Abstract
Background
Left ventricular structure and function abnormalities may be an early marker of cardiomyopathy among African Americans with diabetes (DM) even in the absence of coronary artery disease (CAD), arrhythmia, valvular heart disease and end-stage renal disease (ESRD). This study examined the association of prediabetes (PDM), DM and HbA1c with left ventricular structure and function among Jackson Heart Study (JHS) participants without traditional risk factors.
Methods
Retrospective cross-sectional analyses of the association of PDM, DM and HbA1c with, left ventricular ejection fraction (LV EF), fractional shortening (LV FS), stroke volume index (SVI), cardiac index (CI), left ventricular end diastolic volume index (LVEDVI), left ventricular end systolic volume index (LVESVI), relative wall thickness (RWT), myocardial contraction fraction (MCF) and left ventricular mass index (LVMI). The study was conducted in 2234 adult JHS participants without preexisting CAD, arrhythmia, valvular heart disease or ESRD. Statistical analyses included descriptive, univariate and covariate adjusted linear regression analyses. Sensitivity analyses to explore the impact of hypertension on study outcomes were also carried out.
Results
DM compared with no DM was associated with lower, SVI (− 0.96 ml/m2, p = 0.029), LVEDVI (− 1.44 ml/m2 p = 0.015), and MCF (− 1.90% p = 0.007) but higher CI (0.14 L/min/m2, p < 0.001), RWT (0.01 cm, p = 0.002) and LVMI (2.29 g/m2, p = 0.009). After further control for DM duration, only CI remaining significantly higher for DM compared with no DM participants (0.12 L/min/m2, p = 0.009). PDM compared with no PDM was associated with lower, SVI (− 0.87 ml/m2, P = 0.024), LVEDVI (− 1.15 ml/m2 p = 0.003) and LVESVI (− 0.62 ml/m2 p = 0.025). HbA1c ≥ 8.0% compared with HbA1c < 5.7% was associated with lower SVI (− 2.09 ml/m2, p = 0.004), LVEDVI (− 2.11 ml/m2 p = 0.032) and MCF (− 2.94% p = 0.011) but higher CI (0.11 L/min/m2, p = 0.043) and RWT (0.01 cm, p = 0.035).
Conclusions
Glycemic status is associated with important left ventricular structure and function changes among African Americans without prior CAD, arrhythmia, valvular heart disease and ESRD. Longitudinal studies may further elucidate these relationships.
Similar content being viewed by others
Background
Type I and II diabetes mellitus (DM) affects proximately 30.3 million adults in the United States (US) and is expected to double in prevalence by 2050 [1]. Prediabetes (PDM) affects about 91.8 million adults with an annualized conversion rate to DM of 5–10% [2, 3]. DM has been reported to increase the risk of heart failure (HF) 2 to fivefold and about 19–30% of HF patients have concurrent DM [4,5,6,7,8]. Furthermore, glycemic status may be associated with poor HF outcomes among individuals with DM [9].
Rubler et al. proposed a unique association between DM and structural cardiac changes in the absence of major coronary artery disease (CAD) or valvular heart disease often termed diabetic cardiomyopathy (D-CM) [10]. While its pathophysiology and clinical course remains unclear, D-CM is increasingly recognized as a DM complication. Suggested pathophysiologic mechanisms for D-CM include; hyperglycemia, insulin resistance, myocardial fibrosis, small vessel disease and cardiac autonomic neuropathy [11, 12]. While convention suggests that diastolic dysfunction precedes left ventricular (LV) systolic dysfunction [12], emerging evidence suggests however that LV structural changes and systolic dysfunction may occur early, precede diastolic dysfunction and be an early marker of D-CM [13,14,15,16].
African Americans are at higher risk of DM and HF when compared with other ethnicities [17,18,19]. While traditional risk factors like CAD, valvular heart disease and arrythmia have been associated with HF in individuals with DM, few studies have explored the independent relationship between glycemic status and Left ventricular structure and function (LV SF) among African Americans [20].
Methods
Study aim
This study examined the association of PDM, DM and glycated hemoglobin (HbA1c) with LV SF among African American participants in the Jackson Heart Study (JHS) without prior (CAD), arrhythmia, valvular heart disease or end stage renal disease (ESRD).
Design, setting and data
This was a cross sectional analysis of the JHS baseline data. JHS is a community-based cohort study that explores the risk and etiologic factors for cardiovascular disease among African Americans. JHS commenced in 2000 and includes a cohort of 5,306 participants from the Jackson, Mississippi metropolitan statistical area. Participants were selected from 4 recruitment pools: random (17%), volunteer (22%), Atherosclerosis Risk in Communities (ARIC) Study (30%), and secondary family members (31%). Study design and methods have been described previously [21].
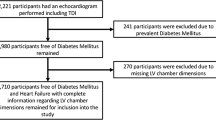
Characteristics of study participants (Fig. 1)
From the 5306 JHS study baseline participants, 4052 participants had complete CAD, arrythmia, valvular heart disease and ESRD data. From these, 1221 participants with prior CAD, arrhythmia, valvular heart disease and ESRD were excluded. From the 2831 participants remaining, 2234 and 1600 participants with 2D and M-Mode echocardiogram data respectively with pertinent study covariates were selected as the final analytical sample. The diabetes duration and beta blocker dosage subset analyses were conducted on 2082 and 1512 participants for the 2D echocardiogram and M-Mode outcomes respectively. Main study exclusion criteria were (a) CAD (self-report, clinical or EKG evidence of prior myocardial infarction), (b) History of significant arrhythmia (atrial flutter or fibrillations and major ventricular tachyarrhythmias) (c) valvular heart disease (moderate to severe aortic, mitral, tricuspid or pulmonary disease) and (d) ESRD on hemodialysis.
