Abstract
Background
Blood pressure (BP) exhibits seasonal variations, with peaks reported in winter. However, the association between seasonal variations and blood pressure variability in patients with new-onset essential hypertension is not fully understood. This study evaluated the potential association of seasonal variations with new-onset essential hypertension.
Methods
This retrospective observational study recruited a total of 440 consecutive patients with new-onset essential hypertension who underwent 24-h ambulatory electrocardiograph (ECG) and BP measurement at our department between January 2019 and December 2019. Demographic and baseline clinical data including BP variability, heart rate variability, and blood tests were retrieved. Multivariate linear regression analysis was performed to identify factors independently associated with mean BP and BP variability.
Results
Among the 440 patients recruited, 93 cases were admitted in spring, 72 in summer, 151 in autumn, and 124 in winter. Univariate analysis revealed that systolic BP (SBP), diastolic BP (DBP), high-sensitivity C-reactive protein, SBP drop rate, DBP drop rate, 24-h standard deviation of SBP, 24-h standard deviation of DBP, 24-h SBP coefficient of variation, and 24-h DBP coefficient of variation were associated with patients admitted in winter (P < 0.05 for all). Multivariate linear regression analysis showed that winter was the influencing factor of 24-h standard deviation of SBP (B = 1.851, t = 3.719, P < 0.001), 24-h standard deviation of DBP (B = 1.176, t = 2.917, P = 0.004), 24-h SBP coefficient of variation (B = 0.015, t = 3.670, P < 0.001), and 24-h DBP coefficient of variation (B = 0.016, t = 2.849, P = 0.005) in hypertensive patients.
Conclusions
Seasonal variations are closely associated with BP variability in patients with new-onset essential hypertension. Our study provides insight into the underlying pathogenesis of new-onset essential hypertension.
Similar content being viewed by others
Introduction
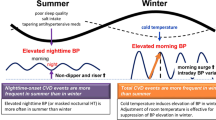
Essential hypertension, also known as primary hypertension, is the most common risk factor for cardiovascular diseases [1, 2]. Therefore, it is imperative to control hypertension in daily life. It has been well documented that many factors, such as age, smoking, and dietary habits, can affect new-onset essential hypertension. Seasonal variations have also been proposed as an additional risk factor. For example, higher environmental temperatures have been linked to lower blood pressure (BP), while lower temperatures have been linked to higher BP [3]. Although an association between seasonal variations and BP fluctuations has long been noted, there is very little agreement regarding how to manage the influence of seasonal changes on BP [3,4,5,6,7,8,9]. It should be pointed out that the effects of seasonal variations could be easily overlooked, leading to failed BP control and often catastrophic adverse events in clinical practice [4].
Previous studies have shown that cardiovascular risk can be appropriately managed by monitoring BP and seasonal changes [4, 8]. Of note, there is evidence supporting an association between BP fluctuation and environmental temperatures [3]. Current clinical and experimental data indicate that there are correlations between winter and autonomic nervous system activation and that a rise in peripheral vascular resistance contributes to the onset of essential hypertension [3]. In addition, BP variability has received much attention because of the wide application of 24-h ambulatory BP monitoring. A higher BP variability serves as a validated predictor of increased risk of cardiovascular events, independently of the average BP values [10]. However, to the best of our knowledge, the potential relationships between mean BP levels, BP variability, and seasonal variations in patients with new-onset essential hypertension have not been well examined. In the present study, we sought to systematically evaluate associations between seasonal variations and aspects of new-onset essential hypertension.
