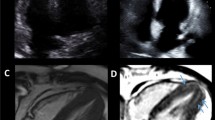
Abstract
Background
Cardiac magnetic resonance (CMR) has been used to diagnose and risk-stratify patients with left ventricular noncompaction (LVNC). The prognostic value of CMR parameters for LVNC, especially feature tracking (CMR-FT), is not well known in LVNC patients with left ventricular dysfunction. The present study aimed to investigate whether the combination of CMR-FT with traditional CMR parameters can increase the prognostic value of CMR for LVNC patients with reduced left ventricular ejection fraction (LVEF).
Methods
A total of 123 candidates were retrospectively included in this multicenter study and 55 LVNC patients (mean age, 45.7 ± 16.2 years; 61.8% men) remained after applying the exclusion criteria. Clinical features, left ventricular (LV) function parameters, global and segment myocardial strain, and late gadolinium enhancement (LGE) were evaluated. The outcomes include the composite events of cardiovascular death, heart transplantation, hospitalization for heart failure, thromboembolic events, and ventricular arrhythmias.
Results
After a median follow-up of 5.17 years (interquartile range: 0.17 to 10.58 years), 24 (36.8%) patients experienced at least one major adverse cardiovascular event (MACE). The myocardial strain parameters of patients with events were lower than those of patients without events. In the univariable Cox analysis, LVEF, the presence of LGE, global longitudinal strain (GLS) and segmental strains, including longitudinal strain at the apical level and radial and circumferential strain at the basal level, were significantly associated with MACEs. In the multivariate analysis, LGE (hazard ratio (HR) 3.452, 95% CI 1.133 to 10.518, p = 0.029) was a strong predictor of MACEs and significantly improved the predictive value (chi-square of the model after adding LGE: 7.51 vs. 13.47, p = 0.009). However, myocardial strain parameters were not statistically significant for the prediction of MACEs after adjusting for age, body mass index, LVEF and the presence of LGE and did not increase the prognostic value (chi-square of the model after adding GLS: 13.47 vs. 14.14, p = 0.411) in the multivariate model.
Conclusions
The combination of CMR-FT with traditional CMR parameters may not increase the prognostic value of CMR in LVNC patients with reduced LVEF, while the presence of LGE was a strong independent predictor of MACEs and significantly improved the predictive value.
Similar content being viewed by others
Background
Left ventricular noncompaction (LVNC) is a rare congenital disorder that is characterized by a bilayer myocardium with compacted and noncompacted layers, prominent trabeculations, and deep intertrabecular recesses connected with the left ventricular cavity [1]. LVNC is considered as a clinical phenotype with prognostic heterogeneity [2]. Myocardial dysfunction/heart failure is one of the major clinical manifestations and the main indicators of treatment and is largely associated with the outcomes of patients with LVNC [3,4,5]. However, predicting the prognosis of LVNC with left ventricular dysfunction is challenging and unclear. To guide individualized treatment strategies and monitoring in LVNC with left ventricular dysfunction, risk stratification tools are necessary.
Cardiac magnetic resonance (CMR) is the technique of choice for the diagnosis, and early detection of cardiomyopathy and the severity grading of LVNC [6]. Left ventricular dilation, left ventricular systolic dysfunction, and late gadolinium enhancement (LGE) have prognostic effects for patients with LVNC as evaluated using CMR [6,7,8,9]. With the emergence and development of new CMR imaging modalities, myocardial strain by CMR feature tracking (CMR-FT) has been proven to be a sensitive indicator for the assessment of abnormal cardiac deformation and plays an important role in the prediction of a series of heart diseases [10]. And CMR-FT significantly increases the diagnosis and prediction efficiency of adverse cardiovascular events when combined with the baseline clinical variables, left ventricular ejection fraction (LVEF) and LGE in a series of cardiomyopathies[11,12,13]. Although the studies on LVNC patients reported that the strain parameters were reduced even if LVEF was normal or supernormal, and the strain parameters are more sensitive to the changes in cardiac function [14,15,16], the prognostic value of myocardial strain by CMR is unknown in LVNC patients with left ventricular dysfunction.
The present study aimed to investigate whether the combination of CMR-FT with traditional MRI parameters can increase the prognostic value of MRI in an LVNC patient cohort with left ventricular dysfunction and to determine the optimal prediction model.