Measures
Outcome measures
Echocardiography was performed using Sonos 4500 echocardiogram Hewlett Packard machines following American Society of Echocardiography recommendations [22]. 2D and M-mode examination assessed all 4 cardiac chamber parasternal, apical, and subcostal windows long axis views. Blinded observers then read and provided quality ratings [23]. Nine left ventricular structure and function measures were examined; (a) Left ventricular ejection fraction % (LV EF) using biplane Simpson’s method. (b) Left ventricular end diastolic volume index (LVEDVI) = left ventricular end diastolic (LVEDV)/Body surface area (BSA) and left ventricular end systolic volume index (LVESVI) = left ventricular end systolic volume (LVESV)/BSA), (c) Stroke volume index (SVI) = LVEDV- LVESV/ BSA; (d) Cardiac index (CI) = heart rate at echocardiogram image acquisition × corresponding stroke volume)/BSA, (e) Left ventricular fractional shortening (LV FS) = Left ventricular end diastolic volume (LVEDD)—Left ventricular end systolic volume (LVEDD)/LVESD) × 100; (f) Left ventricular mass index (LVMI) = left ventricular mass (LVM) in g = [((0.8 × 1.04) (LVEDD + interventricular septal thickness + posterior wall thickness)3)) − (LVEDD)3 + 0.6/BSA], (g) Myocardial contraction fraction (MCF) = ratio of stroke volume to left ventricular myocardial volume (LVM/1.04 g/mL) [24] and (h) Relative wall thickness (RWT) was calculated as 2 × posterior wall thickness/LVEDD. All measurements utilized the 2D echocardiogram values, except for LV FS which utilized M-Mode values.
Main independent measures
DM and PDM were the main independent variables. DM was defined by; self-reported physician diagnosis, medication use (oral or insulin) or HbA1c ≥ 6.5%. PDM was defined as HbA1c 5.7–6.4% in the absence of prior DM diagnosis or medication use. HbA1c was the secondary independent measure categorized as HbA1c < 5.7%, 5.7 to < 6.5%, 6.5% to < 8.0% and > 8.0%.
Covariates
JHS clinic procedures are reported previously [21, 25]. Covariates include (a) Hypertension, (b) Dyslipidemia, (c) CKD stage III-IV, (d) smoking status, (e) Nutrition status (using a 158 question food frequency questionnaire and 24 h dietary recall [26] categorized using American heart association (AHA) criteria [27], (f) physical activity- similarly using AHA’s Life's Simple 7 criteria [27], (g) Socio-demographic variables (age, gender and highest level of education) (h) crack or cocaine use, (i) Alcohol use and (j) Body mass index (BMI). Cardioactive medications; obtained using JHS Medication survey form (MSRA) were considered given their potential impact on cardiac remodeling [28] and include; beta or calcium channel blockers, diuretics, angiotensin converting enzyme inhibitor (ACE) and angiotensin receptor blockers (ARB). Vasodilators were excluded from consideration given insufficient records. Beta blockers were converted to carvedilol equivalent doses for subset analyses using methods described previously by Cohen-Solal et al. [29] Finally, left ventricular hypertrophy patterns were approximated using a composite of LVMI and RWT and classified as; No LVH, concentric remodeling, eccentric hypertrophy and concentric hypertrophy [30].
Statistical analysis
The distribution of all study variables was examined and positively skewed variables were transformed using their natural logarithms. Categorical variables were examined by PDM or DM status using Chi-square and Fishers exact test. Continuous measures were examined using one-way analysis of variance including Kruskal–Wallis tests when normality and homoscedasticity assumptions were not met. Simple linear regression analyses tested the univariate relationship between each LV SF outcomes with PDM or DM status (No PDM/DM, PDM and DM). Underlying linear regression assumption tests determined that all the outcome measures did not meet the normality assumptions and were therefore transformed using their natural logarithm. The coefficients of these log transformed measures were converted to percent differences using the reverse transformation formula (exponentiated (β Coefficients) – 1) × 100. Ninety-five percent confidence intervals, P-values and the reversed transformed mean differences in original units were also reported. Three sets of multivariable regression analyses were then conducted; LV SF outcomes versus. (a) PDM or DM status controlling for main covariates (Table 3), (b) PDM or DM status controlling for main covariates with additional control for diabetes duration (Table 4) and (c) HbA1c categories (< 5.7%, 5.7- < 6 to < 6.5%, 6.5% to < 8.0% and ≥ 8.0%) controlling for main covariates, diabetes duration and carvedilol equivalent dose (Table 5). Analyses of BSA indexed outcomes were not further controlled for body habitus (BMI/BSA). Given the common co-occurrence of DM and hypertension and the potential joint effect on heart disease [31], the interaction of hypertension and either PDM or DM with LV SF outcomes on the multiplicative scale was examined (Figs. 2, 3). Supplementary analysis examined the distribution and means of study variables among study participants compared with excluded participants with CAD, arrhythmia, valvular heart disease and ESRD (Additional file 1: Table 1). All statistical tests were 2-sided at a significance level of α = 0.05 using SAS version 9.4 © (SAS Institute Inc., Cary, N.C.).
