Abstract
Background
The complement system plays an important role in the development of left ventricular hypertrophy. Complement C1q is an initial component of the classical complement pathway and is related to many inflammatory diseases. We aimed to determine whether there was an association between serum complement C1q and left ventricular hypertrophy induced by coarctation of the aorta (CoA).
Methods
Based on whether CoA was combined with a large ventricular septal defect (VSD) or patent ductus arteriosus (PDA), the patients were divided into a simple CoA group (n = 15) and a complex CoA group (n = 13). Meanwhile, we selected simple large VSD (n = 14) patients and normal children (n = 28) as the control group. The serum complement C1q level was compared using immunity transmission turbidity among different groups.
Results
The preoperative content of C1q in the simple CoA group was significantly lower than that in the complex CoA group and normal group (96.97 ± 20.66 vs. 130.73 ± 35.78, 96.97 ± 20.66 vs. 156.21 ± 29.14, P < 0.05). There was no significant difference in the preoperative content of C1q between the complex CoA group and the large VSD group (P > 0.05). There was a negative correlation between the preoperative complement C1q content and the interventricular septal thickness and left ventricular posterior wall thickness (r = − 0.035, r = − 0.288, P < 0.05). The percentage of postoperative decrease in C1q in children with simple CoA or complex CoA was positively correlated with the time of cardiopulmonary bypass and aortic cross clamp, respectively (r = 0.797, r = 0.622, r = 0.898, r = 0.920, P < 0.05). There was no significant difference in the content of preoperative triglycerides (TG), total cholesterol (TCHO), high-density lipoprotein cholesterol (HDL-C) or low-density lipoprotein cholesterol (LDL-C) among the different groups (P > 0.05). In the simple CoA group and complex CoA group, the preoperative complement C1q, TG, TCHO, HDL-C and LDL-C levels were significantly higher than those after the operation (P < 0.05). There was no significant correlation between preoperative complement C1q and TG, TCHO, HDL-C or LDL-C (P > 0.05).
Conclusions
Complement C1q has an inhibitory effect on the formation of left ventricular hypertrophy, which may not be mediated by regulating lipid metabolism. During cardiac surgery, complement C1q may have a protective effect against myocardial injury.
Similar content being viewed by others
Background
Congenital coarctation of the aorta (CoA) is a common congenital heart disease. Its incidence is approximately 1/2500 live birth infants, and it accounts for 6 ~ 8% [1] of all congenital heart diseases. CoA may be seen in isolation or with other congenital heart anomalies, such as bicuspid aortic valve, ventricular septal defect, patent ductus arteriosus, transposition of the great arteries, atrioventricular canal defects, or leftsided obstructive heart defects [2,3,4]. According to whether it is isolated or combined with other congenital heart anomalies, CoA can be classified as isolated/simple CoA and complex CoA [5]. Some simple CoA may have no obvious symptoms in the early stage, which is easy to ignore and escapes diagnosis. If it is not treated in time, patients usually die of complications such as heart failure, aortic rupture or intracranial hemorrhage due to hypertension [6]. Refractory hypertension and blood pressure differences between the upper and lower limbs are the most important clinical manifestations and signs of CoA [6]. CoA can increase the left ventricular afterload, leading to coarctation proximal hypertension [7]. The increase in the left ventricular afterload will lead to changes in the myocardial tissue and ventricular structure [8, 9]. At the cellular level, it will cause activation and pathological proliferation of cardiomyocyte interstitial fibroblasts, which will lead to left ventricular myocardial fibrosis, hypertrophy and congestive heart failure [10].
The complement system is an essential part of innate and adaptive immunity. It comprises of a group of proteins with enzyme activity in the blood, body fluids and on the surface of the cell membrane. It plays a biological function in regulating phagocytosis, clearing aging apoptotic cells, participating in the immune response and mediating the inflammatory response [11]. C1q is an important promoter of the classical complement pathway. It activates the complement cascade reaction by recognizing the complement binding site of the antibody FC segment in an IgG or IgM immune complex, which leads to clearance of the antigen antibody complex [12]. The complement C1q tumor necrosis factor related protein (CTRPs) superfamily is a cluster of adipokines. The family consists of 15 members. They have been proven to have diverse biological influences on the cardiovascular system [13]. Some studies reported that CTRP-3 could attenuate pressure overload-induced cardiac hypertrophy [14] and the CTRP-6 could attenuate postinfarct cardiac fibrosis [15]. The structure of CTRPs is similar to that of the complement component C1q. Some studies have shown that complement C1q can induce skeletal muscle fibrosis by regulating the Wnt/β-Catenin pathway [16].Therefore, we wondered whether complement C1q is involved in left ventricular hypertrophy. It has not been reported until now.