Materials and methods
Study population
This multicenter retrospective cohort study identified patients with the LVNC phenotype via a Boolean search of contrast-enhanced CMR reports between March 2009 and January 2020. Patients were queried in the CMR report database by searching the following keywords that describe LVNC: “left ventricular noncompaction”, “left ventricular hypertrabeculation”, and “spongiform cardiomyopathy”. The inclusion criteria were patients who fulfilled the following diagnostic criteria for LVNC: 1) bilayer myocardium with thin compacted and a thick noncompacted layers; 2) marked trabeculations with deep endomyocardial recesses; and 3) an end-diastolic noncompacted/compacted ratio > 2.3 in any LV segment in any long axis view [17]. The exclusion criteria were as follows: age < 18 years, incomplete or poor-quality CMR images, heart transplantation before CMR examination, any concurrent congenital or acquired heart disease, and normal left ventricular function (LVEF ≥ 55%).
A total of 123 candidates were identified from the CMR report database and 55 subjects remained after exclusion applying the exclusion criteria. The inclusion and exclusion criteria of the patients are summarized in Fig. 1. Clinical and CMR data were recorded in standard form at two centers by two experienced study physicians. In addition, we searched 51 CMR reports of healthy subjects as controls for segmental myocardial strain analysis, aged and sex matched to the LVNC patients. All data were made anonymous and analyzed at center A. The study protocol was approved by the Ethics Committee of the Peking Union Medical College Hospital, Sichuan University (K2019059) and West China Hospital of Sichuan University (756/2019), and written informed consent was obtained from all participants.
CMR protocol
CMR images were obtained with the same type of scanner in two centers by using a 3.0-T whole body scanner (Skyra and Trio Tim; Siemens Medical Solutions, Erlangen, Germany). An 18-channel cardiac phased array coil and electrocardiogram triggering were used for CMR scanning. The adopted CMR protocol was the same in the two centers. Steady-state-free precession (SSFP) cine images were acquired for the assessment of left ventricular function and morphology before the contrast agent was injected, and they included two orientations, the short-axis view covering the full left ventricle from basis to apex and the 2-, 3- and 4-chamber long-axis views. LGE images for detecting myocardial scarring or fibrosis were obtained using a contrast-enhanced inversion recovery TrueFISP sequence for 10 ~ 15 min after an intravenous bolus of gadobenate dimeglumine (MultiHance 0.5 mmol/ml; Bracco, Milan, Italy). The dose of contrast agent was 0.1–0.2 ml/kg body weight at a flow rate of 2.5–3.0 ml/s and then injected with 20 ml of saline flush at a rate of 3.0 ml/s. The scan parameters of the two sequences were as follows: (1) SSFP sequence: slice thickness 6–8 mm, slice gap 0 mm, repetition time 3.42 ms, echo time 1.5 ms, flip angle 60°, and voxel size: 1.6 mm × 1.6 mm × 0.8 mm; (2) inversion recovery TrueFISP sequence: slice thickness 8 mm, repetition time 2.81 ms, echo time 1.04 ms and flip angle 55°.
CMR image analysis
All collected images were transferred to the dedicated postprocessing software Cvi42 (Circle Cardiovascular Imaging, Calgary, Canada) and analyzed at center A by two expert radiologists blinded to patient baseline characteristics and radiological information. The left ventricular end-diastolic volume (EDV), left ventricular end-systolic volume (ESV), stroke volume (SV), and LVEF were calculated from the short-axis cine images in the short 3D module. EDV, ESV, and SV were corrected using BSA (LVEDVi, LVESVi, and LVSVi). Left ventricular epicardial and endocardial borders were manually traced in the 2-, 3-, and 4-chamber views of cine images at the end-diastole and the short-axis view at the end-diastole and end-systole by using the semiautomated feature tracking module. Global radial strain (GRS), global circumferential strain (GCS), and segmental radial and circumferential strain at the three levels, including apex, mid, and base, were calculated from the average of the peak strain of the short axis, and the global longitudinal strain (GLS) and segmental longitudinal strain at the three levels were calculated from the average of the peak strain of the long axis. Papillary muscles and noncompacted myocardium were excluded when the endocardial border was drawn. LGE extent was calculated as a percentage enhancement of myocardial mass from the LGE images. The presence of LGE was defined as myocardial enhancement with a signal intensity of > 5 SD above the mean signal intensity of the remote normal myocardium. The pattern and location of LGE were visually assessed by two experienced radiologists and classified as subendocardial, mid-wall, subepicardial, or transmural in distribution using standard American Heart Association 17 segment model.
Follow-up
All patients were followed up on the telephone by using the standard questionnaire interview and the clinical medical records, electrocardiogram, ultrasound and the results of laboratory tests of all patients after CMR examination were queried, which was performed at center A by experienced physicians blinded to the CMR data. The clinical endpoints were major adverse cardiovascular events (MACEs), including cardiovascular death, heart transplantation, hospitalization for heart failure, thromboembolic events and ventricular arrhythmias defined as sustained or non-sustained ventricular tachycardia and ventricular fibrillation. The duration of follow-up was calculated from the date of the first CMR examination to the first occurrence of an endpoint.