Hypertension and PDM interaction versus LV outcomes (Reference = No PDM, DM or hypertension). Each row represents a separate model for Left Ventricular Measures controlled for age gender physical activity, highest level of education, nutrition pattern, Dyslipidemia smoking status, used crack or cocaine in any form, beta blocker, Calcium channel blockers, diuretics, Angiotensin converting enzyme inhibitor, Angiotensin Receptor Blocker. LVEF, LV FS, MCF and RWT were additionally controlled for log of BMI. SVI: P for prediabetes and hypertension interaction = 0.654. CI: P for prediabetes and hypertension interaction = 0.974. LMVI: P for prediabetes and hypertension interaction = 0.270. LV EF: P for prediabetes and hypertension interaction = 0.354. LV FS: P for prediabetes and hypertension interaction = 0.830. LVEDVI: P for prediabetes and hypertension interaction = 0.767. LVESVI: P for prediabetes and hypertension interaction = 0.062. MCF: P for prediabetes and hypertension interaction = 0.652 and RWT: P for prediabetes and hypertension interaction = 0.174
Hypertension and DM interaction versus LV outcomes (Reference = No PDM, DM or hypertension). Each row represents a separate model for Left Ventricular Measures controlled for age gender physical activity, highest level of education, nutrition pattern, Dyslipidemia smoking status, used crack or cocaine in any form, beta blocker, Calcium channel blockers, diuretics, Angiotensin converting enzyme inhibitor, Angiotensin Receptor Blocker. LVEF, LV FS, MCF and RWT were additionally controlled for log of BMI. SVI P for Diabetes and hypertension interaction = 0.680. CI: P for Diabetes and hypertension interaction = 0.824. LVMI: P for Diabetes and hypertension interaction = 0.625. LV EF:P for Diabetes and hypertension interaction = 0.292. LV FS:P for Diabetes and hypertension interaction = 0.434. LVEDVI P for Diabetes and hypertension interaction = 0.250. LVESVI P for Diabetes and hypertension interaction = 0.085. MCF: P for Diabetes and hypertension interaction = 0.666. RWT:P for Diabetes and hypertension interaction = 0.917
Results
The characteristics of the study sample including outcomes, independent variables and covariates are presented in Table 1. Unadjusted and adjusted linear regression analyses results for each of the LV SF outcomes by PDM and DM status or HbA1c categories are presented in Tables 2, 3, 4, 5 and Figs. 2, 3. For all regression analyses, differences in original unit means and percentage differences when compared with the reference measures are reported.
LV EF and LV FS
No statistically significant difference in LV EF of LV FS was observed by PDM and DM status (Tables 3 and 4).
LVEDVI
In participants with PDM compared with those without PDM or DM, LVEDVI was 2.65% lower (p = 0.006) Table 3. This effect remained even after adjustment for DM duration (− 2.82% p = 0.003) Table 4. Participants with DM compared with the reference group had a 2.66% lower LVEDVI (p = 0.015) Table 3 though the effect was not observed after adjustment for DM duration (p = 0.417) Table 4.
LVESVI
In participants with PDM compared with those without PDM or DM, LVESVI was 3.59% lower (p = 0.031) Table 3. This effect remained even after adjustment for DM duration (− 3.73%, p = 0.025) Table 4. No statistically significant difference in LVESVI was observed by DM status (Tables 3 and 4).
SVI
In participants with PDM compared with those without PDM or DM, SVI was 2.18% lower (p = 0.017) Table 3. This effect remained even after adjustment for DM duration (− 2.36%, p = 0.024) Table 4. Participants with DM compared with the reference group had a 2.61% lower SVI (p = 0.029) Table 3 though the effect was not observed after adjustment for DM duration (p = 0.243) Table 4.
CI
PDM was not associated with any difference in CI (Tables 3 and 4). In participants with DM compared with those without PDM or DM, CI was 6.03% higher (p < 0.001) Table 3. This effect remained even after adjustment for DM duration (5.10%, p = 0.009) Table 4.
MCF
PDM was not associated with any difference in MCF (Tables 3 and 4). In participants with DM compared with those without PDM or DM, MCF was 3.43% lower (p = 0.007) Table 3. This effect was not observed after adjustment for DM duration (p = 0.089) Table 4.
RWT
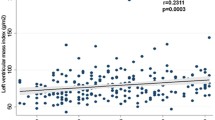
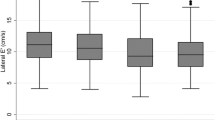
PDM was not associated with any difference in RWT (Tables 3 and 4). In participants with DM compared with those without PDM or DM, RWT was 3.13% higher (p = 0.002) Table 3. This effect was not observed after adjustment for DM duration (p = 0.065) Table 4. The distribution of left ventricular hypertrophy patterns by PDM and DM status is presented in Fig. 4. Eccentric hypertrophy was the predominant LVH pattern occurring with higher frequency among participants with PDM and DM when compared to those without either condition.
LVMI
PDM was not associated with a difference in LVMI (Table 3) In participants with DM compared with those without PDM or DM, LVMI was 3.31% higher (p = 0.009) Table 3. This effect was not observed after adjustment for DM duration (p = 0.065) Table 4.
Stratified analyses by HBA1c (Table 5)
In a subset of the study sample with available DM duration and carvedilol equivalent dose, we examined the relationship between categories of HbA1c and LV SF outcomes. These models controlled for the same similar covariates in Tables 3 and 4 in addition to duration of DM and carvedilol dose equivalent. Results showed that participants with HbA1c of ≥ 8% compared with HbA1c of < 5.7% had; 3.97% lower LVEDVI (p = 0.032), 5.81% lower SVI (p = 0.004), 4.85% higher CI (P = 0.043), 5.38% lower MCF (p = 0.011) and 3.73% higher RWT (p = 0.035). SVI was the only outcome measure that was significantly lower for participants with HbA1c of 6.5 to < 8.0% when compared the reference group (4.84% lower, p = 0.036). Participants with HbA1c of 5.7% to < 6.5% compared with the reference group had; a 2.25% lower LVEDVI (p = 0.016), 2.13% lower SVI (p = 0.036) and 2.28% lower CI (p = 0.048).