Therefore, determining the level of complement C1q in patients with CoA will be of great significance to further clarify the pathogenesis of left ventricular hypertrophy induced by CoA. In this study, we analyzed the content of complement C1q and the changes in complement C1q before and after surgery in patients with CoA and clarified the regulatory effect of complement C1q on CoA-induced left ventricular hypertrophy and the correlation between operation-related factors and complement C1q.
Methods
Materials
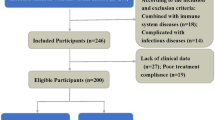
Patients with CoA hospitalized in the pediatric heart center of Beijing Anzhen Hospital between January 1, 2017 and December 31, 2019 were included in this study. Inclusion criteria: (1) Meets the diagnostic criteria of CoA; (2) Clinical diagnosis of simple CoA or complex CoA (CoA with a large ventricular septal defect (VSD) (defect diameter ≥ 10 mm) or CoA with a thick patent ductus arteriosus (PDA) (inner diameter of pulmonary end of arterial catheter ≥ 5 mm), and a left to right shunt at ventricular level or large artery level); (3) Complement C1q examination was performed before and 24 h after the operation, and the clinical data were complete; (4) There were no other systemic diseases. Exclusion criteria: (1) patients with CoA who did not undergo surgery; (2) patients with an early death; (3) CoA with a small ventricular septal defect (defect diameter < 5 mm) or a small patent ductus arteriosus (pulmonary end of the ductus arteriosus < 2 mm), or CoA with other cardiovascular malformations; (4) CoA with a large ventricular septal defect or a thick patent ductus arteriosus and a right to left shunt at the ventricular or large artery level; (5) combined with other systemic diseases; (6) incomplete clinical data before and after the operation. Meanwhile, we selected children with simple large VSD with complete clinical data (defect diameter ≥ 10 mm, left to right shunt at the ventricular level) and normal children as controls. The institutional review board for clinical research at Beijing Anzhen Hospital approved the use of patient medical records for this retrospective review.
Methods
2.2.1 Group division
Based on whether CoA patients had a large VSD or PDA, the patients were divided into a simple CoA group and a complex CoA group (CoA combined with left to right shunt congenital heart disease). The control group was divided into a simple large VSD group and normal group.
2.2.2 Measurement of echocardiographic parameters
All patients were measured by transthoracic echocardiography with an ultrasonic instrument (PHILIP IE33) one day before the operation. The position and length of the aortic constriction were measured by two-dimensional echocardiography through the long axis section of the suprasternal aortic arch, and the blood flow velocity at the constriction was measured by color Doppler flow imaging. Left ventricular end diastolic diameter (LVDd), interventricular septal thickness (IVST) and left ventricular posterior wall thickness (LVPWT) were measured by M-mode echocardiography through the parasternal left ventricular long axis section. The diameter of the VSD and the left to right shunt velocity at the ventricular level were measured through multiple sections by two-dimensional echocardiography and color Doppler flow imaging. The diameter, length and left to right shunt velocity of the patent ductus arteriosus were measured by two-dimensional echocardiography and color Doppler flow imaging through the short axis section of the parasternal artery.
IVST, LVPWT and LVDd were converted to Z scores (number of standard deviations from the expected mean) using the formula from Sluymans and Colan [17].
2.2.3 Measurement of complement C1q
Venous blood was taken from patients with an empty stomach in the case group one day before the operation and 24 h after the operation in the morning. Venous blood was taken from the children in the control group with an empty stomach in the morning. The serum was separated from the blood samples. The content of complement C1q was determined by a complement C1q determination kit (immunoturbidimetry) (Shanghai Beijia Biochemical Reagent Co., Ltd., China).
2.2.4 Measurement of triglyceride (TG), total cholesterol (TCHO), high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C)
The venous blood of patients with an empty stomach was taken before the operation, and serum samples were used to determine the contents of TG, TCHO, HDL-C and LDL-C.