Statistical methods
Statistical analysis was performed using GraphPad Prism version 9.0 (GraphPad Software, La Jolla, California), IBM SPSS Statistics 23.0 software (IBM SPSS Statistics, IBM Corporation, Armonk, New York), and R software version 4.1.0 (R Foundation for Statistical Computing, Vienna, Austria). Continuous variables are presented as the mean ± standard deviation. Categorical variables are presented as absolute numbers (percentage). To compare the patient characteristics and CMR data in the groups, we compared continuous data by the Mann‒Whitney U test or independent Student’s t test and used chi-square analysis for categorial data. Correlations between LVEF and global strain parameters were assessed based on the Pearson correlation coefficient.
The cutoff value of the optimal receiver operating characteristic (ROC) curve for predicting MACEs was selected as the value for maximizing sensitivity and specificity. The survival rate was evaluated by Kaplan–Meier analysis. Univariate Cox regression analysis was used to identify potential clinical and CMR predictors of MACEs. Then these variables with p value ≤ 0.1 were included in multivariate Cox regression analysis to determine the independent predictors of the MACE. The hazard ratio (HR) and 95% confidence interval (CI) were calculated to estimate the risk associated with a particular variable. First, a baseline clinical Cox model building was performed as model 1. To investigate the incremental role, we sequentially added the traditional CMR markers, mainly including LVEF and the presence of LGE, to building model 2 and model 3. Finally, the parameters of CMR-FT (global and segmental myocardial strain) were added into model 3 to build other nested regression models. The -2Loglikelihood and c-index of each model for the prediction of MACEs were calculated. The likelihood ratio test and continuous net reclassification improvement (NRI) were used to evaluate the incremental prognostic value of these nested regression models. The intra- and interobserver reliability and agreement for continuous CMR variables were evaluated using the interclass correlation coefficients (ICCs) and Bland‒Altman analysis.
Results
Population characteristics
The baseline clinical characteristics for the controls and the patients with and without MACEs are shown in Table 1. A total of 24 (36.8%) patients experienced at least one MACE during a median follow-up of 5.17 years (interquartile range: 0.17 to 10.58 years). Four (16.7%) patients developed ventricular arrhythmias, five (20.8%) patients were hospitalized for heart failure, and 15 (62.5%) patients suffered from cardiac death. The causes of death included heart failure (n = 10), acute coronary syndrome (n = 2), malignant ventricular arrhythmias (n = 2) and infection after valve replacement (n = 1). The clinical characteristics were not significantly different between the two groups, except for age. Patients with MACEs were more likely to be older than those without MACEs (51.8 ± 17.1 years vs. 41.0 ± 13.9 years, p = 0.012).
CMR results
The CMR findings are shown in Table 2. Compared with the controls, the patients’ group had significantly lower LVEF, they had significantly larger EDVi and ESVi (p < 0.001). In patients’ group, the LVEF values were lower in patients with MACEs (22.9 ± 11.2% vs. 31.2 ± 13.7%, p = 0.025). All global and segmental strain parameters in patients’ group were significantly lower than in the controls (all p < 0.01). Compared with the patients without MACEs, global strain parameters were mainly attenuated in patients with MACEs, GRS (8.6 ± 5.6% vs. 13.3 ± 8.9%, p = 0.034), GCS (−7.4 ± 3.5% vs. −9.6 ± 4.8%, p = 0.091), and GLS (−4.7 ± 2.5% vs. −7.0 ± 3.2%, p = 0.004); Segmental strain parameters were also reduced, and the longitudinal strain at the apical level, radial and longitudinal strain at the mid level, and radial and circumferential strain at the basal level were significantly lower in patients with events (all p < 0.05). The decrease in global and segment strain at three different levels (apical, mid, and basal) in the same group are illustrated in Additional file 1: Fig. S1 in the appendix. The reduction in radial strain at the basal level, circumferential strain at the apical level, and longitudinal strain at the mid level were most pronounced, while the reduction in global strain was moderate.
The presence of LGE (79.2% vs. 48.4%, p = 0.02) and the LGE extent (12.1 ± 10.6% vs. 8.0 ± 14.2%, p = 0.054) were higher in patients with MACEs. The most common pattern was subendocardial LGE and mid-wall LGE in all LVNC patients, the number of LGE segments was 84 (14.5%) and 91 (15.7%), respectively. The transmural LGE was more prevalent in patients with MACEs than in patients without MACEs (40 (12.4%) vs. 11 (4.3%), p < 0.001). However, no statistical significance was observed in the LGE extent between the two groups. The clinical characteristics and CMR parameters in LVNC patients with or without LGE are shown in Additional file 1: Table S1 in the appendix.
