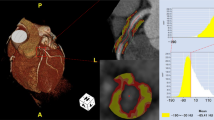
Abstract
Objective
We sought to investigate the correlation of pericoronary adipose tissue with coronary artery disease and left ventricular (LV) function.
Methods
Participants with clinically suspected coronary artery disease were enrolled. All participants underwent coronary computed tomography angiography (CCTA) and echocardiography followed by invasive coronary angiography (ICA) within 6 months. Pericoronary adipose tissue (PCAT) was extracted to analyze the correlation with the Gensini score and LV function parameters, including IVS, LVPW, LVEDD, LVESD, LVEDV, LVESV, FS, LVEF, LVM, and LVMI. The correlation between PCAT and the Gensini score was assessed using Spearman’s correlation analysis, and that between the PCAT volume or FAI and LV function parameters was determined using partial correlation analysis.
Results
One hundred and fifty-nine participants (mean age, 64.55 ± 10.64 years; men, 65.4% [104/159]) were included in the final analysis. Risk factors for coronary artery disease, such as hypertension, diabetes, dyslipidemia, and a history of smoking or drinking, had no significant association with PCAT (P > 0.05), and there was also no correlation between PCAT and the Gensini score. However, the LAD-FAI was positively correlated with the IVS (r = 0.203, P = 0.013), LVPW (r = 0.218, P = 0.008), LVEDD (r = 0.317, P < 0.001), LVESD (r = 0.298, P < 0.001), LVEDV (r = 0.317, P < 0.001), LVESV (r = 0.301, P < 0.001), LVM (r = 0.371, P < 0.001), and LVMI (r = 0.304, P < 0.001). Also, the LCX-FAI was positively correlated with the LVEDD (r = 0.199, P = 0.015), LVESD (r = 0.190, P = 0.021), LVEDV (r = 0.203, P = 0.013), LVESV (r = 0.197, P = 0.016), LVM (r = 0.220, P = 0.007), and LVMI (r = 0.172, P = 0.036), and the RCA-FAI was positively correlated with the LVEDD (r = 0.258, P = 0.002), LVESD (r = 0.238, P = 0.004), LVEDV (r = 0.266, P = 0.001), LVESV (r = 0.249, P = 0.002), LVM (r = 0.237, P = 0.004), and LVMI (r = 0.218, P = 0.008), respectively. Finally, the total volume was positively correlated with FS (r = 0.167, P = 0.042).
Conclusion
The FAI was positively correlated with the LV function but was not associated with the severity of coronary artery disease.
Key points
-
A significant correlation exists between the fat attenuation index (FAI) and left ventricular function parameters.
-
Our results suggest that cardiac mortality caused by an increased FAI may be due to changes in the left ventricular function.
-
Pericoronary adipose tissue did not correlate with the body mass index or risk factors of coronary heart disease.
Similar content being viewed by others
Introduction
Coronary artery disease (CAD) is a heart disease in which atherosclerotic plaque accumulates in the coronary artery, causing coronary lumen stenosis or occlusion and often resulting in myocardial ischemia or necrosis. Coronary atherosclerosis has been considered to be an inflammatory reaction [1]. For patients with CAD, predicting the risk of adverse coronary events is more important than coronary stenosis. The detection of pericoronary inflammation can facilitate risk stratification and risk prediction earlier in patients with coronary heart disease.
In recent years, the pericoronary fat attenuation index (FAI), which is measured using coronary CT angiography (CCTA) images, can be used to reflect pericoronary inflammation [2, 3]. Recent studies have shown that pericoronary adipose tissue (PCAT) participates in the process of coronary inflammation and is significantly related to the type of plaque and degree of stenosis [4,5,6]. One CRISP study showed that an FAI of > − 70.1 HU was associated with a greater risk for cardiac mortality, which could improve the prediction of a heart disease risk [7].
Although PCAT was shown to have great clinical significance in the occurrence and development of CAD [8], there are several important questions that must be explored in this popular field. The Gensini score is a traditional index used to evaluate the severity of CAD [9]; however, it is unknown whether PCAT assessment can determine the severity of CAD. Second, some studies reported that CAD is a cause of left ventricular (LV) dysfunction, and LV dysfunction can increase the mortality of CAD, but the association between PCAT and LV function is still unclear [10, 11].
The aim of this study was to investigate the association of PCAT with CAD and LV function, which can help clinicians to evaluate the prognosis of patients with CAD.