Methods
Patient recruitment and study design
This retrospective observational study recruited 440 consecutive patients with a first diagnosis of essential hypertension who were scheduled to receive 24-h ambulatory electrocardiograph (ECG) and BP measurement at the People's Hospital of Xuancheng City between January 2019 and December 2019. These patients included 93 who were admitted in spring, 72 in summer, 151 in autumn, and 124 in winter. Diagnostic criteria of essential hypertension conformed to criteria recently described in clinical methodology: BP measured at home three times on different days with a systolic BP (SBP) > 140 mmHg or diastolic BP (DBP) > 90 mmHg [11]. Patients were excluded if they had any of the following clinical conditions: previous diagnosis of hypertension, secondary hypertension, declined to accept 24-h ambulatory ECG, BP measurement with non-effective readings > 20%, medications that affected BP or heart rate before admission, acute coronary syndrome, implantation of pacemaker, stroke, cardiopulmonary insufficiency, cancer, any autoimmune diseases, any hematological diseases, severe infection, or acute mental or psychological stress. The study was approved by the Ethics Committee of The People's Hospital of Xuancheng City (No. 2022-1w002-01) before the performance. Because this was a retrospective observational study, the ethics committee of The People's Hospital of Xuancheng City waived the requirement for informed consent from eligible patients. All methods were performed in accordance with the relevant guidelines and regulations. Figure 1 shows the flow chart of patient selection in this study.
24-h ambulatory ECG and BP measurement
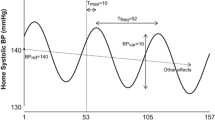
All patients underwent 24-h ambulatory ECG and BP monitoring (CB-2304-A/CB-2306-A, VasoMedical, China) upon admission to the Department of Cardiology. 24-h ambulatory BP monitoring was performed using a cuff-monitor. BP was measured every 25 min during the day (between 08:00 and 22:00) and every 60 min during the night (between 22:00 and 08:00 the next morning) using a cuff size that fit the individual’s arm circumference as previously described [12]. Only recordings rated as of sufficient quality, defined as at least 80% of valid readings over the 24-h period, were included for analysis; at least 20 valid daytime and at least 7 valid nighttime measurements were required. All participants were requested to maintain their normal diet and to refrain from vigorous exercise, alcohol, smoking, and caffeinated beverages the day of the test. The following parameters were recorded: 24-h ambulatory BP, containing 24-h average SBP, 24-h SBP load, 24-h DBP, 24-h DBP load, 24-h average BP, 24-h pulse pressure difference, 24-h SBP standard deviation, 24-h DBP standard deviation, 24-h SBP coefficient of variation, 24-h DBP coefficient of variation, ambulatory arterial stiffness index (AASI), heart rate variability, containing standard deviation of normal R-R intervals (SDNN), standard deviation average of NN intervals (SDANN), root mean square successive difference of normal R-R intervals (rMSSD), percent of the number of times that the difference between adjacent normal RR intervals > 50 ms in the total number of NN intervals (PNN50), high-frequency power (HF), low-frequency power (LF), and LF/HF ratio. All parameters were calculated according to previously published methods using customized and validated software [13, 14]. 24-h SBP coefficient of variation = (24-h SBP standard deviation) / (24-h average SBP; 24-h DBP coefficient of variation = (24-h DBP standard deviation) / (24-h average DBP). BP load was defined as the percentage of readings greater than the normal in total measurements, based on a daytime SBP > 140 mmHg or DBP > 90 mmHg and a nighttime SBP > 120 mmHg or DBP > 80 mmHg. The SBP drop rate = [(mean daytime SBP – mean night SBP) / mean daytime SBP]*100, and the DBP drop rate = [(mean daytime DBP—mean night DBP) / mean daytime DBP]*100. The SDNN and SDANN were regarded as vagal and sympathetic influences [14]. rMSSD and pNN50 were regarded as parasympathetic nerve activity [15]. For frequency domain variables, HF generally reflects cardiac parasympathetic nerve activity, while LF is possibly correlated with sympathetic tone [15]. The LF/HF ratio is an indicator of the balance between sympathetic and parasympathetic activities, with higher LF/HF ratios indicating increased sympathetic nerve excitability [15].
Definition
The date of 24-h ambulatory BP monitoring was used to define the seasons as follows: spring: March, April, May; summer: June, July, August; autumn: September, October, November; and winter: December, January, February [16]. Data on meteorological parameters such as average temperatures, the maximum and minimum temperatures, and precipitation during each BP monitoring period were obtained from the Meteorology Bureau of Xuancheng (http://ah.cma.gov.cn/). Smoking was defined as current smoker. Educational qualifications were classified as illiterate, primary school, middle school, high school, specific college course, or above.
