL. Binary logistic regression analysis was performed to examine independent risk factors for the progression of NCL. Receiver-operating characteristic (ROC) analysis and a calculation of sensitivity and specificity were performed to test the ability of FBG to predict the progression of NCL. A P value of less than 0.05 was considered statistically significant.
All patients were divided into the control group (without NCL progression) and the progression group (with NCL progression) in accordance with the definition of NCL progression.
Results
There were 205 (177 men and 28 women) patients without NCL progression (the control group) and 275 (222 men and 53 women) patients with NCL progression (the progression group) (Fig. 1).
There were no significant differences in age, sex, history of diabetes mellitus, and rates of hyperlipidemia, smoking, myocardial infarction, PCI, CABG, heart rate, systolic arterial pressure, left ventricular ejection fraction (LVEF), cardiac troponin I (cTnI) peak value, triglyceride (TG), total cholesterol (TCHO), high density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), total three-triiodothyronine (TT3), total thyroxine (TT4), free triiodothyronine (FT3), C reactive protein (CRP), uric acid (UA), time from attack to reperfusion, myocardial blush grade (MBG) of 0–1 in the culprit artery, pre-dilation rate, thrombotic lesion rate, ≥ two vessel lesion rate, collateral circulation rate, culprit lesion length, complex lesion rate, and degree of baseline stenosis between the two groups (all P > 0.05) (Table 1).
The average of LVEF seem to be higher than expected ( 53% in average, in both groups) for STEMI patients. It may be involved in the Teichholz method.
There were significant differences in BMI (P < 0.001), FBG (P < 0.001), glycated albumin (GA) (P < 0.01), hemoglobinA1c (HbA1c) (P < 0.01), homocysteine (Hcy) (P < 0.001), serum creatinine (Scr) (P < 0.001), and the degree of follow-up stenosis(P < 0.001) between the two groups (Table 1).
In terms of medications, patients received a similar amount of β-blockers (62% vs. 65%), calcium antagonists (30% vs. 28%), ACEI/ARB (56% vs. 54%), and statins (91% vs. 89%) in each group (all P > 0.05) (Table 1).
Multivariate logistic regression analysis indicated that FBG (OR 1.274, 95% CI 1.077–1.505, P = 0.005) and Scr (OR 1.020, 95% CI 1.002–1.038, P = 0.027) were independent predictors of the progress of NCL after primary PCI in patients with STEMI (P < 0.05) (Table 2).
Partial correlation analysis showed that FBG was positively correlated with NCL progression (r = 0.231, P = 0.001) .
ROC analysis for the predictors of NCL progression indicated that an FBG level ≥ 5.715 mmol/L may predict NCL progression. The sensitivity was 74.4% and the specificity was 76.6% (AUC: 0.613, SEM: 0.041, 95% CI 0.532–0.693, P < 0.008) (Fig. 2).
Discussion
PPCI in a culprit artery is the preferred strategy for treating patients with acute STEMI. However, approximately 40–65% of patients with STEMI present with three-vessel lesions. A clinical follow-up study of patients with three-vessel lesions after successful PCI suggested that NCL progression may be occurring [1]. This may be the most important factor that affects the prognosis of patients with acute myocardial infarction after successful PCI.
However, there have been few studies on the clinical predictors for progression of NCLs. Tsiamis et al. [6] performed follow-up angiography in 117 patients with acute coronary syndrome. These authors suggested that NCLs may have progressed, and acute myocardial infarction may be an independent predictive factor for the progression of NCLs.
Our follow-up study on the progression of NCLs suggested that this progression may be the most important prognostic factor in patients with STEMI after successful PCI [7]. Our data suggested that the progression of NCLs could involve inflammation and a stress mechanism, and a high dosage of ramipril may inhibit NCL progression, which could be the main cause of revascularization after PPCI for patients with STEMI.
In the present study, we investigated the clinical prediction factors of NCL progression in patients with STEMI after PPCI. We carried out a 12-month clinical and angiographic follow-up in 480 patients, and found that there were significant differences in BMI, FBG, GA, HbA1c, Hcy, and Scr between the control group and the progression group, indicating that BMI, FBG, GA, HbA1c, Hcy,and Scr may be clinical correlation factors of NCL progression in patients with STEMI after PPCI.
Elevated plasma Hcy is an independent risk factor for atherosclerosis, which is recognized as inflammatory and immune responses. In the present study, we suggest that Hcy promote NCL progression by regulating inflammatory and immune responses.