Methods
This retrospective study was approved by the ethics committee of Changzhou No. 2 People's Hospital affiliated with Nanjing Medical University, and the need to obtain informed consent was waived.
Study patients
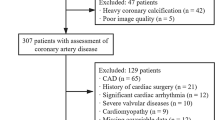
The study subjects were all patients treated at Changzhou No. 2 People's Hospital affiliated with Nanjing Medical University from September 2019 to September 2021. We retrospectively included patients with clinically suspected CAD in our institution who underwent CCTA and echocardiography followed by invasive coronary angiography (ICA) within 6 months. Exclusion criteria included the following: (a) history of coronary myocardial infarction and cardiac surgery; (b) anatomical variation of the heart or coronary artery; (c) diseases that seriously affect heart function, such as heart space–occupying lesions, cardiomyopathy, or severe heart valve disease; (d) family history of CAD; and (e) failure to reconstruct images (Fig. 1).
Clinical data
The clinical data of patients were obtained from the inpatient care system. Hypertension, diabetes, and dyslipidemia were diagnosed according to clinical indicators. A diagnosis of hypertension was defined by a systolic blood pressure ≥ 140 mmHg and/or diastolic blood pressure ≥ 90 mmHg or taking anti-hypertensive drugs. The diagnosis of diabetes was made based on the guideline for the prevention and treatment of type 2 diabetes and defined by a fasting blood glucose > 6.1 mmol/L and/or glycosylated hemoglobin > 6.5% or hypoglycemic drugs. Dyslipidemia was diagnosed according to the guidelines for the prevention and treatment of dyslipidemia in adults and defined as meeting ≥ 1 of the following requirements: (a) total cholesterol ≥ 5.18 mmol/L, (b) triglyceride ≥ 1.70 mmol/L, (c) high-density lipoprotein cholesterol < 1.04 mmol/L, (d) low-density lipoprotein cholesterol ≥ 3.37 mmol/L, and (e) taking blood lipid–regulating drugs.
CCTA image acquisition
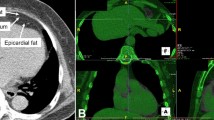
CCTA was performed on Siemens third-generation dual-source CT system (SOMATOM definition force; Siemens AG, Munich, Germany). Before scanning, the patient rested quietly for ≥ 15 min, and no patients took drugs such as β-blockers to slow down their heart rate. The scanning range was from the superior sternal fossa to the diaphragmatic surface of the heart. The prospective electrocardiogram gating sequence was used for scanning, and the scanning parameters were as follows: tube voltage, 100 kV; tube current, 228–300 mA; CT rotation time, 0.25 s; layer thickness, 0.75 mm; reconstruction interval, 0.50 mm; and display matrix, 512 × 512. Iodixanol injection (100 mL: 32 g; Qing Liming, NJCTTQ, China) was injected through the middle elbow vein. The injection volume was 60–80 mL, and the injection speed was 5–6 mL/s.
Coronary artery reconstruction
The scanned images were transmitted to the Skviewer software program (Coronary System; Shukun Technology, Beijing, China). At first, each image was processed for image consistency to eliminate the impact of different window widths and window levels on the quality of the reconstructed image. Then, Coronary System was used for longitudinal and axial multiplanar reconstruction of the coronary artery. Finally, an experienced radiologist evaluated the image quality of coronary artery reconstruction, awarding a score of 1–4 points, respectively, for poor image quality (images were subsequently excluded), acceptable image quality, good image quality, and excellent image quality.
Perivascular fat analysis
PCAT was extracted automatically by using the Skviewer software FAI intelligent analysis system (Skviewer FAI; Shukun Technology, Beijing, China) [12]. The volume and FAI of PCAT were measured using the method described by Oikonomou et al. [7]; the threshold value of PCAT ranged from − 190 to − 30 HU, the measured length was 40 mm, and the extracted radial distance was the average diameter of the target vessel. The segment analyzed was the proximal 40 mm of the left anterior descending (LAD) and left circumflex (LCX) arteries and the proximal 10–50 mm of the right coronary artery (RCA). The software could extract PACT volume and FAI automatically, and when the automatic extraction was inaccurate, we adjusted the extraction range manually. Subsequently, the PCAT volume and FAI were calculated by the software automatically (Fig. 2). The above measurement results were completed by an experienced radiologist independently. In order to test the consistency of the measurement results, 20% (32) of patients were randomly selected 1 month later, and a second measurement session was carried out by 2 radiologists. An intraobserver consistency test was carried out on the results measured by the same doctor, and an interobserver consistency test was carried out on the results measured by 2 doctors (Additional file 1).