Interaction of DM or PDM with hypertension on left ventricular structure and outcomes measures
Figures 2 and 3 presents results of several multivariable regression analyses exploring the multiplicative interaction of HTN on PDM and DM (respectively) on left ventricular structure and function outcomes. Results showed no statistically significant interaction on the multiplicative scale.
Additional file 1: Table 1
Compares the distribution of study outcome variables and covariates by apriori excluded participants with CAD, arrythmia, valvular heart disease and ESRD status compared with selected participants without these conditions.
Discussion
This study represents an examination of left ventricular structure and function among a population of African Americans who have DM without concurrent CAD, arrhythmia, valvular heart disease or ESRD. Similar studies are sparse in this population. Study findings show significantly lower LVEDVI, SVI, MCF but higher CI, RWT and LVMI among African Americans with DM compared with those without DM. These finding appear DM duration dependent except for CI which remained higher among individuals with DM compared to those without DM when DM duration was considered. PDM was associated with lower LVEDVI, LVESVI and SVI among compared with no PDM. We also observed important differences in the cardiac structure and function measures by DM control using HbA1c. Sensitivity analysis failed to show that interaction of DM with hypertension modified observed effects significantly. Study findings are further discussed below.
Left ventricular end diastolic and systolic volumes and Stroke volume index
SVI is essentially the difference between the LVEDVI and LVESVI and has been strongly associated with all-cause mortality and adverse cardiac events [32, 33]. While the pathophysiology of cardiomyopathy and structural heart changes in DM has not been fully elucidated, some evidence suggests that left ventricular function and contractility is impaired early in the course of the disease [34]. In our study, PDM but not DM was associated with decreased LVEDVI and LVESVI. SVI was lower among participants with DM compared with those without PDM/DM, though appeared confounded by DM duration. In contrast, SVI was lower among participants with PDM compared with those without PDM/DM. While temporal relationships are difficult to ascertain from cross sectional studies, these findings may suggest that changes in SVI occur early in the PDM to DM spectrum among African Americans. It remains unclear if the decrease in SVI results in adaptive physiologic changes in participants with DM but not PDM. The relationship between HbA1c and SVI however showed a clearer relationship between SVI and glycemic status. Specifically, SVI progressively decreased as HbA1c increased in comparison to the normal HbA1c (< 5.7%). LVEDVI also similarly decreased with increasing HbA1c. These findings should however be interpreted with caution as HbA1c is dynamic and may not represent long term glycemic status.
This study’s results compare with prior studies including Jensen et al. UK biobank cardiovascular magnetic resonance sub-study that found a decrease in SVI among participants with DM and Bertoni et al. study that found a decrease in stroke volume among African Americans with DM with prior Cardiovascular disease [35]36. Both studies did not however account for DM duration or HbA1c in their models like we did in this study. The pathophysiologic basis for the findings in our study is supported by prior evidence. Specifically, metabolic abnormalities (affecting glucose and free fatty acid), abnormal calcium homeostasis, myocardial apoptosis, myocardial fibrosis, small vessel ischemia and microangiopathy theoretically result in myocardial stiffness, impaired relaxation and lower end diastolic volumes [11, 37,38,39,40,41,42]. Our study suggests that LVEDVI and SVI may be a clinically relevant measure to consider in African Americans with abnormal glycemic status though further longitudinal studies are required to more clearly elucidate these relationships.
Cardiac Index
In our study, CI was higher for participants with DM compared to those without PDM/DM and similarly higher for participants with HbA1c ≥ 8.0% compared with normal HbA1c (< 5.7%). Paradoxically, HbA1c of 5.7 to < 6.5% compared with normal HbA1c was associated with lower CI. Study finding of higher CI are similar though not of the same magnitude as findings in the Strong Heart Study [43]. Specifically, this study’s observed mean CI difference was lower though the Strong Heart Study was of Native American not African Americans and did not control for similar covariates including DM duration as done in this study.
CI is typically the product of SV and heart rate at time of volumetric assessment. As discussed in the previous section, lower SVI and LVEDVI among individuals with DM or poor control (higher HbA1c) may reflect impaired ventricular filling secondary to relaxation impairments or decreased filling time [11, 37]. Our observed higher CI for DM and HbA1c ≥ 8.0% is thus likely attributable to a higher resting heart rate [44]. The higher resting heart rate among individuals with DM is often attributed to cardiac autonomic neuropathy (CAN) which is increasingly recognized as an important physiologic change among individuals with DM resulting from early cardiac parasympathetic denervation [11, 45]. Ewing and Balcıoğlu suggest that among individuals with DM, vagal denervation results a dominant sympathetic tone and resting tachycardia. They reported that while tachycardia eventually diminishes secondary to progressive sympathetic nerve fiber damage, increased resting heart rate persists among individuals with DM [46, 47]. CAN may also affect myocardial blood flow in denervated neuropathic individuals with DM when compared with non -neuropathic patients with DM [48]. In our study, we observed a progressive increase in CI as HbA1c increased that may give an insight to the glycemic control range at which CAN effect on CI occurs. This observed relationship should be interpreted with caution as HbA1c is dynamic and may not always represent long-term glycemic status. While we did not explicitly study CAN, further studies to elucidate the role of heart rate on CI among African Americans with DM may be relevant. Furthermore, elevated CI is likely not sustainable as clinical cardiomyopathy and HF eventually may occur in individuals with DM. The exact progression to HF and pathogenesis of these changes cannot however be elucidated from this study and requires further prospective studies.
Myocardial contraction fraction
MCF is a unitless three-dimensional volumetric measure of myocardial shortening proposed by King et al. as potentially outperforming traditional shortening measures like LV EF [24]. Several studies have since demonstrated a strong association between MCF and adverse cardiovascular outcomes [49, 50]. MCF is essentially a ratio of stroke volume to left ventricular volume and is independent of chamber size and geometry. In fact, King et al. hypothesized that MCF is perhaps a more useful measure of myocardial function in part because its derivation lacks the influence of LVEDV on ventricular shortening. This is of importance in our study because we sought to evaluate the independent effect of glycemic status on MCF as an LV structure and function without the influence of underlying cardiac chamber changes.