In the current study, there were no significant differences in the patient characteristics and medical history between the control group and the progression group. Additionally, all patients received comparable medication.
Multivariate logistic regression analysis indicated that FBG and Scr were independent predictors of the progress of NCL after primary PCI in patients with STEMI. It showed glycometabolism disorder and renal insufficiency may promote the progress of NCL after primary PCI in patients with STEMI.
Partial correlation analysis showed that FBG levels were positively correlated with NCL progression. ROC analysis for the predictors of NCL progression indicated that a fasting glucose level ≥ 5.715 mmol/L may predict NCL progression, and the sensitivity was 74.4% and the specificity was 76.6%. These results indicated that elevated FBG may be an independent predictor of NCL progression in STEMI patients who underwent primary PCI.
Both the R square value of the partial correlation analysis and the AUC of the ROC curve were rather low, in particular the AUC which is just over 60% and whose lower limit is just 0.532,
although there were significant difference. This may be related to patients that enrolled were not enough in this study.
-
The cut-off for blood glucose, which also involves satisfactory sensitivity and specificity, is 5.715 mmol/L, and very close to normal values. It indicated that strict glycaemic control is needed in the post-infarction period, and consequently an aggressive treatment to keep glycaemia low is recommended.
Diabetes is an independent risk factor for coronary artery disease. Compared with the non-diabetic population, diabetes is associated with a two–three-fold increase in the risk of cardiovascular disease and mortality because of cardiovascular disease [6]. Previous studies have shown that glucose metabolism plays a role in the development and prognosis of coronary heart disease. Even patients with mildly elevated blood glucose levels are more prone to acute myocardial infarction than patients with healthy blood glucose levels [7]. Berry et al. [8] found that FBG, HbA1c and a history of diabetes are associated with the severity and progression of coronary atherosclerosis. In the current study, fasting glucose and creatinine levels were found to be independent predicters of NCL progression, and fasting glucose was positively correlated with NCL progression in a partial correlation analysis. Increased secretion of high levels of catecholamine, glucocorticoids and other hormones in acute myocardial infarction can enhance liver glycogen decomposition and inhibit glycogen production [9].In addition to upregulating glucose production, insulin resistance and impaired glucose uptake mechanisms during critical disease jointly lead to the occurrence of hyperglycemia [10, 11]. Hyperglycemia leading to plaque progression may involve the following mechanisms [12] : (1) Non-enzymatic glycation of proteins and lipids increases, and the formation of reactive higher glycation end products, resulting in mechanical dysfunction of the vascular wall. This obstructs circulating blood cells and causes them to adhere to the blood vessel wall, and also interferes with cell function by binding to a variety of receptors on macrophages, endothelial cells and other cells, increasing pro-inflammatory signal transduction and promoting inflammation of the blood vessel wall. (2) During hyperglycemia, insulin receptor substrate 1 is down-regulated and the cells become resistant to insulin. The insulin-like growth factor 1 receptor sends signals through other alternative scaffold proteins to induce vascular smooth muscle cells to dedifferentiate, migrate and proliferate. (3) By activating protein kinase C, hyperglycemia causes many abnormal changes related to atherosclerosis, such as increased vascular permeability, endothelial dysfunction and reduced production of nitric oxide, resulting in impaired vasodilation, increased apoptosis, and increased production of extracellular matrix.
Conclusion
The results of the current study suggest that BMI, FBG, GA, HbA1c, and Hcy may be clinical correlation factors for NCL progression in patients with STEMI after PPCI. FBG and Scr were independent predictors of the progress of NCL after primary PCI in patients with STEMI. This study is a single-center retrospective analysis, with a relatively small sample size and a lack of detailed intravascular imaging data, and there is a need for further randomized prospective controlled studies.
Availability of data and materials
Please contact author for data requests.
Abbreviations
- STEMI:
-
ST-segment elevation myocardial infarction
- PPCI:
-
Primary percutaneous coronary intervention
- BMI:
-
Body mass index
- FBG:
-
Fasting blood glucose
- GA:
-
Glycated albumin
- HbA1c:
-
Hemoglobin A1c
- PCI:
-
Percutaneous coronary intervention
- CABG:
-
Coronary artery bypass grafting
- ACEI/ARB:
-
Angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker
- LVEF:
-
Left ventricular ejection
- TG:
-
Triglyceride
- TCHO:
-
Total cholesterol
- HDL-C:
-
High density lipoprotein cholesterol
- LDL-C:
-
Low-density lipoprotein cholesterol
- Hcy:
-
Homocysteine
- Scr:
-
Serum creatinine
- TT3:
-
Total three-triiodothyronine
- TT4:
-
Total thyroxine
- FT3:
-
Free triiodothyronine
- CRP:
-
C-reactive protein
- UA:
-
Uric acid
- MBG:
-
Myocardial blush grade
References
Varenhorst C. Hasvold P, Johansson S. Janzon M, Albertsson P. Leosdottir M, et al Culprit and nonculprit recurrent ischemic events in patients with myocardial infarction: data from SWEDEHEART (swedish web system for enhancement and development of evidence-based care in heart disease evaluated according to recommended therapies). J Am Heart Assoc. 2018; 7:e007174.