2.2.5 Patients with simple or complex CoA were all treated with surgery.
The intraoperative cardiopulmonary bypass time, aortic cross clamp time and mechanical ventilation time were collected.
Statistical analysis
SPSS 20.0 was used for statistical analysis. Measurement data are presented as the mean ± standard deviation (\(\overline{X} \pm S\)), and count data are expressed as the frequency (percentage). The χ2 test was performed to analyze the sex distribution. A t-test was used to compare the differences between the two groups of normally distributed measurement data, and one-way ANOVA was used to compare the differences among three or more groups. The Mann–Whitney U test was used to compare the nonnormally distributed measurement data between the two groups. For the correlation between complement C1q and IVSTZ, LVPWTZ, LVDdZ, TG, TCHO, HDL-C, LDL-C, cardiopulmonary bypass time, aortic cross clamp time and mechanical ventilation time, if the data conformed to a normal distribution, pearson correlation analysis was used; if the data did not conform to a normal distribution, rank correlation analysis was used. P < 0.05 was considered statistically significant.
Results
Clinical characteristics
There were 28 cases in the case group, including 15 cases (53.6%) in the simple CoA group and 13 cases (46.4%) in the complex CoA group (11 cases (39.3%) were CoA combined with large VSD, and 2 cases (7.1%) were CoA combined with thick PDA). There were 42 cases in the control group, including 14 cases (33.3%) in the simple large VSD group and 28 cases (66.7%) in the normal group.
There was no significant difference in sex, age, weight or height among the simple CoA group, complex CoA group, simple large VSD group and normal group (P > 0.05) (Table 1).
The differences in IVSTZ, LVPWTZ and LVDdZ in different groups
The IVSTZ and LVPWTZ of patients in the simple CoA group were significantly higher than those in the complex CoA group and normal group (P < 0.05). There was no significant difference in LVDdZ between the complex CoA group and the normal group (P > 0.05). There was no significant difference in IVSTZ and LVPWTZ between patients in the complex CoA group and the large VSD group (P > 0.05), but the LVDdZ in the complex CoA group was significantly lower than that in the large VSD group (P < 0.05) (Table 2).
TG, TCHO, HDL-C and LDL-C values and their correlation with complement C1q in different groups
The preoperative content of complement C1q in the simple CoA group was significantly lower than that in the complex CoA group and normal group (96.97 ± 20.66 vs. 130.73 ± 35.78, 96.97 ± 20.66 vs. 156.21 ± 29.14, t = 3.109, t = − 6.973, P < 0.05) (Fig. 1). There was no significant difference in preoperative complement C1q content between the complex CoA group and the large VSD group (130.73 ± 35.78 vs. 147.62 ± 50.38, t = − 0.997, P > 0.05) (Fig. 1). The postoperative content of complement C1q in the simple CoA group or complex CoA group was significantly lower than it was pre-operation (69.40 ± 24.19 vs 96.97 ± 20.66, 78.38 ± 28.12 vs. 130.73 ± 35.78, t = 6.853, t = 5.914, P < 0.05) (Table 3).
The distribution of preoperative content of complement C1q in normal group, simple CoA group, complex CoA group and large VSD group. (The preoperative content of complement C1q in the simple CoA group (n = 15) was significantly lower than that in the complex CoA group (n = 13) and normal group (n = 28) (P < 0.05); There was no significant difference in preoperative complement C1q content between complex CoA group (n = 13) and large VSD group (n = 14) (P > 0.05).)
There was no significant difference in the contents of TG, TCHO, HDL-C and LDL-C before operation in each group (P > 0.05) (Table 4). In simple CoA group and complex CoA group, the preoperative complement C1q, TG, TCHO, HDL-C and LDL-C levels were significantly higher than those after the operation, respectively (P < 0.05) (Table 4). There was no significant correlation between preoperative complement C1q and TG, TCHO, HDL-C or LDL-C (P > 0.05) (Table 4). In the simple CoA group, there was no significant correlation between postoperative complement C1q and TG, TCHO, HDL-C or LDL-C (P > 0.05) (Table 4).However, in the complex CoA group, postoperative complement C1q was positively correlated with TCHO, HDL and LDL (r = 0.893, 0.929, 0.819, P < 0.05) (Table 4).