Statistical analysis
Normally distributed continuous variables are presented as mean ± standard deviation (SD), and medians and interquartile ranges (IQRs) are otherwise presented. Categorical variables are presented as percentages. A chi-square (χ2) test was used to analyze the differences in categorical variables between groups, and analysis of variance (ANOVA) was used for comparisons of data among multiple groups. The Mann–Whitney U test or Kruskal–Wallis variance analysis was used for non-normally distributed data between groups. The multivariate linear regression analysis was conducted with 24-h average SBP, 24-h average DBP, 24-h standard deviation of SBP, 24-h standard deviation of DBP, 24-h SBP coefficient of variation, or 24-h DBP coefficient of variation as the dependent variable, and the variables that were statistically significant in the univariate analysis were considered independent variables. Multiple linear regression analysis was used to study the association of seasonal variations with mean BP levels and BP coefficient of variation. A P-value less than 0.05 was considered statistically significant. All statistical analyses were performed using SPSS23 ( IBM).
Results
Demographic and baseline clinical characteristics of patients
A total of 440 patients were registered in this study. The patients were divided into four groups according to the dates of ECG and BP measurements: 93 in spring, 72 in summer, 151 in autumn, and 124 in winter. The demographic and baseline clinical characteristics and biochemical parameters of patients in these four groups are presented in Table 1. There were no differences in the demographic and baseline clinical characteristics between these four groups. However, compared with other groups, the winter group were more likely to have higher clinic SBP (P < 0.001), higher clinic DBP (P < 0.001), higher high-sensitivity C-reactive protein (hs-CRP) (P = 0.003), lower maximum temperature (P < 0.001), lower minimum temperature (P < 0.001), lower average temperature (P < 0.001), and lower mean precipitation.
24-h ambulatory ECG and BP measurements of patients
The winter group had the highest SBP drop rate, DBP drop rate, 24-h SBP standard deviation, 24-h DBP standard deviation, 24-h SBP coefficient of variation, and 24-h SBP coefficient of variation among the four groups (all P < 0.05; Table 2). 24-h SBP standard deviation, 24-h DBP standard deviation, 24-h SBP coefficient of variation, and 24-h SBP coefficient of variation were higher in winter than in spring, summer, and autumn (all P < 0.05). Heart rate, mean daytime heart rate, LF, and HF were higher in summer and winter than in spring and autumn (all P < 0.05).
Linear regression analysis of 24-h average SBP
The demographic and baseline clinic characteristics and laboratory data of patients based on 24-h average SBP are shown in Table 3. Those with higher 24-h average SBP were mostly male, current smoker, and higher educational participants (all P < 0.05; (Table 4), Table 5). Multivariate linear regression analysis revealed that educational qualifications were independently and positively associated with 24-h average SBP (P < 0.05; Table 3).
Linear regression analysis of 24-h average DBP
The demographic and clinic characteristics and laboratory data of patients based on 24-h average DBP are shown in Table 4. Those with higher 24-h average DBP were mostly male and current smoker and had lower age and higher triglyceride levels without coronary artery disease (all P < 0.05; Table 4). Multivariate linear regression analysis revealed that age and smoking were independently associated with 24-h average DBP (all P < 0.05; Table 4).
Linear regression analysis of 24-h standard deviation of SBP
The demographic and clinic characteristics and laboratory data of patients based on 24-h standard deviation of SBP are presented in Table 5. Those with higher 24-h average SBP were mostly older, had lower educational qualifications, and were admitted in winter (all P < 0.05; Table 5). Multivariate linear regression analysis showed that winter was independently associated with 24-h standard deviation of SBP (P < 0.05; Table 5).
Linear regression analysis of 24-h standard deviation of DBP
The demographic and clinical characteristics and laboratory data of patients based on 24-h standard deviation of DBP are shown in Table 6. Those with 24-h standard deviation of DBP were mostly current smoker and had higher body mass index (BMI) and admitted in winter (all P < 0.05; Table 6). Multivariate linear regression analysis showed that current smoker, BMI, and winter were independently associated with 24-h standard deviation of DBP (P < 0.05; Table 6).
Linear regression analysis of 24-h SBP coefficient of variation
The demographic and clinical characteristics and laboratory data of patients based on 24-h SBP coefficient of variation are presented in Table 7. Those with 24-h SBP coefficient of variation were mostly older, had differences in educational qualifications and triglycerides, and were admitted in winter (all P < 0.05; Table 7). Multivariate linear regression analysis showed that educational qualifications and admission in winter were independently associated with 24-h standard deviation of SBP (P < 0.05; Table 7).