ICA and Gensini score calculation
According to the 2021 American College of Cardiology/American Heart Association angiography guidelines of the United States, the patients were punctured through the right femoral artery or radial artery with the conventional Seldinger standard method. The left and right coronary arteries were then successively imaged with multi-position irradiation imaging [13].
The ICA results were evaluated by lumen diameter stenosis (DS). The segment score was obtained by multiplying the corresponding scores of the vascular segment where the plaque was located by the score of stenosis. The sum of the total scores of each segment was the total Gensini score [14] (Table 1).
LV function parameter acquisition
According to the current guidelines and diagnostic criteria set by the American Society of Echocardiography and European Association of Cardiovascular Imaging [15], echocardiographs were acquired by the Vivid E9 (GE Vingmed Ultrasound AS; Horten, Norway) echocardiography system with an M5S transducer (3.5 MHz). The patient adopted the left-lying position, laid still for 5 min, and breathed calmly; then, we imaged the long-axis section of the LV to obtain the interventricular septum (IVS), LV posterior wall (LVPW), LV end-diastole diameter (LVEDD), LV end-systolic diameter (LVESD), 1eft ventricular end-diastolic volume (LVEDV), and left ventricular end-systolic volume (LVESV), then calculated the LV fractional shortening (FS), LV ejection fraction (LVEF), LV mass (LVM), and LV mass index (LVMI). The calculation formulae used are as follows:
LVEF = (LVEDV − LVESV)/LVEDV × 100%
FS (%) = (LVEDD − LVESD)/LVEDD
LVM (g) = 0.8*1.04*[(IVS + LPWT + LVEDD)3 − LVESD3] + 0.6
LVMI (g/m2) = LVM/body surface area
Body surface area (m2) = 0.0061 × height (cm) + 0.0128 × weight (kg) − 0.1529
Statistical analysis
All statistical analyses were performed using SPSS (SPSS Statistics for Windows Version 23.0; IBM Corporation, Armonk, NY, USA). The counting data were expressed in quantity and percentage, and the continuous variables were described using mean ± standard deviation (SD) or median (upper quartile, lower quartile) values. Two subgroup comparisons of continuous variables used 2 independent-samples Student’s t-tests or Mann–Whitney U tests. The correlation between PCAT volume or FAI and Gensini score and LV function parameters was assessed using Spearman’s correlation analysis. In order to eliminate the influence of interference factors, we used a partial correlation analysis to analyze the correlation between the PCAT volume or FAI and Gensini score and LV function parameters. P < 0.05 was considered to indicate statistical significance. The intragroup correlation coefficient (ICC) was used to evaluate intra- and interobserver consistency.
Results
Baseline characteristics
Initially, 197 participants with clinically suspected CAD were included. According to strict exclusion criteria, 159 patients (mean age, 64.55 ± 10.64 years; range, 43–89 years) were finally enrolled in this study (Fig. 1), including 104 men (mean age, 62.03 ± 10.58 years; range, 43–89 years) and 55 women (mean age, 69.33 ± 9.05 years; range, 51–85 years). Other baseline characteristics are shown in Table 2.
Relationship between PCAT volume and CAD risk factors
The PCAT volumes of all participants were divided into 2 subgroups according to whether they had hypertension, diabetes, dyslipidemia, a smoking history, or a drinking history. Table 3 shows the distribution of volume among the subgroups. The results showed that there were statistically significant differences in the LCX volume and total volume between the subgroups (P = 0.028 and P = 0.025). The results of the other subgroups were not significant (P > 0.05). The distribution range of PCAT volume is shown in Fig. 3. [The PCAT intra- and interobserver consistencies were good (P < 0.001) (Additional file 1)].
Relationship between FAI and CAD risk factors
Table 4 shows the distribution of FAI among different subgroups. The results showed that there was no significant difference in FAI between the subgroups (P > 0.05). Figure 4 shows the distribution range of FAI.