The correlation between complement C1q and left ventricular hypertrophy
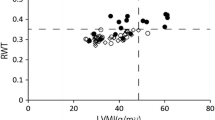
The content of preoperative complement C1q was negatively correlated with the IVSTZ and LVPWTZ (r = − 0.035, r = − 0.288, P < 0.05) (Fig. 2, Fig. 3), and there was no significant correlation with LVDdZ (r = − 0.233, P > 0.05) (Fig. 4).
The correlation between complement C1q and intraoperative related factors
Patients in the simple CoA group and complex CoA group were treated with surgery. In the simple CoA group, 4 children did not undergo cardiopulmonary bypass during the operation, and in the complex CoA group, 5 children did not undergo cardiopulmonary bypass. There was no significant difference in the percentage of postoperative complement C1q decrease between the simple CoA group and the complex CoA group (0.40 ± 0.17 vs. 0.36 ± 0.21, t = 0.515, P > 0.05). There was no significant difference in cardiopulmonary bypass time, aortic cross clamp time or mechanical ventilation time between the simple CoA group and the complex CoA group (P > 0.05) (Table 5).
The percentage of postoperative decrease in C1q in children with simple CoA or complex CoA was positively correlated with the time of cardiopulmonary bypass and aortic cross clamp, respectively (r = 0.797, r = 0.622, r = 0.898, r = 0.920, P < 0.05) (Table 5, Fig. 5–6). In simple CoA group, the percentage of postoperative decrease in C1q was positively correlated with the time of mechanical ventilation (r = 0.620, P < 0.05) (Table 5, Fig. 7). But in complex CoA group, there was no significant correlation between the percentage of postoperative decrease in C1q and the time of mechanical ventilation (r = 0.699, P > 0.05) (Table 5, Fig. 7).
The correlation of CPB time and the percentage decrease of complement C1q before and after operation in simple CoA group and complex CoA group. A shows the correlation of CPB time and the percentage decrease of complement C1q before and after operation in simple CoA group (n = 11); B shows the correlation of CPB time and the percentage decrease of complement C1q before and after operation in complex CoA group (n = 8). The percentage of postoperative decrease in C1q in children with simple CoA or complex CoA was positively correlated with the time of cardiopulmonary bypass (CPB), respectively (r = 0.797, r = 0.898, P < 0.05).)
The correlation of aortic cross clamp time and the percentage decrease of complement C1q before and after operation in simple CoA group and complex CoA group. (A shows the correlation of aortic cross clamp time and the percentage decrease of complement C1q before and after operation in simple CoA group (n = 11); B shows the correlation of aortic cross clamp time and the percentage decrease of complement C1q before and after operation in complex CoA group (n = 8). The percentage of postoperative decrease in C1q in children with simple CoA or complex CoA was positively correlated with aortic cross clamp time, respectively (r = 0.622, r = 0.920, P < 0.05).)
The correlation of mechanical ventilation time and the percentage decrease of complement C1q before and after operation in simple CoA group and Complex CoA group. (A shows the correlation of mechanical ventilation time and the percentage decrease of complement C1q before and after operation in simple CoA group (n = 11); B showed the correlation of mechanical ventilation time and the percentage decrease of complement C1q before and after operation in complex CoA group (n = 8). In simple CoA group, the percentage of postoperative decrease in C1q was positively correlated with the time of mechanical ventilation (r = 0.620, P < 0.05). However in complex CoA group, there was no significant correlation between the percentage of postoperative decrease in C1q and the time of mechanical ventilation (r = 0.699, P > 0.05).)
Discussion
Congenital coarctation of the aorta (CoA) is a kind of localized aortic stenosis near the arterial catheter that was first described by Morgagni in 1760 [18]. CoA can be divided into simple CoA and complex CoA according to whether it is accompanied by important intracardiac pathological changes. Simple CoA refers to CoA with or without patent ductus arteriosus (PDA), while complex CoA refers to CoA with important intracardiac pathological changes, including ventricular septal defect (VSD), atrial septal defect (ASD), aortic valve stenosis, and double outlet of the right ventricle. The most common combined intracardiac malformation is VSD [5]. The hemodynamic changes in CoA are closely related to the severity of the coarctation and the combined intracardiac malformations. The main hemodynamic changes caused by CoA include upper limb hypertension and left ventricular hypertrophy. The increase in blood flow above the coarctation and the ejection resistance of the left ventricle increase the blood pressure of the upper limb, while the decrease in blood flow below coarctation leads to a decrease in the blood pressure of the lower limb. At the same time, the main response of the left ventricle to outflow tract obstruction is compensatory left ventricular hypertrophy. Over time, continuous hypertension can further aggravate the left ventricular hypertrophy and myocardial fibrosis, resulting in reduced left ventricular compliance and diastolic heart failure. If not treated in time, it can progress to total heart failure.