Park M, Seung K, Kim P, Park H, Yoon S, Baek J, et al. Long-term percutaneous coronary intervention rates and associated independent predictors for progression of nonintervened nonculprit coronary lesions. Am J Cardiol. 2009;104:648–52.
Zheng JL. Lu L, Hu J. Zhang RY, Zhang Q. Chen QJ, et al Increased serum YKL-40 and C-reactive protein levels are associated with angiographic lesion progression in patients with coronary artery disease. Atherosclerosis. 2010; 210:590–5.
Wang JG. Chia YC, Chen CH. Park S, Hoshide S. Tomitani N, et alWhat is new in the 2018 chinese hypertension guideline and the implication for the management of hypertension in Asia? J Clin Hypertens (Greenwich). 2020; 22:363–8.
Chinese Elderly Type 2 Diabetes Prevention and Treatment of Clinical Guidelines Writing Group, Geriatric Endocrinology and Metabolism Branch of Chinese Geriatric Society, Geriatric Endocrinology and Metabolism Branch of Chinese Geriatric Health Care Society, et al. Clinical guidelines for prevention and treatment of type 2 diabetes mellitus in the elderly in China (2022 edition). Zhonghua nei ke za zhi. 2022;61:12–50.
Cosentino F. Grant PJ, Aboyans V. Bailey CJ, Ceriello A. Delgado V, et al2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020; 41:255–323.
Khan I. Siddiqui AH, Singhal S. Aslam M, Perwez A. Faraz A. Glycaemic status in patients of acute myocardial infarction: a detailed analysis. Diabetes Metab Syndr. 2016;10(1 Suppl 1):140–3.
Berry C. Noble S, Grégoire JC. Ibrahim R, Levesquie S. Lavoie MA, et al Glycaemic status influences the nature and severity of coronary artery disease. Diabetologia. 2010; 53:652–8.
Langouche L. Glucose metabolism and insulin therapy. Crit Care Clin. 2006;22:119–29., Van den Berghe G.
Zhang JW. Zhou YJ, Cao SJ. Yang Q, Yang SW. Nie B. Impact of stress hyperglycemia on in-hospital stent thrombosis and prognosis in nondiabetic patients with ST-segment elevation myocardial infarction undergoing a primary percutaneous coronary intervention. Coron Artery Dis. 2013;24:352–6.
Bartnik M, Rydén L, Ferrari R, Malmberg K, Pyörälä K, Simoons M, et al. The prevalence of abnormal glucose regulation in patients with coronary artery disease across Europe. The Euro Heart Survey on diabetes and the heart. Eur Heart J. 2004;25:1880–90.
Katakami N. Mechanism of development of athersclerosis and cardiovascular disease in diabetes mellitus. J Atheroscler Thromb. 2018;25(1):27–39.
Acknowledgements
We thank Conn Hastings, PhD, from Liwen Bianji, Edanz Editing China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Funding
The study was supported by Natural Science Foundation of Qinghai Province (2019-ZJ-929). The funders of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Author information
Authors and Affiliations
Contributions
JW was responsible for the study concept and design. CYY, WW, and TZW were responsible for the acquisition and analysis of data. All authors contributed to the interpretation of the data. JW and TZW drafted the manuscript. The corresponding author attests that all listed authors meet authorship criteria. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
and consent to participate:This study was a retrospective study.This study conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in approval by the Ethics Committee of Beijing Anzhen Hospital,Capital Medical University (2015-43) for our team to access the clinical/personal patient data used in our research. Written informed consent was obtained from all participants before this study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, J., Yan, Cy., Wang, W. et al. The clinical prediction factors for non-culprit lesion progression in patients with acute ST elevation myocardial infarction after primary percutaneous coronary intervention. BMC Cardiovasc Disord 22, 529 (2022). https://doi.org/10.1186/s12872-022-02974-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-022-02974-2