Correlation analysis of PCAT volume or FAI and Gensini score or BMI
Spearman’s correlation or Pearson’s analysis was used to analyze the correlation between PCAT and Gensini score and BMI. The results showed that the LCX and total volumes were positively correlated with BMI (r = 0.170, P = 0.032; r = 0.157, P = 0.048). However, there was no significant correlation between the LAD and RCA volumes and BMI and no correlation between FAI and BMI. The PCAT also was not correlated with the Gensini score, as shown in Fig. 5.
Correlation analysis of PCAT volume or FAI and LV function parameters.
We performed a partial correlation analysis of control variables, excluding confounding factors such as age, gender, height, weight, BMI, smoking, drinking, heart rate, hypertension, diabetes and hyperlipidemia, then analyzed the correlation between PCAT and LV function parameters (Fig. 6).
Partial correlation analysis results of PCAT and LV function parameters. The results are presented in the form of a matrix. The LAD-FAI was positively correlated with IVS (r = 0.203, P = 0.013), LVPW (r = 0.218, P = 0.008), LVEDD (r = 0.317, P < 0.001), LVESD (r = 0.298, P < 0.001), LVEDV (r = 0.317, P < 0.001), LVESV (r = 0.301, P < 0.001), LVM (r = 0.371, P < 0.001), and LVMI (r = 0.304, P < 0.001). The LCX-FAI was positively correlated with LVEDD (r = 0.199, P = 0.015), LVESD (r = 0.190, P = 0.021), LVEDV (r = 0.203, P = 0.013), LVESV (r = 0.197, P = 0.016), LVM (r = 0.220, P = 0.007), and LVMI (r = 0.172, P = 0.036). The RCA-FAI was positively correlated with LVEDD (r = 0.258, P = 0.002), LVESD (r = 0.238, P = 0.004), LVEDV (r = 0.266, P = 0.001), LVESV (r = 0.249, P = 0.002), LVM (r = 0.237, P = 0.004), and LVMI (r = 0.218, P = 0.008). The total volume was positively correlated with FS (r = 0.167, P = 0.042)
Discussion
While using CCTA to diagnose coronary artery stenosis, we can predict the prognosis of patients with CAD through the analysis of PCAT.
Some studies on the correlation between epicardial fat volume (EFV) and CAD reported that the increase of EFV was positively correlated with the risk of CAD [16, 17]. Severe CAD often leads to changes in cardiac function, and studies have shown that there is a correlation between EVF and LV function parameters [18, 19]. Thus, we analyzed the relationship between PCAT volume, Gensini score, and LV function parameters and found that there was no correlation between them. The possible reason for this is that the measurement methods are different. The extraction range of EFV is all adipose tissue from the aortic root to the apex of the heart, and the radial range of extraction of PCAT is equal to the diameter of the target vessel. Many factors are related to coronary heart disease, including obesity, diabetes, and hypertension [20, 21]. Previous studies have shown that EVF is correlated with traditional risk factors of coronary heart disease [22,23,24] and positively correlated with BMI [25]. However, some research results suggest that EVF can be an independent predictor of CAD [26] and is not associated with the traditional risk factors of CAD [27, 28]. Therefore, we analyzed the correlation between PCAT volume, BMI, and risk factors for CAD. Our results showed that PCAT volume did not correlate with BMI or risk factors for coronary heart disease. These differences in results may be related to different measurement methods, pathological changes in the natural course of the disease, lifestyle factors, and drug intervention [29, 30]. Our results showed that only the results of LCX and total volumes were statistically significant (P < 0.05). This may be related to the reduced amount of adipose tissue around the LCX; the method we adopted can cover a more complete vessel volume. The correlation shown with the total volume may be affected by LCX. In a study of pericoronary epicardial adipose tissue by Vos et al. [31], only the fat volume around LCX showed different results from LAD and RCA, which was similar to our results. Inflammation is a critical factor not only for the development but also the progression of atherosclerosis [1, 32, 33]. Inflammatory factors released by the arterial wall can induce lipolysis, inhibit lipogenesis, and promote perivascular edema [34]. Meanwhile, the cytokines secreted by adipose tissue play a significant role in atherogenesis and myocardial ischemia [35, 36]. These changes showed attenuation from lipids (close to − 190 Hu) to water (close to − 30 Hu) on CT [37, 38]. Therefore, coronary inflammation can be detected by FAI.