ROC analysis was performed for CMR parameters with significant differences between groups, and the results are displayed in Additional file 1: Table S2 in the appendix. The area under the curve of GRS, GLS, and LVEF was statistically significant (GRS: 0.669, GLS: 0.733, LVEF: 0.677, all p < 0.05). The cutoff values for GRS, GLS, and LVEF were ≤ 11.41%, > -6.23%, and ≤ 23.89%, respectively.
Univariate analysis
In univariate Cox regression analysis (reported in Table 3), GLS (HR: 1.238; 95% CI: 1.018 to 1.506; p = 0.033) and LVEF (HR: 0.964; 95% CI: 0.929 to 0.999; p = 0.046) were univariate predictors of MACEs. Longitudinal strain at the apical level and radial and circumferential strain at the basal level were also univariate predictors of MACEs. The presence of LGE (HR: 2.768; 95% CI: 1.033 to 7.419; p = 0.043) was significantly associated with adverse events. However, the LGE extent (HR: 11.385; 95% CI: 0.441 to 293.9; p = 0.143) did not have prognostic value in the univariate Cox regression analysis.
Multivariate analysis
The different models by multivariate analysis are shown in Table 4. In multivariate analysis, which included age, body mass index (BMI), LVEF, the presence of LGE, and GLS (p < 0.1 in univariate Cox regression analysis), LGE was a strong and independent predictor of MACEs in model 3 and model 4. The presence of LGE (in model 3) significantly improved the model fit compared with model 2, including the clinical characteristics and LVEF (global chi-square test: 7.51 vs. 13.47, p = 0.015). Moreover, the continuous NRI increased significantly (model 2 vs. model 3: continuous NRI = 0.235, p = 0.049). In comparison with model 3, which includes the traditional CMR imaging markers of LVEF and the presence of LGE, the addition of GLS did not improve the model fit (global chi-square test: 13.47 vs. 14.14, p = 0.411) and GLS was no longer statistically significant for the prediction of MACEs in model 4. Figure 2 depicts the incremental values in predicting the MACEs by sequentially adding the traditional CMR imaging markers and CMR-FT strain parameter. The models including segmental strains by multivariate analysis are shown in Additional file 1: Table S3. The results showed that the addition of segmental strain parameters, longitudinal strain at the apical level and radial and circumferential strain at the basal level did not improve the model fit.
Incremental prognostic value of traditional CMR imaging markers and CMR-FT strain parameters. Nested multivariable survival models illustrate that feature tracking myocardial strain did not improve the prediction for major adverse cardiac event, and LGE was a robust independent predictor. CMR cardiovascular magnetic resonance; LVEF left ventricular ejection fraction; GLS global longitudinal strain; LGE late gadolinium enhancement; FT feature tracking
Incidence of MACEs and LGE
The Kaplan‒Meier survival curve of the presence of LGE is displayed in Fig. 3 (log-rank = 4.522, p = 0.0335). The survival rates free from MACEs were 55.2% and 33.1% in patients without and with LGE, respectively. The median survival time was 4.25 years in patients with LGE.
Myocardial strain and LVEF
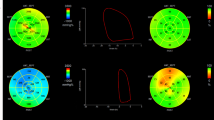
The strain parameters and LVEF were not independent predictors of MACEs in multivariate analysis. A strong correlation was observed between these parameters (correlation between LVEF and GRS: Pearson r = 0.7964; correlation between LVEF and GCS: Pearson r = −0.8163; correlation between LVEF and GLS: Pearson r = −0.6802; all p < 0.0001) (Fig. 4).
Correlation analysis of global myocardial strain by CMR-FT and LVEF. The global strain parameters were significantly correlated with LVEF. GRS: Pearson r = 0.7964, GCS: Pearson r = −0.8163, GLS: Pearson r = −0.6802, all p < 0.0001. CMR, cardiovascular magnetic resonance; FT, feature tracking; GRS, global radial strain; GCS, global circumferential strain; GLS, global longitudinal strain; LVEF, left ventricular ejection fraction
Reproducibility analysis
A total of 20 patients were analyzed for intra- and inter-observer reliability and agreement. Additional file 1: Table S4 presents both intra- and interobserver variability for global and segmental myocardial strains. Consistency of measurements was good for all strain measurements and no significant differences were seen. With the exception of apical radial strain, mid radial strain and basal longitudinal strain, both intra- and interobserver ICC values (ICC = 0.770–0.993) were good to excellent for all analyzed strain measurements.