Linear regression analysis of 24-h DBP coefficient of variation
The demographic and clinical characteristics and laboratory data of patients based on 24-h DBP coefficient of variation are shown in Table 8. Those with 24-h DBP coefficient of variation were mostly older, had differences in diabetes mellitus, family history of hypertension, and higher educational qualifications, and were admitted in winter (all P < 0.05; Table 8). Multivariate linear regression analysis showed that age and admission in winter were independently associated with 24-h standard deviation of DBP (P < 0.05; Table 8).
Discussion
In this retrospective observational cohort study of patients with a first diagnosis of essential hypertension, we found that SBP, DBP, hs-CRP, SBP drop rate, DBP drop rate, 24-h standard deviation of SBP, 24-h standard deviation of DBP, 24-h SBP coefficient of variation, and 24-h DBP coefficient of variation were associated with new-onset essential hypertension in patients admitted in winter. It is noteworthy that those in the winter group had the highest 24-h standard deviation of SBP, 24-h standard deviation of DBP, 24-h SBP coefficient of variation, and 24-h DBP coefficient of variation.
Underlying mechanisms of seasonal variations affecting new-onset essential hypertension
The significant effects of seasonal changes on cardiovascular diseases, including hypertension, have been well documented. In particular, BP has been reported to be higher in winter [17,18,19]. With seasonal shifts and corresponding temperature changes, there is a concomitant shift in the seasonal peaks of BP [20]. Epidemiological studies revealed that an inverse correlation between BP and temperature is likely the result of more physiological thermoregulation: vasoconstriction and increased peripheral resistance in cold environments, and vasodilatation and decreased peripheral vascular resistance in warm environments [21]. Consistent with the above findings, we found that SBP and DBP were highest in winter. Our findings are also consistent with previous findings showing that the circadian rhythm of BP fluctuation is affected by seasonal changes and temperatures, with a larger nocturnal BP decline and a more common dipping pattern in winter compared to summer [22]. We speculated that seasonal variation-linked BP changes are related to sympathetic nervous system activity, which is also affected by large seasonal changes in temperature [23]. In response to chronic cold exposure, circulating norepinephrine levels increase and are independently and negatively correlated with outdoor temperatures [23]. Our findings are inconsistent with one previous study showing that LF, as an index of sympathetic system activity, and HF, as an indicator of parasympathetic activity, were higher in summer and winter than in spring and autumn [15]. In the present study, we did not observe an association between 24-h average SBP and DBP and seasonal variations. It is likely that 24-h average BP was affected by stable ambient temperature modulated by an internal air conditioning system or floor heating system indoors. Notably, the winter group had the highest clinic BP among these four groups. This may be due to the fact that white coat effect leading to increased sympathetic nervous activity [24]. Moreover, in outpatient clinic setting, cold-induced peripheral vasoconstriction leading to a rise in peripheral vascular resistance may account for the increase in clinical BP with cold exposure. More research is needed to understand the sympathetic nervous activity that is affected by seasonal variations.
Hypertension is a multi-factorial chronic inflammatory disease, and a tight link between atherosclerosis and inflammation has emerged [25, 26]. The present study indicated that markers of inflammation were highest in winter. We found that changes in hs-CRP levels also varied seasonally and may be a potential mechanism underlying seasonal-related cases of new-onset essential hypertension. The inflammatory response is mediated to a large extent by oxidative stress and reactive oxygen species, which causes structural and functional changes in blood vessels and endothelial dysfunction, contributing to increased BP [27].