One study by Antonopoulos et al. [38] found that FAI was positively correlated with atherosclerotic plaque load, and a greater perivascular FAI was associated with higher inflammatory expression levels. Some other studies have found that FAI is related to coronary hemodynamic changes and myocardial ischemia; furthermore, FAI around culprit lesions was increased significantly in patients with acute myocardial infarction [39, 40]. Therefore, we analyzed the correlation between FAI and Gensini score and found that there was no correlation between PCAT FAI and Gensini score (P > 0.05). However, what triggered this result? It may be that we did not stratify the population according to the Gensini score, and there were only 26 patients (16%) with severe CAD (Gensini score > 60 points) in our study population. Meanwhile, some studies have shown that FAI can present dynamic changes with the prognosis of the disease, and statins, aspirin, or anti-diabetic drugs can also alter the FAI [41, 42]. In addition, coronary artery calcification is closely correlated with atherosclerotic plaque formation and cardiovascular disease, but some studies have shown the potential impact of calcification on machine learning [43, 44]. These may cause our results to be different from others.
Further analysis found that there was no significant difference between FAI and BMI (LAD: r = 0.137, P = 0.086; LCX: r = 0.124, P = 0.118; RCA: r = 0.021, P = 0.790). Between the results of FAI and risk factors of CAD, there was no relationship. These findings can further support the idea that FAI is a reliable imaging index to quantify coronary artery inflammation and is not affected by other risk factors [45]. The study of Antonopoulos et al. [38] found that FAI was not associated with traditional cardiovascular risk factors, similar to our results.
Since the parameters of LV function are affected by individual differences [46, 47], we analyzed the PCAT and LV function parameters by partial correlation analysis to exclude the effects of interference factors. There was no correlation between PCAT and LV function parameters, but a significant correlation between FAI and LV function parameters existed. This may be because the coronary artery and myocardium have no obvious boundary, and the PCAT can release cytokines that reach the myocardium and coronary artery through paracrine signaling mechanisms, which results in changes in myocardial and LV function [48,49,50]. Hoshino et al. [40] mentioned that FAI was associated with cardiac mass, and some studies have reported a correlation between LV function and EAT [51, 52]; notably, these results are similar to ours. In addition, studies have shown that LV dysfunction and increased FAI (> 70.1 HU) can significantly increase the cardiac mortality [42, 53]. Our results suggest that cardiac mortality caused by increased FAI may be due to changes in LV function.
There are several limitations to this study that should be pointed out. First, this study is a single-center retrospective analysis with a small sample size that failed to obtain the treatment and lifestyle intervention details of patients. Second, in our study, the number of patients with severe CAD was small, and we did not stratify the severity of patients, which may lead to bias in our results. Third, previous studies have shown that CAC is a reliable and independent predictor of future cardiovascular events [54], so we will evaluate the relationship between calcification and PCAT in future research. Fourth, some studies have shown that there are differences in FAI between different genders [55], and women account for less than half of the population in our study (34.6%), which may also cause deviation in the results. Further research is needed to identify sex-specific variables that explain the correlation between PCAT and LV function accurately.
Conclusion
The results showed that there was no correlation between PCAT volume, FAI, and the severity of CAD, but there was a positive correlation between FAI and LV function parameters, and the correlation involving LAD was the strongest. Moreover, our study found that FAI has no significant relationship with BMI or traditional risk factors of CAD. It further supports that FAI can be used as a reliable imaging index of coronary artery inflammation, potentially playing a significant role in clinical diagnosis and evaluation of CAD.
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available to protect patient privacy but are available from the corresponding author upon reasonable request.
Abbreviations
- CAD:
-
Coronary artery disease
- FAI:
-
Pericoronary fat attenuation index
- CCTA:
-
Coronary computed tomography angiography
- PCAT:
-
Pericoronary adipose tissue
- ICA:
-
Invasive coronary angiography
- BMI:
-
Body mass index
- LAD:
-
Left anterior descending
- LCX:
-
Left circumflex
- RCA:
-
Right coronary artery
- DS:
-
Diameter stenosis
- IVS:
-
Inter ventricular septum
- LVPW:
-
Left ventricular posterior wall
- LVEDD:
-
Left ventricular end-diastole diameter
- LVESD:
-
Left ventricular end-systolic diameter
- LVEDV:
-
Left ventricular end-diastolic volume
- LVESV:
-
Left ventricular end-systolic volume
- FS:
-
Fractional shortening
- LVEF:
-
Left ventricular ejection fraction
- LVM:
-
Left ventricular mass
- LVMI:
-
Left ventricular mass index
- EFV:
-
Epicardial fat volume
References
Ridker PM, Libby P, MacFadyen JG, et al. Modulation of the interleukin-6 signalling pathway and incidence rates of atherosclerotic events and all-cause mortality: analyses from the Canakinumab Anti-Inflammatory Thrombosis Outcomes Study (CANTOS). Eur Heart J. 2018;39(38):3499–507.