The mechanisms of cardiac hypertrophy include cellular metabolism, proliferation, noncoding RNAs, immune responses, translational regulation, and epigenetic modifications [19]. Immune cell infiltration has been shown to be involved in the pathogenesis of CoA [20]. So, in the left ventricular hypertrophy induced by congenital CoA, whether immune related factors are involved? To date, no studies have been reported.
The complement system is an important part of innate and adaptive immunity. Under physiological conditions, the activation and regulation of the complement system are in a balanced state. When the balance is broken, the complement system will attack the body’s own cells and tissues, which will lead to a variety of inflammatory reactions and autoimmune diseases [21]. C1q is an important promoter of the classical complement pathway. It activates the complement cascade reaction by recognizing the complement binding site of the antibody FC segment in an IgG or IgM immune complex, triggering the clearance of the antigen antibody complex [4]. It has been reported that complement C1q can lead to left ventricular hypertrophy by promoting β-catenin activation [22]. The C1q complement/TNF-related protein (CTRP) family has a spherical region at the C-end of the protein, its structure is similar to that of the complement component C1q, and there is a signal peptide at the N-end of the protein [23]. In 2019, Zhang B et al. reported that CTRP-3 could attenuate pressure overload-induced cardiac hypertrophy by suppressing the p38/CREB pathway and p38-induced ER stress [14]. Therefore, whether complement C1q is involved in the pathogenesis of congenital CoA and the regulation of left ventricular hypertrophy caused by CoA has not been reported in the literature.
Based on our study, we found that the content of complement C1q in the plasma of patients with simple CoA was significantly lower, and their IVSTZ and LVPWTZ were significantly higher. By further analyzing the relationship between complement C1q and left ventricular hypertrophy, we found that the content of complement C1q before the operation was negatively correlated with IVSTZ and LVPWTZ. These data indicate that complement C1q has a certain inhibitory effect on the formation of left ventricular hypertrophy. Meanwhile, we also found that the IVSTZ and LVPWTZ were not significantly different between the complex CoA group and the large VSD group, which may be related to the large left to right shunts at the ventricular level, but the LVDdZ in the complex CoA group was significantly lower than that in the large VSD group. We think that this may be related to the reduction in the left to right shunt flow through the ventricular septum, which was induced by the obstruction of the left ventricular outflow tract caused by CoA.
During cardiac surgery with cardiopulmonary bypass and aortic cross clamp, the heart is isolated from the circulation. This inevitably induces myocardial ischemia. In addition to this ischemic insult, an additional hit will occur upon reperfusion, which may worsen the extent of tissue damage and organ dysfunction [24]. In 2020, Miyamoto T found that in pediatric cardiac surgery with cardiopulmonary bypass, the C1q concentration was significantly lower. For patients who were given C1 esterase inhibitors intravenously 60 min after CPB, the classical complement pathway was activated through the replenishment of complement [25].
Mechanical ventilation can also promote the release of inflammatory mediators and activate the inflammatory response. Silvia Marchesi et al. reported that the blood IL6 and TNF-α ratio increased significantly in patients on mechanical ventilation [26]. Dhanireddy S found that mechanical ventilation could exacerbate both pulmonary and systemic inflammation in response to bacteria and contribute to the pathogenesis of both acute lung injury and multiple organ dysfunction syndromes, it could augment pulmonary production of the proinflammatory cytokines KC, MIP-2, TNF-alpha, and IL-6 and increase the alveolar-capillary permeability to proteins [27].
By analyzing complement C1q in children with CoA before and 24 h after the operation, we found that the decreased percentage of C1q in children with simple CoA or complex CoA was positively correlated with the time of cardiopulmonary bypass and aortic cross clamp. In the simple CoA group, the percentage of postoperative decrease in C1q was positively correlated with the time of mechanical ventilation. However, in the complex CoA group, there was no significant correlation between the percentage of postoperative decrease in C1q and the time of mechanical ventilation. These results show that with the extension of cardiopulmonary bypass time and aortic cross clamp time, complement C1q is gradually consumed and reduced. Complement C1q may have a certain protective effect on myocardial ischemia reperfusion during cardiopulmonary bypass and aortic cross clamp. In terms of mechanical ventilation time, with the extension of mechanical ventilation time, complement C1q gradually decreases, which may be due to mechanical ventilation causing inflammatory reactions, and complement C1q protects other organs and tissues by inhibiting inflammatory reactions. The continuous activation and consumption of complement C1q in this process eventually leads to a gradual decrease in complement C1q.