Discussion
In the present study, we first evaluated the prognostic value of the combination of CMR-FT with traditional CMR markers, particularly LGE, in LVNC patients. The major findings are as follows: 1) the presence of LGE was a significant independent predictor of MACEs and can effectively improve the predictive ability of the risk prediction model in LVNC patients with reduced LV systolic function, 2) the global and segmental strain were lower in LVNC patients with MACEs than in those without MACEs, and 3) LV strain parameters from CMR-FT had no significant incremental prognostic value in these patients with reduced LV systolic function.
Differential prognosis of LVNC according to LV strain
Strain parameters are the main noninvasive strategy for assessing systolic function of the left ventricle and are useful for the early detection and outcome evaluation of some heart diseases [18, 19]. Our results demonstrated that the global and segmental strain decreased in LVNC patients with MACEs. The same results were found in the pediatric population [20]. In univariate analysis, GLS was a predictor of MACEs. However, it was not an independent predictor of MACEs in multivariate analysis and could not increase the predictive value of the nested regression models in LVNC patients with reduced LVEF. However, recent publications highlight the predictive role of CMR-FT in cohorts of patients with the cardiovascular disease, especially GLS [11, 13, 21,22,23]. Myocardial deformation is an essential factor for maintaining normal global systolic function. Therefore, LV myocardial strain is associated with LVEF. Our results support this conclusion. The difference among these studies can mainly be attributed to the differences in the study cohort, in which all patients had myocardial dysfunction in our study. Compared with studies in which myocardial strain had incremental independent prognostic value, the LVEF of our subjects was generally lower (30.5% vs 55.9%, 46.4%, 44.9%) [12, 13, 24]. The study by Marciniak et al. [25] showed that when stroke volume was maintained, myocardial strain changed less and less as the left ventricular diameter increased. This may suggest that the sensitivity of myocardial strain gradually decreases with the decrease of decreasing LV function. Some studies have reported that myocardial strains and LVEF had no independent prognostic value in patients with dilated cardiomyopathy [26, 27]. In the present study, LVEF also failed to predict adverse events. Similar to these studies, the LVEF of the enrolled subjects was significantly reduced. The relationship between LVEF and survival probability was weaker when LVEF < 25% [28]. Therefore, LVEF may have superior predictive power in a cohort where most LVNC patients do not have severe left ventricular systolic dysfunction [29]. Based on the different results of the abovementioned studies, our findings are applicable to LVNC patients with reduced LV systolic function. Moreover, left ventricular function could be improved in patients who received medical therapy. Therefore, it may limit the application of global strain in predicting prognosis in LVNC patients with impaired systolic dysfunction.
In comparison with the global strain parameters, the segmental strain parameters can reveal the change in myocardial deformation in each segment. However, the inter- and interobserver reproducibility of some segmental strain values, such as apical radial strain, were not good and they should be used with caution within clinical studies. In the present study, the reduction of global strains was moderate compared with the three segmental strains. The reduction in circumferential and longitudinal strain at the mid and apical levels were more pronounced than that at the basal level. This result supported that myocardial noncompaction often occurs in the apical and middle segments [30, 31], and the range of myocardial circumferential and longitudinal motion increases gradually from the basal segment to the apical segment [32]. In the present study, the longitudinal strain at the apical level and radial and circumferential strains at the basal level were predictors of MACEs in univariate analysis. The addition of these segmental strains, especially the circumferential strain at the basal level, resulted in a higher c-index and chi-square value for the nested model compared with the addition of GLS, although they were not independent predictors. Some studies have shown that some segmental strain parameters can enhance the predictive performance in patients with cardiovascular disease [33, 34]. More research is required to explore the predictive value of segmental strains in LVNC patients.
Prognostic importance of LGE for LVNC
LGE by cardiac MRI is a reference method for the noninvasive detection and quantification of myocardial fibrosis; moreover, it is a robust independent predictor of poor prognosis and is used extensively in various cardiomyopathy studies [35]. The results showed that the presence of LGE was a reliable predictor of MACEs, including hospitalization caused by heart failure, ventricular arrhythmias, and cardiovascular death. The patients with LGE had a 3.45-fold risk of MACEs than patients without LGE during a median follow-up of 5.17 years. Our results are consistent with the previous studies [7, 8, 36]. Moreover, the addition of the presence of LGE significantly improved the model fit compared with the model of age, BMI and LVEF based on a series of nested multivariable models.