In this study, we observed that MCF was lower for participants with DM compared to those without PDM/DM. In the model controlled for DM duration however, we found no clear association of DM with MCF. In contrast, HbA1c ≥ 8.0% compared with the reference HbA1c < 5.7% was associated with a lower MCF. Glycemic control may thus be a more important determinant of MCF. Few studies have evaluated the relationship of glycemic status on MCF among African Americans as explored in this study. Abdalla et al. using the Multi-Ethnic Study of Atherosclerosis (MESA) did demonstrate that DM was associated with the lower MCF, though utilized cardiac magnetic resonance imaging (cMRI) rather than echocardiography [51]. The findings of this study may inform further studies regarding glycemic status and MCF among African Americans.
Left ventricular mass index and relative wall thickness
No statistically significant relationship between DM or HbA1c and LVMI was obsereved after we controlled for DM duration. While larger LVMI has been among individuals with DM compared with no DM further confering poorer cardiovascular outcomes [36, 43, 52, 53], few studies have examined this relationship among African Americans without CAD, arrhythmia, valvular heart disease or ESRD. The LVMI finding in this study is in contrast to these prior studies and should be interpreted with caution as LVMI is generally higher for African Americans and detection of large differences in a homogenous sample may be difficult [54].
In our study, RWT was higher among participants with HbA1c ≥ 8.0% compared with HbA1c < 5.7% though was not significantly different for DM versus no PDM/DM participants. Glycemic control may thus be an important determinant of RWT. There is mixed evidence regarding the influence of co-occurring hypertension and DM on left ventricular hypertrophy with some studies reporting that it is hypertension dependent others maintain that it is hypertension independent [55,56,57]. In our study the interaction between PDM or DM with hypertension on both RWT and LVMI was not statistically significant. Analyses presented in Fig. 4 shows that eccentric remodeling was the predominant hypertrophy pattern for individuals with PDM and DM. This finding may underly the independent influence of DM on hypertrophy patterns though both concentric and eccentric hypertrophy patterns may can occur in normotensive individuals with DM [58, 59].
Left ventricular ejection fraction and left ventricular fractional shortening
In our study, no statistically significant difference in LV EF or LV FS was observed for PDM, DM or HbA1c. This finding is not inconsistent with emerging evidence despite evidence to the contrary [60]. The sensitivity of LV EF for detecting early systolic dysfunction may in fact be poor despite its wide clinical use [61]. Nakai el al for example demonstrated that 2D speckle tracking echocardiography (STE) evaluation of longitudinal strain showed evidence of subclinical LV longitudinal dysfunction preferentially and frequently in asymptomatic DM patients with normal LV EF [14]. Further studies to evaluate the relationship between more sensitive measures of left ventricular dysfunction with DM may elucidate these relationships more appropriately. STE data was not evaluated in JHS.
Conclusion
From a clinical perspective, study findings highlight important abnormalities in LV structure and function among individuals with abnormal glycemic status that may present intervention opportunities though further causal studies are required. While the magnitude of observed differences may not be of overt clinical utility, they should be considered in context of the cross-sectional design of the study and the potential for observing larger effect sizes in longitudinal studies. Many of the LV structure and function parameters that we examined were DM duration and HbA1c level dependent further highlighting the need for early case finding and DM control. The strong association of PDM with SVI and DM or HbA1c with CI requires further pathophysiologic enquiry not addressed in our study. Specifically, is there a temporal relationship between glycemic status, CI, stroke volume and heart rate?. Is heart rate perhaps an important prognostic marker for subclinical LV changes and ultimately clinical HF? Do changes in LVEDVI occur much earlier than thought particularly even among individuals with PDM? Study findings also suggest a potential prognostic value of MCF among individuals with uncontrolled DM especially given the potential limitations of LV EF as a screening tool for subclinical and early systolic changes. Overall, this study in our impression contributes to the limited literature regarding LV structure and function in African Americans who are at considerable risk for DM associated structural cardiac changes.
Study limitations
This study is subject to important limitations. First, the cross-sectional design limits the ability to make clear inference regarding causality. The findings however suggest potential causal hypotheses in support of further studies. Despite efforts to exclude participants with silent ischemia using for example EKG evidence of prior ischemia, some potential for missing underlying ischemia still exists. Data regarding DM duration and beta blocker use was not available for all participants resulting in smaller subset analytic samples.DM duration is subject to potential recall bias and systematic error. The lack of tissue doppler studies in JHS limited our ability to evaluate diastolic parameters. Finally, limited M-mode LV FS outcome data may affect the power study observations.
Availability of data and materials
The data that support the findings of this study are available from [Jackson Heart Study] but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of [Jackson Heart Study].