Abnormal lipid metabolism of cardiomyocytes can cause left ventricular hypertrophy. Celentano A found that hypercholesterolemia in normotensive nondiabetic adults is independently associated with a mildly concentric left ventricular geometry [28]. Increased left ventricular afterload leads to myocyte hypertrophy and interstitial fibrosis, which has been shown to cause concentric left ventricular hypertrophy [29]. The energy supply of cardiomyocytes mainly depends on the oxidation of long-chain fatty acids, producing ATP through mitochondria, while the obstruction of fatty acid oxidation and the enhancement of the myocardial glucose metabolism pathway are related to myocardial hypertrophy [30]. Therefore, there is a close relationship between lipid metabolism and left ventricular hypertrophy.
In 2004, Wong GM et al. identified C1q complement/TNF-related protein (CTRP) [23]. To date, the literature has reported that there are 15 CTRP family members (CTRP 1–15). The CTRP protein family has a spherical region at the C-end of the protein and its structure is similar to that of the complement component C1q. There is a signal peptide at the N-end of the CTRP protein. Serum CTRP levels are negatively correlated with TCHO and TG and positively correlated with high-density lipoprotein cholesterol (HDL-C) [31, 32]. Complement C1q and CTRP partially share the same structure, but whether complement C1q is related to lipid metabolism in patients with CoA has not been reported in the literature. Through this study, we found that there was no significant difference in plasma TG, TCHO, HDL-C or LDL-C between CoA children and children without an increased left ventricular afterload before operation, and there was no correlation between the complement C1q level and lipid metabolism-related indicators before operation. This may be because the children we included in this study were young and the course of the disease was short, so they did not develop abnormal lipid metabolism. Additionally, complement C1q may not inhibit the formation of left ventricular hypertrophy by regulating lipid metabolism in CoA-induced left ventricular hypertrophy. After operation, the complement C1q was positively correlated with TCHO, HDL and LDL in complex CoA group, but there was no correlation between the complement C1q level and TG. This may be related to the effect of surgical trauma on lipid metabolism. This mechanism needs further study.
In conclusion, in this study, by analyzing the content of complement C1q in the plasma of children with simple and complex CoA and the relationship between the content of complement C1q and LVPW, IVS and LVDd, we found that complement C1q has an inhibitory effect on CoA-induced left ventricular hypertrophy, which is negatively correlated with left ventricular myocardial hypertrophy. By analyzing the content of complement C1q in children with CoA before and 24 h after the operation, it was found that the decreased percentage of complement C1q after the operation was positively correlated with the time of cardiopulmonary bypass, aortic cross clamp and mechanical ventilation. We also found that there was no significant correlation between complement C1q and related indicators of lipid metabolism. The above results have certain clinical significance for clarifying the pathogenesis of left ventricular hypertrophy caused by congenital CoA and the role of complement C1q in myocardial injury during cardiac surgery.
There were some limitations in our study. First, this was a retrospective study, there was only the value of complement C1q at 24 h after operation, and the values at other times after operation were incomplete. Therefore, we only analyzed the complement C1q level at 24 h after the operation. The time of echocardiogram reexamination after operation was inconsistent, so the correlation between postoperative complement C1q and LVDd, IVST and LVPWT could not be analyzed. Second, the number of patients included in this study is small. In the future, we will conduct a large clinical prospective study to further investigate the correlation between complement C1q levels and left ventricular hypertrophy induced by coarctation of the aorta.