Season and ambulatory BP
Seasonal BP variations are a global phenomenon that affect both sexes, all age groups, normotensive individuals, and untreated and treated hypertensive patients [3]. A previous cross-sectional study of 24 populations from 15 countries in Europe, Asia, and Australia revealed seasonal variations in SBP of 2.9 and 3.4 mmHg in the Northern and Southern Hemispheres, with the lowest points occurring in June and January, respectively [28]. In the present study, BP changed concomitantly with seasonal changes, but 24-h average SBP and DBP were not associated with seasonal variations. In contrast, other studies have revealed a stronger correlation between indoor temperatures and dynamic BP fluctuation compared to associations between outdoor temperatures and BP fluctuation [29]. The inconsistency between these studies is probably due to different criteria used for patient enrollment. It is also likely attributed to the fact that previous studies did not address the association between seasonal variations and new-onset essential hypertension as we did in this study. Importantly, the present study confirmed an association between BP variability and seasonal variations in new-onset essential hypertension. It is now widely accepted that BP variability, as a predictor of poor outcomes for stable coronary heart disease [30], is also a cardiovascular risk factor [31]. In this study, we found that standard deviation of SBP and DBP, 24-h SBP coefficient of variation, and 24-h DBP coefficient of variation were higher in winter than in summer, even after adjusting for comorbidities, which may explain why the incidence of cardiovascular events is affected in winter. Nevertheless, since we did not include weighted measures of BP variability, the “winter” associated differences in variability could be due to higher nighttime dipping during winter. Thus, a large difference between day and nighttime measurements would result in high standard deviation of SBP and DBP, 24-h SBP coefficient of variation, and 24-h DBP coefficient of variation over 24 h. On the basis of seasonal-linked BP fluctuations, ambulatory BP measurements can increase the comprehensiveness and accuracy of assessing BP changes during seasonal variations, which is conducive to making optimal treatment decisions. It should be noted that seasonal variations could be easily overlooked, leading to ineffective management of hypertension and potentially disastrous consequences. Therefore, the risk stratifications for new-onset essential hypertension patients require further optimization, particularly for identifying prognostic factors of unfavorable diseases.
Study limitations
First, our work was a retrospective observational study with a relatively small sample size, and thus there could be selection bias in our study. Second, our results might have been influenced by other environmental factors including low atmospheric air pressure, high wind velocity, shorter sunshine duration, and air pollution. Although we chose to focus on environmental temperature in the present study, we cannot rule out the potential effects of other factors on the association between environmental temperature and BP fluctuations observed in this study. Third, all patients were divided into four groups based on the date of examination. Therefore, we did not account for individual differences in the current study. However, age, sex, and cardiovascular risk factors were not significantly different between these groups. Five, 24-h-weighted indices of BP variability were not analyzed [32], because of the retrospective nature of this study, the 24-h ambulatory BP monitoring was not digitally recorded. Therefore, we were unable to investigate all parameters in detail. Six, the 24-h ambulatory BP monitoring device used in this study has not been clinically validated. Seven, 24-h ambulatory ECG and BP monitoring device was different from the traditional method for 24-h ambulatory BP monitoring. Finally, in the present study, we did not perform multiple examinations. Hence, further studies with multiple testing are needed to corroborate our findings from this study.
Conclusions
Winter was independently associated with the highest 24-h standard deviations of SBP, 24-h standard deviations of DBP, 24-h SBP coefficient of variation, and 24-h DBP coefficient of variation. It is important to integrate seasonal variations into clinical practice to make appropriate prevention and management decisions of new-onset essential hypertension.
Availability of data and materials
We collected the demographic data, clinical characteristics, risk factors, blood samples, biochemical data, data of 24-h ambulatory ECG and BP measurement in the People's Hospital of Xuancheng City between January 2019 and December 2019. The data that support the fndings of this study are available from the People's Hospital of Xuancheng City, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. The datasets used and/or analyzed during this study are available from the corresponding author on reasonable request. Requests to access these datasets should be directed to Jun Wang, 1,057,958,292@qq.com.