Abbreviations
- DM:
-
Diabetes Mellitus
- CAD:
-
Coronary artery disease
- ESRD:
-
End-stage renal disease
- PDM:
-
Prediabetes
- JHS:
-
Jackson Heart Stud
- LV EF:
-
Left ventricular ejection fraction
- LV FS:
-
Left ventricular fractional shortening
- SVI:
-
Stroke volume index
- CI:
-
Cardiac Index
- LVEDVI:
-
Left ventricular end diastolic volume index
- LVESVI:
-
Left ventricular end systolic volume index
- RWT:
-
Relative wall thickness
- MCF:
-
Myocardial contraction fraction
- LVMI:
-
Left ventricular mass index
- D-CM:
-
Diabetic cardiomyopathy
- LV:
-
Left ventricular
- LV SF:
-
Left ventricular structure and function
- LVEDD:
-
Left Ventricular End Diastolic Diameter
- LVESD:
-
Left Ventricular End Systolic Diameter
- LVM:
-
Left Ventricular Mass
- HbA1c:
-
Glycated Hemoglobin
- LDL-C:
-
Low density lipoproteins
- HDL-C:
-
High density lipoproteins
- CKD:
-
Chronic Kidney Disease
- FFQ:
-
Food Frequency Questionnaire
- MDRD:
-
Modification of Diet in Renal Disease
- AHA:
-
American Heart Association
- MSRA:
-
Medication Survey Form
- ACE:
-
Angiotensin converting enzyme inhibitor
- ARB:
-
Angiotensin receptor blockers
- BSA:
-
Body surface area
- BMI:
-
Body mass index
References
Centers for Disease Control and Prevention. National diabetes statistics report, 2017. Atlanta: Centers for Disease Control and Prevention, US Dept of Health and Human Services. 2017.
Tabák AG, Herder C, Rathmann W, Brunner EJ, Kivimäki M. Prediabetes: a high-risk state for developing diabetes. Lancet. 2012;379(9833):2279.
Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics—2020 update: a report from the american heart association. Circulation. 2020;141(9):e139–596.
Kannel WB, McGee DL. Diabetes and cardiovascular disease: the Framingham study. JAMA. 1979;241(19):2035–8.
Soläng L, Malmberg K, Ryden L. Diabetes mellitus and congestive heart failure. Eur Heart J. 1999;20(11):789–95.
Aronow WS, Ahn C. Incidence of heart failure in 2,737 older persons with and without diabetes mellitus. Chest. 1999;115(3):867–8.
Thrainsdottir IS, Aspelund T, Thorgeirsson G, Gudnason V, Hardarson T, Malmberg K, et al. The association between glucose abnormalities and heart failure in the population-based Reykjavik study. Diabetes Care. 2005;28(3):612–6.
Rydén L, Armstrong PW, Cleland JGF, Horowitz JD, Massie BM, Packer M, et al. Efficacy and safety of high-dose lisinopril in chronic heart failure patients at high cardiovascular risk, including those with diabetes mellitus. Results from the ATLAS trial. Eur Heart J. 2000;21(23):1967–78.
Stratton IM, Adler AI, Neil HAW, Matthews DR, Manley SE, Cull CA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321(7258):405–12.
Rubler S, Dlugash J, Yuceoglu YZ, Kumral T, Branwood AW, Grishman A. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am J Cardiol. 1972;30(6):595–602.
Fang ZY, Prins JB, Marwick TH. Diabetic cardiomyopathy: evidence, mechanisms, and therapeutic implications. Endocr Rev. 2004;25(4):543–67.
Jia G, Hill MA, Sowers JR. Diabetic cardiomyopathy: an update of mechanisms contributing to this clinical entity. Circ Res. 2018;122(4):624–38.
Ernande L, Bergerot C, Rietzschel ER, De Buyzere ML, Thibault H, Pignonblanc PG, et al. Diastolic dysfunction in patients with type 2 diabetes mellitus: is it really the first marker of diabetic cardiomyopathy? J Am Soc Echocardiogr. 2011;24(11):1268.
Nakai H, Takeuchi M, Nishikage T, Lang RM, Otsuji Y. Subclinical left ventricular dysfunction in asymptomatic diabetic patients assessed by two-dimensional speckle tracking echocardiography: correlation with diabetic duration. Eur J Echocardiogr J Work Group Echocardiogr Eur Soc Cardiol. 2009;10(8):926–32.
Ng ACT, Delgado V, Bertini M, van der Meer RW, Rijzewijk L, Shanks M, et al. Findings from left ventricular strain and strain rate imaging in asymptomatic patients with type 2 diabetes mellitus. 2009.
Zoroufian A, Razmi T, Taghavi-Shavazi M, Lotfi-Tokaldany M, Jalali A. Evaluation of subclinical left ventricular dysfunction in diabetic patients: longitudinal strain velocities and left ventricular dyssynchrony by two-dimensional speckle tracking echocardiography study. Echocardiography (Mount Kisco, NY). 2014;31(4):456.
Dries DL, Sweitzer NK, Drazner MH, Stevenson LW, Gersh BJ. Prognostic impact of diabetes mellitus in patients with heart failure according to the etiology of left ventricular systolic dysfunction. J Am Coll Cardiol. 2001;38(2):421–8.
Chang PP, Chambless LE, Shahar E, Bertoni AG, Russell SD, Ni H, et al. Incidence and survival of hospitalized acute decompensated heart failure in four US communities (from the Atherosclerosis Risk in Communities Study). Am J Cardiol. 2014;113(3):504–10.
Brancati FL, Kao WL, Folsom AR, Watson RL, Szklo M. Incident type 2 diabetes mellitus in African American and white adults: the atherosclerosis risk in communities study. JAMA. 2000;283(17):2253–9.
Elliott P, Andersson B, Arbustini E, Bilinska Z, Cecchi F, Charron P, et al. Classification of the cardiomyopathies: a position statement from the European Society Of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2007;29(2):270–6.
Taylor HA Jr, Wilson JG, Jones DW, Sarpong DF, Srinivasan A, Garrison RJ, et al. Toward resolution of cardiovascular health disparities in African Americans: design and methods of the Jackson Heart Study. Ethn Dis. 2005;15(4 Suppl 6):S6–S4.
Carpenter MA, Crow R, Steffes M, Rock W, Skelton T, Heilbraun J, et al. Laboratory, reading center, and coordinating center data management methods in the Jackson Heart Study. Am J Med Sci. 2004;328(3):131–44.