Conclusions
C1q is an important promoter of the classical complement pathway. In our study, we found that serum complement C1q levels in simple CoA patients showed lower level. In CoA patients, serum complement C1q had a negative association with interventricular septal thickness and left ventricular posterior wall thickness. The decreasing of serum complement C1q was an unfavorable factor for acute myocardial injury during cardiac surgery. Complement C1q may be related to the process of left ventricular hypertrophy induced by CoA and have a protective effect against myocardial injury during cardiac surgery.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- CoA:
-
Coarctation of the aorta
- VSD:
-
Ventricular septal defect
- PDA:
-
Patent ductus arteriosus
- TG:
-
Triglycerides
- TCHO:
-
Total cholesterol
- HDL-C:
-
High-density lipoprotein cholesterol
- LDL-C:
-
Low-density lipoprotein cholesterol
- LVDd:
-
Left ventricular end diastolic diameter
- IVST:
-
Interventricular septal thickness
- LVPWT:
-
Left ventricular posterior wall thickness
- CTRP:
-
C1q complement/TNF-related protein
References
Kenny D, Hijazi ZM. Coarctation of the aorta: from fetal life to adulthood. Cardiol J. 2011;18:487–95.
Anderson RH, Lenox CC, Zuberbuhler JR. Morphology of ventricular septal defect associated with coarctation of aorta. Br Heart J. 1983;50:176–81.
Warnes CA. Bicuspid aortic valve and coarctation: two villains part of a diffuse problem. Heart. 2003;89:965–6.
Becker AE, Becker MJ, Edwards JE. Anomalies associated with coarctation of aorta: particular reference to infancy. Circulation. 1970;41:1067–75.
Beekman RH. Coarctation of the aorta.In: Allen HD, Gutgesell HP, Clark EB, Driscoll DJ. Moss and Adams’ heart disease in infant, children, and adolescents. 6th ed. Philadephia: Lippncott Williams & wilkins; 2001. p. 990–1010.
Karaosmanoglu AD, Khawaja RD, Onur MR, Kalra MK. CT and MRI of aortic coarctation: pre-and postsurgical findings. AJR Am J Roentgenol. 2015;204:W224–33.
Jashari H, Lannering K, Mellander M, Ibrahimi P, Rydberg A, Henein MY. Coarctation repair normalizes left ventricular function and aorto-septal angle in neonates. Congenit Heart Dis. 2017;12:218–25.
Becker RC, Owens AP 3rd, Sadayappan S. Tissue-level inflammation and ventricular remodeling in hypertrophic cardiomyopathy. J Thromb Thrombolysis. 2020;49:177–83.
Kwiecinski J, Lennen RJ, Gray GA, Borthwick G, Boswell L, Baker AH, et al. Progression and regression of left ventricular hypertrophy and myocardial fibrosis in a mouse model of hypertension and concomitant cardiomyopathy. J Cardiovasc Magn Reson. 2020;22:57.
Reant P, Metras A, Detaille D, Reynaud A, Diolez P, Jaspard-Vinassa B, et al. Impact of afterload increase on left ventricular myocardial deformation indices. J Am Soc Echocardiogr. 2016;29:1217–28.
Kolev M, Le Friec G, Kemper C. Complement tapping into new sites and effector systems. Nat Rev Immunol. 2014;14:811–20.
Sjoberg AP, Trouw LA, Blom AM. Complement activation and inhibition: a delicate balance. Trends Immunol. 2009;30:83–90.
Wang H, Wang R, Du D, Li F, Li Y. Serum levels of C1q/TNF-related protein-1 (CTRP-1) are closely associated with coronary artery disease. BMC Cardiovasc Disord. 2016;16:92.
Zhang B, Zhang P, Tan Y, Feng P, Zhang Z, Liang H, et al. C1q-TNF-related protein-3 attenuates pressure overload-induced cardiac hypertrophy by suppressing the p38/CREB pathway and p38-induced ER stress. Cell Death Dis. 2019;10:520.
Lei H, Wu D, Wang JY, Li L, Zhang CL, Feng H, et al. C1q/tumor necrosis factor-related protein-6 attenuates post-infarct cardiac fibrosis by targeting RhoA/MRTF-A pathway and inhibiting myofibroblast differentiation. Basic Res Cardiol. 2015;110:35.
Naito AT, Sumida T, Nomura S, Liu ML, Higo T, Nakagawa A, et al. Complement C1q activates canonical Wnt signaling and promotes aging-related phenotypes. Cell. 2012;149:1298–313.
Sluymans T, Colan SD. Structural measurements and adjustment for growth. In: Lai WW, Mertens LL, Cohen MS, Geva T, editors. Echocardiography in pediatric and congenital heart disease. Wiley-Blackwell, FL: West Sussex Press; 2016. p. 66–7.