Myocardial fibrosis can lead to changes in myocardial electrical properties. Wan J et al. showed that ventricular arrhythmias are common in patients with LGE [37]. In the present study, we found that the severe arrhythmia may occur in patients with LGE (50% vs. 28.6%). Nucifora et al. reported that myocardial fibrosis, qualitative and quantitative by LGE was observed in 55% patients with isolated LVNC and was correlated with clinical severity and ventricular dysfunction [6]. LVNC patients with LGE had low LVEF and high EDV, ESV, and NYHA functional class in our study. These results suggested that LVNC patients with LGE have lower cardiac function and severe clinical manifestations.
In the present study, the quantitative extent of LGE was considered, but no significant correlation was observed with clinical results. The discrepancy with some previous studies may be associated with the different study populations, small sample size, definitions of different outcomes, or the use of different statistical methods [6,7,8]. More studies with sufficient sample sizes are needed to further verify its prognostic value in the future.
Study limitations
This study has some limitations. First, although this study was a multicenter study, the final patient cohort included in the study was limited. Uncontrollable confounders and selection bias may have been present. Second, most LVNC patients were treated with drugs because of cardiac dysfunction. However, we did not explore whether the different types and doses of drugs would affect the prognosis. Third, considering that the population of the study included the LVNC patients with reduced LVEF, the findings may not be universally applicable to all LVNC populations. In the future, we will further study the prognostic value of myocardial strain in patients with normal left ventricular function.
Conclusions
This study further reinforces the predictive value of CMR-LGE in LVNC patients with reduced LVEF, while LV strain parameters from CMR-FT could not increase the prediction efficiency of the risk prognostic model in these patients.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- LVNC:
-
Left ventricular noncompaction
- CMR:
-
Cardiac magnetic resonance
- LGE:
-
Late gadolinium enhancement
- CMR-FT:
-
Cardiac magnetic resonance feature tracking
- LVEF:
-
Left ventricular ejection fraction
- SSFP:
-
Steady-state-free precession
- EDV:
-
End-diastolic volume
- ESV:
-
End-systolic volume
- SV:
-
Stroke volume
- GRS:
-
Global radial strain
- GCS:
-
Global circumferential strain
- GLS:
-
Global longitudinal strain
- MACEs:
-
Major adverse cardiovascular events
- HR:
-
Hazard ratio
- CI:
-
Confidence interval
- NRI:
-
Net reclassification improvement
- ICC:
-
Interclass correlation coefficients
- BMI:
-
body mass index
References
Chin TK, Perloff JK, Williams RG, Jue K, Mohrmann R. Isolated noncompaction of left ventricular myocardium. A study of eight cases Circulation. 1990;82:507–13.
Aras D, Tufekcioglu O, Ergun K, Ozeke O, Yildiz A, Topaloglu S, et al. Clinical features of isolated ventricular noncompaction in adults long-term clinical course, echocardiographic properties, and predictors of left ventricular failure. J Card Fail. 2006;12:726–33.
Towbin JA, Lorts A, Jefferies JL. Left ventricular non-compaction cardiomyopathy. Lancet. 2015;386:813–25.
Murphy RT, Thaman R, Blanes JG, Ward D, Sevdalis E, Papra E, et al. Natural history and familial characteristics of isolated left ventricular non-compaction. Eur Heart J. 2005;26(2):187–92.
Ichida F. Left ventricular noncompaction-Risk stratification and genetic consideration. J Cardiol. 2020;75(1):1–9.
Nucifora G, Aquaro GD, Pingitore A, Masci PG, Lombardi M. Myocardial fibrosis in isolated left ventricular non-compaction and its relation to disease severity. Eur J Heart Fail. 2011;13:170–6.
Andreini D, Pontone G, Bogaert J, Roghi A, Barison A, Schwitter J, et al. Long-Term Prognostic Value of Cardiac Magnetic Resonance in Left Ventricle Noncompaction: A Prospective Multicenter Study. J Am Coll Cardiol. 2016;68:2166–81.
Mavrogeni S, Sfendouraki E, Theodorakis G, Kolovou G. Diagnosis, severity grading and prognosis of left ventricular non-compaction using cardiovascular magnetic resonance. Int J Cardiol. 2013;31(167):598–9.
Ashrith G, Gupta D, Hanmer J, Weiss RM. Cardiovascular magnetic resonance characterization of left ventricular non-compaction provides independent prognostic information in patients with incident heart failure or suspected cardiomyopathy. J Cardiovasc MagnReson. 2014;16:64.
Voigt JU, Cvijic M. 2- and 3-Dimensional Myocardial Strain in Cardiac Health and Disease. JACC Cardiovasc Imaging. 2019;12:1849–63.