Zelnick LR, Katz R, Young BA, Correa A, Kestenbaum BR, de Boer IH, et al. Echocardiographic measures and estimated GFR decline among African Americans: the Jackson heart Study. Am J Kidney Dis. 2017;70(2):199–206.
King DL, Coffin LE-K, Maurer MS. Myocardial contraction fraction: a volumetric index of myocardial shortening by freehand three-dimensional echocardiography. J Am Coll Cardiol. 2002;40(2):325–9.
Fuqua SR, Wyatt SB, Andrew ME, Sarpong DF, Henderson FR, Cunningham MF, et al. Recruiting African-American research participation in the Jackson Heart Study: methods, response rates, and sample description. Ethn Dis. 2005;15(4 Suppl 6):S6-18.
Carithers TC, Talegawkar SA, Rowser ML, Henry OR, Dubbert PM, Bogle ML, et al. Validity and calibration of food frequency questionnaires used with African-American adults in the Jackson Heart Study. J Am Diet Assoc. 2009;109(7):1184–93.
Folsom AR, Shah AM, Lutsey PL, Roetker NS, Alonso A, Avery CL, et al. American Heart Association’s Life’s Simple 7: avoiding heart failure and preserving cardiac structure and function. Am J Med. 2015;128(9):970–6.
Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling—concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. J Am Coll Cardiol. 2000;35(3):569–82.
Cohen-Solal A, Jacobson AF, Piña IL. Beta blocker dose and markers of sympathetic activation in heart failure patients: interrelationships and prognostic significance. ESC Heart Failure. 2017;4(4):499–506.
Dimitrijevic ZM, Salinger Martinovic SS, Nikolic VN, Cvetkovic TP. Protein carbonyl content is a predictive biomarker of eccentric left ventricular hypertrophy in hemodialysis patients. Diagnostics. 2019;9(4):202.
Schutta MH. Diabetes and hypertension: epidemiology of the relationship and pathophysiology of factors associated with these comorbid conditions. J Cardiometab Syndr. 2007;2(2):124–30.
Kato M, Kitada S, Kawada Y, Nakasuka K, Kikuchi S, Seo Y, et al. Left ventricular end-systolic volume is a reliable predictor of new-onset heart failure with preserved left ventricular ejection fraction. Cardiol Res Pract. 2020;2020:3106012.
Mele D, Pestelli G, Molin DD, Trevisan F, Smarrazzo V, Luisi GA, et al. Echocardiographic evaluation of left ventricular output in patients with heart failure: a per-beat or per-minute approach? J Am Soc Echocardiogr. 2020;33(2):135-47.e3.
Boyer JK, Thanigaraj S, Schechtman KB, Pérez JE. Prevalence of ventricular diastolic dysfunction in asymptomatic, normotensive patients with diabetes mellitus. Am J Cardiol. 2004;93(7):870–5.
Jensen MT, Fung K, Aung N, Sanghvi MM, Chadalavada S, Paiva JM, et al. Changes in cardiac morphology and function in individuals with diabetes mellitus: the UK Biobank cardiovascular magnetic resonance substudy. Circ Cardiovasc Imaging. 2019;12(9):e009476.
Bertoni AG, Goff DC, D’Agostino RB, Liu K, Hundley WG, Lima JA, et al. Diabetic cardiomyopathy and subclinical cardiovascular disease. Diabetes Care. 2006;29(3):588.
Dhalla NS, Liu X, Panagia V, Takeda N. Subcellular remodeling and heart dysfunction in chronic diabetes. Cardiovasc Res. 1998;40(2):239–47.
Anversa P, Leri A, Beltrami CA, Guerra S, Kajstura J. Myocyte death and growth in the failing heart. Lab Investig J Tech Methods Pathol. 1998;78(7):767.
Belke DD, Larsen TS, Gibbs EM, Severson DL. Altered metabolism causes cardiac dysfunction in perfused hearts from diabetic (db/db) mice. Am J Physiol Endocrinol Metab. 2000;279(5):E1104–13.
Cai L, Li W, Wang G, Guo L, Jiang Y, Kang YJ. Hyperglycemia-induced apoptosis in mouse myocardium: mitochondrial cytochrome C-mediated caspase-3 activation pathway. Diabetes. 2002;51:1938–48.
Rodrigues B, Cam MC, Kong J, Goyal RK, McNeill JH. Strain differences in susceptibility to streptozotocin-induced diabetes: effects on hypertriglyceridemia and cardiomyopathy. Cardiovasc Res. 1997;34(1):199–205.
Rodrigues B, Cam MC, McNeill JH. Metabolic disturbances in diabetic cardiomyopathy. Mol Cell Biochem. 1998;180(1–2):53–7.
Devereux RB, Roman MJ, Paranicas M, O’grady MJ, Lee ET, Welty TK, et al. Impact of diabetes on cardiac structure and function: the strong heart study. Circulation. 2000;101(19):2271–6.
Sacre JW, Franjic B, Jellis CL, Jenkins C, Coombes JS, Marwick TH. Association of cardiac autonomic neuropathy with subclinical myocardial dysfunction in type 2 diabetes. JACC Cardiovasc Imaging. 2010;3(12):1207.
Turpeinen AK, Vanninen E, Kuikka JT, Uusitupa MI. Demonstration of regional sympathetic denervation of the heart in diabetes: comparison between patients with NIDDM and IDDM. Diabetes Care. 1996;19(10):1083–90.
Ewing D, Campbell I, Clarke B. Heart rate changes in diabetes mellitus. The Lancet. 1981;317(8213):183–6.
Balcıoğlu AS, Müderrisoğlu H. Diabetes and cardiac autonomic neuropathy: clinical manifestations, cardiovascular consequences, diagnosis and treatment. World J Diabetes. 2015;6(1):80–91.