Lindesmith GG, Stanton RE, Stiles QR, Meyer BW, Jones JC. Coarctation of the thoracic aorta. Ann Thorac Surg. 1971;11:482–97.
Nakamura M, Sadoshima J. Mechanisms of physiological and pathological cardiac hypertrophy. Nat Rev Cardiol. 2018;15:387–407.
Rodriguez-Iturbe B, Quiroz Y, Kim CH, Vaziri ND. Hypertension induced by aortic coarctation above the renal arteries is associated with immune cell infiltration of the kidneys. J Hypertens. 2005;18(11):1449–56.
Zipfel PF, Skerka C. Complement regulators and inhibitory proteins. Nat Rev Immunol. 2009;9:729–40.
Zhu Y, Ohama T, Kawase R, Chang J, Inui H, Kanno K, et al. Progranulin deficiency leads to enhanced age-related cardiac hypertrophy through complement C1q-induced β-catenin activation. J Mol Cell Cardiol. 2020;138:197–211.
Wong GW, Wang J, Hug C, Tsao TS, Lodish HF. A family of Acrp30/adiponectin structural and functional paralogs. Proc Natl Acad Sci U S A. 2004;101:10302–7.
De Hert S, Moerman A. Myocardial injury and protection related to cardiopulmonary bypass. Best Pract Res Clin Anaesthesiol. 2015;29:137–49.
Miyamoto T, Ozaki S, Inui A, Tanaka Y, Yamada Y, Matsumoto N. C1 esterase inhibitor in pediatric cardiac surgery with cardiopulmonary bypass plays a vital role in activation of the complement system. Heart Vessels. 2020;35:46–51.
Marchesi S, Hedenstierna G, Hata A, Feinstein R, Larsson A, Larsson AO, et al. Effect of mechanical ventilation versus spontaneous breathing on abdominal edema and inflammation in ARDS: an experimental porcine model. BMC Pulm Med. 2020;20:106.
Dhanireddy S, Altemeier WA, Matute-Bello G, O’Mahony DS, Glenny RW, Martin TR, et al. Mechanical ventilation induces inflammation, lung injury, and extra-pulmonary organ dysfunction in experimental pneumonia. Lab Invest. 2006;86:790–9.
Celentano A, Crivaro M, Roman MJ, Pietropaolo I, Greco R, Pauciullo P, et al. Left ventricular geometry and arterial function in hypercholesterolemia. Nutr Metab Cardiovasc Dis. 2001;11:312–9.
Saliba LJ, Maffett S. Hypertensive heart disease and obesity: a review. Heart Fail Clin. 2019;15:509–17.
van Bilsen M, Smeets PJ, Gilde AJ, van der Vusse GJ. Metabolic remodelling of the failing heart: the cardiac burn-out syndrome? Cardiovasc Res. 2004;61:218–26.
Shanaki M, Fadaei R, Moradi N, Emamgholipour S, Poustchi H. The circulating CTRP13 in type 2 diabetes and non-alcoholic fatty liver patients. PLoS ONE. 2016;11: e0168082.
Bai B, Ban B, Liu Z, Zhang MM, Tan BK, Chen J. Circulating C1q complement/TNF-related protein (CTRP) 1, CTRP9, CTRP12 and CTRP13 concentrations in type 2 diabetes mellitus: In vivo regulation by glucose. PLoS ONE. 2017;12: e0172271.
Acknowledgements
Not applicable.
Funding
This work was supported by research grants from the National Natural Science Foundation of China 81541119, 81400846.
Author information
Authors and Affiliations
Contributions
HZD and LC conceptualized the research hypothesis and analyses, and LC researched the data, performed all of the statistical analyses and wrote the manuscript. HZD and XYL reviewed and edited the manuscript. CZ, GL, YTW and DW obtained informed consent, performed surgery and checked the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The current retrospective study was approved by the Ethics Committee at Beijing Anzhen Hospital and conducted in accordance with the Declaration of Helsinki. Informed consent given by the patients’ guardians enrolled in the current study was obtained at the time of surgery or visit to our hospital.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chen, L., Duan, Hz., Zhang, C. et al. Serum complement C1q level is associated with left ventricular hypertrophy induced by coarctation of the aorta: A retrospective observational study. BMC Cardiovasc Disord 22, 367 (2022). https://doi.org/10.1186/s12872-022-02807-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-022-02807-2