Romano S, Judd RM, Kim RJ, Kim HW, Klem I, Heitner JF, et al. Feature-Tracking Global Longitudinal Strain Predicts Death in a Multicenter Population of Patients with Ischemic and Nonischemic Dilated Cardiomyopathy Incremental to Ejection Fraction and Late Gadolinium Enhancement. JACC Cardiovasc Imaging. 2018;11:1419–29.
Mordi I, Bezerra H, Carrick D, Tzemos N. The Combined Incremental Prognostic Value of LVEF, Late Gadolinium Enhancement, and Global Circumferential Strain Assessed by CMR. JACC Cardiovasc Imaging. 2015;8:540–9.
Fischer K, Obrist SJ, Erne SA, Stark AW, Marggraf M, Kaneko K, et al. Feature Tracking Myocardial Strain Incrementally Improves Prognostication in Myocarditis Beyond Traditional CMR Imaging Features. JACC Cardiovasc Imaging. 2020;13(9):1891–901.
Gastl M, Gotschy A, Polacin M, Vishnevskiy V, Meyer D, Sokolska J, et al. Determinants of myocardial function characterized by CMR-derived strain parameters in left ventricular non-compaction cardiomyopathy. Sci Rep. 2019;9:15882.
Kalam K, Otahal P, Marwick TH. Prognostic implications of global LV dysfunction: a systematic review and meta-analysis of global longitudinal strain and ejection fraction. Heart. 2014;100:1673–80.
Bellavia D, Michelena HI, Martinez M, Pellikka PA, Bruce CJ, Connolly HM, et al. Speckle myocardial imaging modalities for early detection of myocardial impairment in isolated left ventricular non-compaction. Heart. 2010;96:440–407.
Petersen SE, Selvanayagam JB, Wiesmann F, Robson MD, Francis JM, Anderson RH, et al. Left ventricular non-compaction: insights from cardiovascular magnetic resonance imaging. J Am Coll Cardiol. 2005;46:101–5.
Nucifora G, Sree Raman K, Muser D, Shah R, Perry R, Awang Ramli KA, et al. Cardiac magnetic resonance evaluation of left ventricular functional, morphological, and structural features in children and adolescents vs. young adults with isolated left ventricular non-compaction. Int J Cardiol. 2017;246:68–73.
Zhang KW, French B, May Khan A, Plappert T, Fang JC, Sweitzer NK, et al. Strain improves risk prediction beyond ejection fraction in chronic systolic heart failure. J Am Heart Assoc. 2014;13;3(1):e000550.
Arunamata A, Stringer J, Balasubramanian S, Tacy TA, Silverman NH, Punn R. Cardiac Segmental Strain Analysis in Pediatric Left Ventricular Noncompaction Cardiomyopathy. J Am Soc Echocardiogr. 2019;32(6):763-773.e1.
Kammerlander AA. Feature Tracking by Cardiovascular Magnetic Resonance Imaging: The New Gold Standard for Systolic Function? JACC Cardiovasc Imaging. 2020;13:948–50.
Motoki H, Borowski AG, Shrestha K, Troughton RW, Tang WH, Thomas JD, et al. Incremental prognostic value of assessing left ventricular myocardial mechanics in patients with chronic systolic heart failure. J Am Coll Cardiol. 2012;60:2074–81.
Buss SJ, Breuninger K, Lehrke S, Voss A, Galuschky C, Lossnitzer D, et al. Assessment of myocardial deformation with cardiac magnetic resonance strain imaging improves risk stratification in patients with dilated cardiomyopathy. Eur Heart J Cardiovasc Imaging. 2015;16(3):307–15.
Podlesnikar T, Pizarro G, Fernández-Jiménez R, Montero-Cabezas JM, Sánchez-González J, Bucciarelli-Ducci C, et al. Five-Year Outcomes and Prognostic Value of Feature-Tracking Cardiovascular Magnetic Resonance in Patients Receiving Early Prereperfusion Metoprolol in Acute Myocardial Infarction. Am J Cardiol. 2020;133:39–47.
Marciniak A, Claus P, Sutherland GR, Marciniak M, Karu T, Baltabaeva A, et al. Changes in systolic left ventricular function in isolated mitral regurgitation. A strain rate imaging study. Eur Heart J. 2007;28(21):2627–36.
Pi SH, Kim SM, Choi JO, Kim EK, Chang SA, Choe YH, et al. Prognostic value of myocardial strain and late gadolinium enhancement on cardiovascular magnetic resonance imaging in patients with idiopathic dilated cardiomyopathy with moderate to severely reduced ejection fraction. J Cardiovasc MagnReson. 2018;20:36.