Antonopoulos AS, Angelopoulos A, Tsioufis K, et al. Cardiovascular risk stratification by coronary computed tomography angiography imaging: current state-of-the-art. Eur J Prev Cardiol. 2022;29(4):608–24.
Antoniades C, Antonopoulos AS, Deanfield J. Imaging residual inflammatory cardiovascular risk. Eur Heart J. 2020;41(6):748–58.
Ma R, van Assen M, Ties D, et al. Focal pericoronary adipose tissue attenuation is related to plaque presence, plaque type, and stenosis severity in coronary CTA. Eur Radiol. 2021;31(10):7251–61.
Zhou Y, Wei Y, Wang L, et al. Decreased adiponectin and increased inflammation expression in epicardial adipose tissue in coronary artery disease. Cardiovasc Diabetol. 2011;10:2.
Zhu X, Chen X, Ma S, et al. Dual-layer spectral detector CT to study the correlation between pericoronary adipose tissue and coronary artery stenosis. J Cardiothorac Surg. 2021;16(1):325.
Oikonomou EK, Marwan M, Desai MY, et al. Non-invasive detection of coronary inflammation using computed tomography and prediction of residual cardiovascular risk (the CRISP CT study): a post-hoc analysis of prospective outcome data. Lancet. 2018;392(10151):929–39.
Konwerski M, Gromadka A, Arendarczyk A, et al. Atherosclerosis pathways are activated in pericoronary adipose tissue of patients with coronary artery disease. J Inflamm Res. 2021;14:5419–31.
Yao Y, Li X, Wang Z, et al. Interaction of lipids, mean platelet volume, and the severity of coronary artery disease among chinese adults: a mediation analysis. Front Cardiovasc Med. 2022;9: 753171.
Park S, Ahn JM, Kim TO, et al. Revascularization in patients with left main coronary artery disease and left ventricular dysfunction. J Am Coll Cardiol. 2020;76(12):1395–406.
He XY, Gao CQ. Peri-operative application of intra-aortic balloon pumping reduced in-hospital mortality of patients with coronary artery disease and left ventricular dysfunction. Chin Med J (Engl). 2019;132(8):935–42.
Qin B, Li Z, Zhou H, Liu Y, Wu H, Wang Z. The predictive value of the perivascular adipose tissue CT fat attenuation index for coronary in-stent restenosis. Front Cardiovasc Med. 2022;9: 822308.
Writing Committee Members, Lawton JS, Tamis-Holland JE et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022; 79(2):197–215.
Gensini GG. A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol. 1983;51(3):606.
Schiller NB, Shah PM, Crawford M, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2(5):358–67.
Mohammadzadeh M, Mohammadzadeh V, Shakiba M, et al. Assessing the relation of epicardial fat thickness and volume, quantified by 256-slice computed tomography scan, with coronary artery disease and cardiovascular risk factors. Arch Iran Med. 2018;21(3):95–100.
Yu W, Liu B, Zhang F, et al. Association of epicardial fat volume with increased risk of obstructive coronary artery disease in Chinese patients with suspected coronary artery disease. J Am Heart Assoc. 2021;10(6): e018080.
Liu J, Li J, Pu H, et al. Cardiac remodeling and subclinical left ventricular dysfunction in adults with uncomplicated obesity: a cardiovascular magnetic resonance study. Quant Imaging Med Surg. 2022;12(3):2035–50.
de Wit-Verheggen VHW, Altintas S, Spee RJM, et al. Pericardial fat and its influence on cardiac diastolic function. Cardiovasc Diabetol. 2020;19(1):129.
Gorter PM, van Lindert AS, de Vos AM, et al. Quantification of epicardial and peri-coronary fat using cardiac computed tomography; reproducibility and relation with obesity and metabolic syndrome in patients suspected of coronary artery disease. Atherosclerosis. 2008;197(2):896–903.
Wilson S, Mone P, Kansakar U, et al. Diabetes and restenosis. Cardiovasc Diabetol. 2022;21(1):23.