Abbreviations
- BMI:
-
Body mass index
- SBP:
-
Systolic blood pressure
- DBP:
-
Diastolic blood pressure
- TC:
-
Total cholesterol
- TG:
-
Triglyceride
- HDL-c:
-
High-density lipoprotein cholesterol
- LDL-c:
-
Low-density lipoprotein-cholesterol
- Apo-AI:
-
Apolipoprotein A1
- Apo-B:
-
Apolipoprotein B
- Lp(a):
-
Lipoprotein (a)
- CAD:
-
Coronary artery disease
- hs-CRP:
-
High-sensitivity C-reactive protein
- PCI:
-
Percutaneous transluminal coronary intervention
- SDNN:
-
Standard deviation of normal R-R intervals
- SDANN:
-
Standard deviation average of NN intervals
- rMSSD:
-
Root mean square successive difference of normal R-R intervals
- PNN50:
-
Percent of the number of times that the difference between adjacent normal RR intervals > 50 ms in the total number of NN intervals
- HF:
-
High-frequency power
- LF:
-
Low-frequency power
References
Mahajan S, Danwei Z, Siyun H, et al. Prevalence awareness and treatment of isolated diastolic hypertension: insights from the China PEACE million persons project. J Am Heart Assoc. 2019;8:012954.
Nambiar L, Dauerman H, Vanburen P, et al. Decade long temporal trends in hypertension-related deaths, 2007–2017[J]. J Am Coll Cardiol. 2020;75(11):13.
Messerli FH, Hofstetter L, Rimoldi SF, et al. Risk factor variability an cardiovascular outcome: JACC review topic of the week. J Am Coll Cardiol. 2019;73:2596–603. https://doi.org/10.1016/j.jacc.2019.02.063.
Hanazawa T, Asayama K, Watabe D, et al. Association between amplitude of seasonal variation in self-measured home blood pressure and cardiovascular outcomes: HOMED-BP (hypertension objective treatment based on measurement by electrical devices of blood pressure) Study. J Am Heart Assoc. 2018;7(10):e008509.
Fedecostante M, Barbatelli P, Guerra F, et al. Summer does not always mean lower: seasonality of 24 h, daytime, and night-time blood pressure. J Hypertens. 2012;30:1392–8.
Stergiou GS, Myrsilidi A, Kollias A, et al. Seasonal variation in meteorological parameters and clinical, ambulatory and home blood pressure: predicting factors and clinical implications. Hypertens Res. 2015;38:869–75.
Hanazawa T, Asayama K, Watabe D, et al. Seasonal variation in self-measured home blood pressure among patients on antihypertensive medications: HOMED-BP study. Hypertens Res. 2017;40(3):284–90.
Umishio W, Ikaga T, Kario K, et al. Cross-sectional analysis of the relationship between home blood pressure and indoor temperature in winter: a nationwide smart wellness housing survey in Japan. Hypertension. 2019;74(4):756–66.
Stergiou George S, Paolo P, Modesti Pietro A, et al. Seasonal variation in blood pressure: evidence consensus and recommendations for clinical practice consensus statement by the European society of hypertension working group on blood pressure monitoring and cardiovascular variability. J Hypertens. 2020;38(7):1235–43.
Schutte AE, Kollias A, Stergiou GS. Blood pressure and its variability: classic and novel measurement techniques. Nat Rev Cardiol. 2022;19:1–12.
Joint Committee for Guideline Revision. 2018 Chinese guidelines for prevention and treatment of hypertension-a report of the revision committee of Chinese guidelines for prevention and treatment of hypertension. J Geriatr Cardiol. 2019;16(3):182–241.
Kazuomi K, Satoshi H, Yook-Chin C, et al. Guidance on ambulatory blood pressure monitoring: A statement from the HOPE Asia Network. J Clin Hypertens. 2021;23:411–21.
Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93:1043–65.
O'Brien Eoin, White William B,Parati Gianfranco et al. Ambulatory blood pressure monitoring in the 21st century. J Clin Hypertens (Greenwich) 2018, 20(7): 1108–1111.
Xhyheri B, Manfrini O, Mazzolini M, et al. Heart rate variability today. Prog Cardiovasc Dis. 2012;55(3):321–31.
Narita K, Hoshide S, Fujiwara T, et al. Seasonal variation of home blood pressure and its association with target organ damage: the J-HOP Study (Japan morning surge-home blood pressure). Am J Hypertens. 2020;7(7):620–8.
Spencer FA, Goldberg RJ, Becker RC, et al. Seasonal distribution of acute myocardial infarction in the second National registry of myocardial infarction. J Am Coll Cardiol. 1998;31(6):1226–33.
Gallerani M, Portaluppi F, Maida G, et al. Circadian and circannual rhythmicity in the occurrence of subarachnoid hemorrhage. Stroke. 1996;27(10):1793–7.