Fu H, Wen L, Xu H, Liu H, Xu R, Xie L, et al. Prognostic value of multiple cardiac magnetic resonance imaging parameters in patients with idiopathic dilated cardiomyopathy. Int J Cardiol. 2021;325:89–95.
Dec GW, Fuster V. Idiopathic dilated cardiomyopathy. N Engl J Med. 1994;8;331(23):1564–75.
Casas G, Limeres J, Oristrell G, Gutierrez-Garcia L, Andreini D, Borregan M, et al. Clinical Risk Prediction in Patients With Left Ventricular Myocardial Noncompaction. J Am Coll Cardiol. 2021;78(7):643–62.
Niemann M, Liu D, Hu K, Cikes M, Beer M, Herrmann S, et al. Echocardiographic quantification of regional deformation helps to distinguish isolated left ventricular non-compaction from dilated cardiomyopathy. Eur J Heart Fail. 2012;14:155–61.
Yubbu P, Nawaytou HM, Calderon-Anyosa R, Banerjee A. Diagnostic value of myocardial deformation pattern in children with noncompaction cardiomyopathy. Int J Cardiovasc Imaging. 2018;34(10):1529–39.
Bogaert J, Rademakers FE. Regional nonuniformity of normal adult human left ventricle. Am J Physiol Heart Circ Physiol. 2001;280(2):H610–20.
Demissei BG, Fan Y, Qian Y, Cheng HG, Smith AM, Shimamoto K, et al. Left ventricular segmental strain and the prediction of cancer therapy-related cardiac dysfunction. Eur Heart J Cardiovasc Imaging. 2021;22(4):418–26.
Musa TA, Uddin A, Swoboda PP, Fairbairn TA, Dobson LE, Singh A, et al. Cardiovascular magnetic resonance evaluation of symptomatic severe aortic stenosis: association of circumferential myocardial strain and mortality. J Cardiovasc Magn Reson. 2017;19(1):13.
Ambale-Venkatesh B, Lima JA. Cardiac MRI: a central prognostic tool in myocardial fibrosis. Nat Rev Cardiol. 2015;12(1):18–29.
Cheng H, Lu M, Hou C, Chen X, Li L, Wang J, et al. Comparison of cardiovascular magnetic resonance characteristics and clinical consequences in children and adolescents with isolated left ventricular non-compaction with and without late gadolinium enhancement. J Cardiovasc MagnReson. 2015;17:44.
Wan J, Zhao S, Cheng H, Lu M, Jiang S, Yin G, et al. Varied distributions of late gadolinium enhancement found among patients meeting cardiovascular magnetic resonance criteria for isolated left ventricular non-compaction. J Cardiovasc MagnReson. 2013;15:20.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural Science Foundation of China (Nos. 82120108015, 82102020, 82071874, 81971586), Key Project of Sichuan Science and Technology Department (Nos. 2020YFS0050, 2020YJ0029, 2017TD0005, 21ZDYF1967), Fundamental Research Funds for the Central Universities (No. SCU2020D4132) and Clinical Research Finding of Chinese Society of Cardiovascular Disease of 2019 (No. HFCSC2019B01) and “1·3·5 Project for Disciplines of excellence, West China Hospital, Sichuan University” (ZYGD18019).
Author information
Authors and Affiliations
Contributions
GY, WY, BW, XR. and LX designed the study. BW, XR and LX performed literature search, analyzed and interpreted the data, and drafted the manuscript. HR, HW, and ZZ was responsible for data collection. BW, XR and LX made a contribution to the data analysis and interpretation. XH and FH contributed to the literature search and data interpretation. All authors contributed to the manuscript preparation, read and approved the final text.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was performed in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of the Peking Union Medical College Hospital, Sichuan University (K2019059) and West China Hospital of Sichuan University (756/2019). All participants have signed a written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
: Fig. S1. Absolute value of global and segmental peak strain difference between controls and LVNC patients. Table S1 Clinical characteristics and CMR parameters in LVNC patients with or without LGE. Table S2 The ROC analysis for traditional CMR features and CMR feature tracking. Table S3 Incremental value of the segmental strain. Table S4 Intra- and inter-observer reproducibility for the myocardial strain parameters in LVNC patients.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Bai, W., Xu, R., Li, X. et al. Prognostic value of cardiac magnetic resonance imaging parameters in left ventricular noncompaction with left ventricular dysfunction. BMC Cardiovasc Disord 22, 526 (2022). https://doi.org/10.1186/s12872-022-02963-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-022-02963-5