Azab M, Al-Shudifat AE, Johannessen A, et al. Are risk factors for coronary artery disease different in persons with and without obesity? Metab Syndr Relat Disord. 2018;16(8):440–5.
Qu Y, Yang J, Zhang F, et al. Relationship between body mass index and outcomes of coronary artery disease in Asian population: insight from the FOCUS registry. Int J Cardiol. 2020;300:262–7.
Guan B, Liu L, Li X, et al. Association between epicardial adipose tissue and blood pressure: a systematic review and meta-analysis. Nutr Metab Cardiovasc Dis. 2021;31(9):2547–56.
Rabkin SW. The relationship between epicardial fat and indices of obesity and the metabolic syndrome: a systematic review and meta-analysis. Metab Syndr Relat Disord. 2014;12(1):31–42.
Xie Z, Zhu J, Li W, et al. Relationship of epicardial fat volume with coronary plaque characteristics, coronary artery calcification score, coronary stenosis, and CT-FFR for lesion-specific ischemia in patients with known or suspected coronary artery disease. Int J Cardiol. 2021;332:8–14.
Yin R, Tang X, Wang T, et al. Cardiac CT scanning in coronary artery disease: epicardial fat volume and its correlation with coronary artery lesions and left ventricular function. Exp Ther Med. 2020;20(4):2961–8.
Mahabadi AA, Berg MH, Lehmann N, et al. Association of epicardial fat with cardiovascular risk factors and incident myocardial infarction in the general population: the Heinz Nixdorf Recall Study. J Am Coll Cardiol. 2013;61(13):1388–95.
Iacobellis G. Epicardial adipose tissue in endocrine and metabolic diseases. Endocrine. 2014;46(1):8–15.
Iacobellis G, Bianco AC. Epicardial adipose tissue: emerging physiological, pathophysiological and clinical features. Trends Endocrinol Metab. 2011;22(11):450–7.
de Vos AM, Prokop M, Roos CJ, et al. Peri-coronary epicardial adipose tissue is related to cardiovascular risk factors and coronary artery calcification in post-menopausal women. Eur Heart J. 2008;29(6):777–83.
Williams KJ, Tabas I. Atherosclerosis–an inflammatory disease. N Engl J Med. 1999;340(24):1928–9.
Han X, Hao Li, Jing-Jing W, et al. IL-18 cleavage triggers cardiac inflammation and fibrosis upon β-adrenergic insult. Eur Heart J. 2018;39:60–9.
Antonopoulos AS, Margaritis M, Verheule S, et al. Mutual regulation of epicardial adipose tissue and myocardial Redox State by PPAR-γ/Adiponectin signalling. Circ Res. 2016;118(5):842–55.
Zhang F, Xia Y, Yan W, et al. Sphingosine 1-phosphate signaling contributes to cardiac inflammation, dysfunction, and remodeling following myocardial infarction. Am J Physiol Heart Circ Physiol. 2016;310(2):H250–61.
Ma S, Chen X, Ma Y, et al. Lesion-specific peri-coronary fat attenuation index is associated with functional myocardial ischemia defined by abnormal fractional flow reserve. Front Cardiovasc Med. 2021;8: 755295.
Oikonomou EK, Antonopoulos AS, Schottlander D, et al. Standardized measurement of coronary inflammation using cardiovascular computed tomography: integration in clinical care as a prognostic medical device. Cardiovasc Res. 2021;117(13):2677–90.
Antonopoulos AS, Sanna F, Sabharwal N, et al. Detecting human coronary inflammation by imaging perivascular fat. Sci Transl Med. 2017;9(398):eaa12658.
Sun JT, Sheng XC, Feng Q, et al. Pericoronary fat attenuation index is associated with vulnerable plaque components and local immune-inflammatory activation in patients With Non-ST elevation acute coronary syndrome. J Am Heart Assoc. 2022;11(2): e022879.
Hoshino M, Yang S, Sugiyama T, et al. Peri-coronary inflammation is associated with findings on coronary computed tomography angiography and fractional flow reserve. J Cardiovasc Comput Tomogr. 2020;14:483–9.
Liu Y, Sun Y, Hu C, et al. Perivascular adipose tissue as an indication, contributor to, and therapeutic target for atherosclerosis. Front Physiol. 2020;11: 615503.