Stevens MJ, Dayanikli F, Raffel DM, Allman KC, Sandford T, Feldman EL, et al. Scintigraphic assessment of regionalized defects in myocardial sympathetic innervation and blood flow regulation in diabetic patients with autonomic neuropathy. J Am Coll Cardiol. 1998;31(7):1575–84.
Matthews S, Deng L, Flint K. Myocardial contraction fraction-a simple echocardiographic index with prognostic significance in patients with hfpef: a secondary analysis of the topcat trial. J Am Coll Cardiol. 2020;75(11):719.
Maurer MS, Ginns J, Maron B, Olivotto I, Lesser J, Gruner C, et al. THE myocardial contraction fraction (MCF) is associated with Nyha class as well as delayed enhancement by cardiac MRI in hypertrophic cardiomyopathy and predicts sudden cardiac death. J Am Coll Cardiol. 2016;67(13S):1508.
Abdalla M, Akwo EA, Bluemke DA, Lima JA, Shimbo D, Maurer MS, et al. Association between reduced myocardial contraction fraction and cardiovascular disease outcomes: The Multi-Ethnic Study of Atherosclerosis. Int J Cardiol. 2019;293:10–6.
Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic Implications of Echocardiographically Determined Left Ventricular Mass in the Framingham Heart Study. N Engl J Med. 1990;322(22):1561–6.
Biton Y, Goldenberg I, Kutyifa V, Baman JR, Solomon S, Moss AJ, et al. Relative wall thickness and the risk for ventricular tachyarrhythmias in patients with left ventricular dysfunction. J Am Coll Cardiol. 2016;67(3):303–12.
Gardin JM, Wagenknecht LE, Anton-Culver H, Flack J, Gidding S, Kurosaki T, et al. Relationship of cardiovascular risk factors to echocardiographic left ventricular mass in healthy young black and white adult men and women: the CARDIA study. Circulation. 1995;92(3):380–7.
Bella JN, Devereux RB, Roman MJ, Palmieri V, Liu JE, Paranicas M, et al. Separate and joint effects of systemic hypertension and diabetes mellitus on left ventricular structure and function in American Indians (the Strong Heart Study). Am J Cardiol. 2001;87(11):1260–5.
Eguchi K, Boden-Albala B, Jin Z, Rundek T, Sacco RL, Homma S, et al. Association between diabetes mellitus and left ventricular hypertrophy in a multiethnic population. Am J Cardiol. 2008;101(12):1787–91.
Shimizu M, Umeda K, Sugihara N, Yoshio H, Ino H, Takeda R, et al. Collagen remodelling in myocardia of patients with diabetes. J Clin Pathol. 1993;46(1):32.
Yap J, Tay Wan T, Teng Tiew-Hwa K, Anand I, Richards AM, Ling Lieng H, et al. Association of diabetes mellitus on cardiac remodeling, quality of life, and clinical outcomes in heart failure with reduced and preserved ejection fraction. J Am Heart Assoc. 2019;8(17):e013114.
Pareek M, Aharaz A, Nielsen ML, Gerke O, Leósdóttir M, Møller JE, et al. Untreated diabetes mellitus, but not impaired fasting glucose, is associated with increased left ventricular mass and concentric hypertrophy in an elderly, healthy, Swedish population. IJC Metabolic Endocrine. 2015;9:39–47.
Fraser GE, Luke R, Thompson S, Smith H, Carter S, Sharpe N. Comparison of echocardiographic variables between type I diabetics and normal controls. Am J Cardiol. 1995;75(2):141–5.
Sanderson JE, Fraser AG. Systolic dysfunction in heart failure with a normal ejection fraction: echo-Doppler measurements. Prog Cardiovasc Dis. 2006;49(3):196–206.
Acknowledgements
The Jackson Heart Study (JHS) is supported and conducted in collaboration with Jackson State University (HHSN268201800013I), Tougaloo College (HHSN268201800014I), the Mississippi State Department of Health (HHSN268201800015I) and the University of Mississippi Medical Center (HHSN268201800010I, HHSN268201800011I and HHSN268201800012I) contracts from the National Heart, Lung, and Blood Institute (NHLBI) and the National Institute on Minority Health and Health Disparities (NIMHD). The authors also wish to thank the staffs and participants of the JHS. The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services.
Funding
None.
Author information
Authors and Affiliations
Contributions
CA conceptualized, designed, analyzed, interpretated, drafted and edited the final manuscript. DS designed, analyzed, interpreted, drafted, and edited the final manuscript. CA conceptualized, designed, interpreted, and edited the final manuscript. IA conceptualized, designed, interpreted, and edited the final manuscript. JB designed, interpreted, and edited the final manuscript. EO designed, interpreted, and edited the final manuscript. RJM conceptualized, designed, interpreted, and edited the final manuscript. AGB conceptualized, designed, interpreted, and edited the final manuscript. GF conceptualized, designed, analyzed, interpretated, drafted and edited the final manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
In the original Jackson Heart Study, IRB review including informed consent was obtained at the Mississippi universities involved with this work. This secondary analysis of JHS data was reviewed by the Loma Linda University Institutional Review Board and determined to be non-human participants research and exempted from IRB review.
Consent for publication
Not applicable.
Competing interests
No conflict/Competing interest to report.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
. Supplementary Table 1. Distribution of study variables by exclusion criteria (presence or absence of CAD, arrhythmia, valvular Disease or ESRD).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ani, C., Shavlik, D., Knutsen, S. et al. Glycemic status, non-traditional risk and left ventricular structure and function in the Jackson Heart Study. BMC Cardiovasc Disord 22, 186 (2022). https://doi.org/10.1186/s12872-022-02605-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-022-02605-w