Mehta Rajendra H, Manfredini Roberto, Hassan Fauziya et al. Chronobiological patterns of acute aortic dissection. Circulation, 2002, 106(9): 1110–5.
Wataru U, Toshiharu I, Kazuomi K, et al. Cross-sectional analysis of the relationship between home blood pressure and indoor temperature in winter: a nationwide smart wellness housing survey in Japan. Hypertension. 2019;74(4):756–66.
Modesti P, Season A. temperature and blood pressure: a complex interaction. Eur J Intern Med. 2013;24:604–7.
Fedecostante M, Barbatelli P, Guerra F, et al. Summer does not always mean lower: seasonality of 24h, daytime, and night-time blood pressure. J Hypertens. 2012;30:1392–8.
Winnicki M, Canali C, Accurso V, et al. Relation of 24-hour ambulatory blood pressure and short-term blood pressure variability to seasonal changes in environmental temperature in stage I hypertensive subjects results of the harvest trial. Clin Exp Hypertens. 1996;18(8):995–1012.
Adiyaman A, Aksoy I, Deinum J, et al. Influence of the hospital environment and presence of the physician on the white-coat effect. J Hypertens. 2015;11(11):2245–9.
Federico C, Edorado E, Matteo C, et al. Baseline hs-CRP predicts hypertension remission in metabolic syndrome. Eur J Clin Invest. 2019;49(8):13128.
Zanoli Luca, Briet Marie,Empana Jean P et al. Vascular consequences of inflammation: a position statement from the ESH working group on vascular structure and function and the ARTERY society. J Hypertens, 2020, 38(9): 1682–1698.
Zanoli L, Briet M, Empana JP, et al. Vascular consequences of inflammation: a position statement from the ESH working group on vascular structure and function and the ARTERY society. J Hypertens. 2020;38(9):1682–98.
Helena M-S, Cédric G, Stefanie A, et al. Seasonality of cardiovascular risk factors: an analysis including over 230 000 participants in 15 countries. Heart. 2014;100(19):1517–23.
Keigo S, Kenji O, Junko I, et al. Stronger association of indoor temperature than outdoor temperature with blood pressure in colder months. J Hypertens. 2014;32(8):1582–9.
Vidal-Petiot Emmanuelle,Stebbins Amanda,Chiswell Karen et al. Visit-to-visit variability of blood pressure and cardiovascular outcomes in patients with stable coronary heart disease. Insights from the STABILITY trial. Eur Heart J, 2017, 38(37): 2813–2822.
Authors/Task Force Members,Piepoli Massimo F,Hoes Arno W et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The sixth joint task force of the European society of cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of 10 societies and by invited experts): Developed with the special contribution of the European association for cardiovascular prevention & rehabilitation (EACPR).[J] .Eur J Prev Cardiol, 2016, 23: NP1-NP96.
Grzegorz B, Alessia G, Katarzyna S, et al. A new method for assessing 24-h blood pressure variability after excluding the contribution of nocturnal blood pressure fall. J Hypertens. 2007;25(10):2058–66.
Acknowledgements
Thanks to everyone for supporting our final study.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Conceived and designed the study: JW and YY. Data collection and analyzed the data: LT and JZ.Quality control the study and revision: TX and YX. Wrote the paper: LT and JW. LT, JZ and YX contributed to the work equally and should be regarded as co-first authors. The manuscript was approved by all above authors. All authors read and approved by the final manuscript.
Corresponding authors
Ethics declarations
Ethical approval and consent to participate
The study was approved by the Ethics Committee of The People's Hospital of Xuancheng City (No. 2022-1w002-01) before the performance. And all methods were performed in accordance with the relevant guidelines and regulations.
Consent for publication
Not applicable.
Informed consent
Because this was a retrospective observational study, the ethics committee of The People's Hospital of Xuancheng City waived the requirement for informed consent from eligible patients.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Tang, L., Zhang, J., Xu, Y. et al. Novel insights into the association between seasonal variations, blood pressure, and blood pressure variability in patients with new-onset essential hypertension. BMC Cardiovasc Disord 22, 401 (2022). https://doi.org/10.1186/s12872-022-02840-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-022-02840-1