Dai X, Yu L, Lu Z, Shen C, Tao X, Zhang J. Serial change of perivascular fat attenuation index after statin treatment: Insights from a coronary CT angiography follow-up study. Int J Cardiol. 2020;319:144–9.
Gambardella J, Wang X, Mone P, Khondkar W, Santulli G. Genetics of adrenergic signaling drives coronary artery calcification. Atherosclerosis. 2020;310:88–90.
Tesche C, Otani K, De Cecco CN, et al. Influence of coronary calcium on diagnostic performance of machine learning CT-FFR: results from machine registry. JACC Cardiovasc Imaging. 2020;13(3):760–70.
Goeller M, Tamarappoo BK, Kwan AC, et al. Relationship between changes in pericoronary adipose tissue attenuation and coronary plaque burden quantified from coronary computed tomography angiography. Eur Heart J Cardiovasc Imaging. 2019;20(6):636–43.
Appiah D, Nwabuo CC, Ebong IA, et al. The association of age at natural menopause with pre- to postmenopausal changes in left ventricular structure and function: the Coronary Artery Risk Development in Young Adults (CARDIA) study. Menopause. 2022;29(5):564–72.
Kwok CS, Bachmann MO, Mamas MA, et al. Effect of age on the prognostic value of left ventricular function in patients with acute coronary syndrome: a prospective registry study. Eur Heart J Acute Cardiovasc Care. 2017;6(2):191–8.
Siontis GC, Branca M, Serruys P, et al. Impact of left ventricular function on clinical outcomes among patients with coronary artery disease. Eur J Prev Cardiol. 2019;26(12):1273–84.
Matsuura N, Nagasawa K, Minagawa Y, et al. Restraint stress exacerbates cardiac and adipose tissue pathology via β-adrenergic signaling in rats with metabolic syndrome. Am J Physiol Heart Circ Physiol. 2015;308(10):H1275–86.
Petkevicius K, Bidault G, Virtue S, et al. Macrophage beta2-adrenergic receptor is dispensable for the adipose tissue inflammation and function. Mol Metab. 2021;48: 101220.
Nerlekar N, Muthalaly RG, Wong N, et al. Association of volumetric epicardial adipose tissue quantification and cardiac structure and function. J Am Heart Assoc. 2018;7(23): e009975.
Cavalcante JL, Tamarappoo BK, Hachamovitch R, et al. Association of epicardial fat, hypertension, subclinical coronary artery disease, and metabolic syndrome with left ventricular diastolic dysfunction. Am J Cardiol. 2012;110(12):1793–8.
Honold S, Wildauer M, Beyer C, et al. Reciprocal communication of pericoronary adipose tissue and coronary atherogenesis. Eur J Radiol. 2021;136: 109531.
Hermann DM, Gronewold J, Lehmann N, et al. Coronary artery calcification is an independent stroke predictor in the general population. Stroke. 2013;44(4):1008–13.
Bengs S, Haider A, Warnock GI, et al. Quantification of perivascular inflammation does not provide incremental prognostic value over myocardial perfusion imaging and calcium scoring. Eur J Nucl Med Mol Imaging. 2021;48(6):1806–12.
Acknowledgements
Thanks to Professor Changjie Pan for his support of this scientific research. This study received funding from major science and technology projects of the Changzhou Science and Technology Bureau (CE20205047) and the Natural Science Foundation of the Xinjiang Autonomous Region (2022D01F52). Institutional Review Board approval was obtained from the Changzhou No. 2 People’s Hospital affiliated with Nanjing Medical University. We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Funding
Major science and technology projects of the Changzhou Science and Technology Bureau (CE20205047) and the Natural Science Foundation of Xinjiang Autonomous Region (2022D01F52).
Author information
Authors and Affiliations
Contributions
DY: design, acquisition, analysis, interpretation of data; HY: interpretation of data; ZW: interpretation of data; XW: the creation of new software used in the work; XW: analysis; CP: revised manuscript, guided the experimental process. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This retrospective study was approved by the ethics committee of the Affiliated Changzhou No. 2 People's Hospital of Nanjing Medical University ([2016] LYA003), and the need to obtain informed consent was waived. All methods were carried out in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
The PCAT intra- and interobserver consistencies.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
You, D., Yu, H., Wang, Z. et al. The correlation of pericoronary adipose tissue with coronary artery disease and left ventricular function. BMC Cardiovasc Disord 22, 398 (2022). https://doi.org/10.1186/s12872-022-02843-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-022-02843-y