Abstract
Purpose
The purpose of this study was to investigate the safety and efficacy of high-power short-duration (HP-SD) ablation compared to conventional ablation in patients with atrial fibrillation (AF).
Methods
We enrolled consecutive 158 drug-refractory symptomatic AF patients (119 males, mean age 63 ± 10 years) who had undergone first radiofrequency pulmonary vein isolation (PVI). PVI was performed using the conventional setting (20–35 W) in 73 patients (Conventional group) and using the HP-SD setting (45–50 W) in 85 patients (HP-SD group). The rate of first pass isolation, remaining gaps after circumferential ablation, dormant conduction, and the radiofrequency application time in each pulmonary vein (PV) were compared between the groups.
Results
The first pass isolation ratio was significantly higher in the HP-SD group than in the Conventional group (81% vs. 65%, P = 0.027) in the right PV, but did not differ in the left PV. The remaining gaps were fewer in the right superior PV (4% vs. 21%, P = 0.001) and left inferior PV (1% vs. 8%, P = 0.032) areas, and the radiofrequency application time in each PV was shorter (right PV, 12.0 ± 8.9 min vs. 34.0 ± 31.7 min, P < 0.001; left PV, 10.6 ± 3.6 min vs. 25.7 ± 22.3 min, P < 0.001) in the HP-SD group than in the Conventional group.
Conclusion
The use of the HP-SD setting might contribute to improve the first pass isolation rate and to shorten the radiofrequency application time in each PV.
Similar content being viewed by others
Introduction
Atrial fibrillation (AF) has been recognized as the most common sustained arrhythmia and is associated with both cardiovascular mortality and substantial morbidity. [1,2,3] Pulmonary vein isolation (PVI) is an established therapy because of its efficacy. [4, 5] However, it is still a major concern to decrease AF recurrence after PVI. Therefore, the development of an innovative approach to decrease AF recurrence after PVI is important. Recently, high-power short-duration (HP-SD) ablation has been introduced as a new PVI method, and its efficacy and safety have been validated in previous studies. [6,7,8] The HP-SD setting might shorten the conductive heating phase and lead to the reduction of collateral tissue damage. However, lesion characteristics during the HP-SD ablation procedure have not yet been evaluated. The aim of the present study was to investigate procedural characteristics of PVI using the HP-SD setting compared to the conventional setting.
Methods
Study population
We retrospectively enrolled 173 consecutive AF patients who had successfully undergone first PVI for symptomatic drug-refractory AF at our hospital between January 2018 and October 2019, during when we replaced AF ablation with the HP-SD setting (Fig. 1). Echocardiography and computed tomography revealed no structural heart disease in any patient. The patients were divided into two groups based on the used radiofrequency power output setting. We analyzed the first 73 patients who underwent ablation in a conventional setting with a radiofrequency power of 20–35 W before the introduction of HP-SD setting (Conventional group), and the following 85 patients who underwent HP-SD ablation with a radiofrequency power of 45–50 W (HP-SD group). Fifteen patients were excluded because they had undergone the procedure at a radiofrequency power of 36–44 W. Finally, 158 patients (119 males, mean age 63 ± 10 years, 99 paroxysmal AF) were enrolled in the study. Written informed consent was obtained from all study subjects, and the study was approved by the ethics committee of Fukushima Medical University.
Catheter ablation of atrial fibrillation
Our institution has 1 electrophysiological laboratory and 4 electrophysiologists. We had performed 275 catheter ablation procedures per year in average during study period, the 10% of these procedures for ventricular tachyarrhythmias and the remaining 90% for atrial arrhythmias such as AF, supraventricular tachycardia and atrial tachycardia. The detailed procedural protocol of PVI using radiofrequency catheter ablation at our hospital was as previously described. [9] Each patient underwent an electrophysiologic study and first radiofrequency catheter ablation. Anticoagulative drugs were continued or minimally discontinued during perioperative days at the individual operator’s discretion. [10, 11] Anti-arrhythmic drugs were discontinued for at least five half-lives. PVI was performed under sedation with intravenous dexmedetomidine and fentanyl. A 10-polar catheter was positioned at the His bundle area via the right femoral vein to record a His potential. A 20-polar 3-site (CS, lateral right atrium and SVC) mapping catheter (BeeAT, Japan-Life-Line, Tokyo, Japan) was inserted via the right subclavian vein. Proximal 4-polar electrodes were located at the superior vena cava (SVC), middle 8-polar electrodes at the right atrium, and distal 10-polar at the coronary sinus (CS). Twelve-lead surface electrocardiograms (ECGs) and intracardiac electrograms were recorded simultaneously by digital multichannel systems (Prucka CardioLab®, General Electrical Healthcare, Milwaukee, WI, USA and RMC-5000, Nihon Kohden Corp. Tokyo, Japan), filtered at 30–400 Hz for bipolar and 0.05–400 Hz for unipolar electrograms.
All PVI was performed using a 3-dimensional electro-anatomical mapping system (CARTO system, Biosense Webster, Diamond Bar, CA, USA). In the Conventional group, an 8-Fr irrigation catheter with a 3.5-mm distal electrode and real-time contact force (CF) monitoring (ThermoCool SmartTouch, Biosense Webster) or an 8-Fr surround flow irrigation catheter with a 3.5-mm distal electrode and real-time CF monitoring, which has a 56-hole porous tip for surround flow irrigation (ThermoCool SmartTouch SF, Biosense Webster) was used for radiofrequency energy delivery. Radiofrequency energy was delivered at 20–35 W using a point-by-point or dragging technique at the individual operator’s discretion. In the HP-SD group, ThermoCool SmartTouch SF was used for radiofrequency energy delivery. Radiofrequency energy was delivered at 45–50 W using a point-by-point technique and ablation index (AI) module (Biosense Webster). AI is a novel lesion quantity parameter that incorporates CF, time, and power in a weighted formula, and has been shown to accurately estimate lesion depth in canine studies. [12, 13] The duration of radiofrequency application was determined by AI value in the present study. The targeted AI was set at 380–400 in the left posterior PV, and at 400–430 at other sites in the left PV (LPV) and right PV (RPV). The targeted CF were 10–25 g in the Conventional group and 5–20 g in the HP-SD group. Force time integral (FTI) was defined as the total CF integrated over the duration of radiofrequency current delivery at each ablation point. After completion of PVI, intravenous adenosine (20 mg) was administered to check for dormant LA-PV conduction in the right PVs during sinus rhythm and in the left PVs during distal CS pacing with continuous administration of isoproterenol (1.0–3.0 mg/min).
Analysis of procedural parameters during PVI
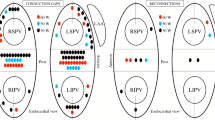
PV was divided in six areas: the superior, carina, and inferior areas of each side of the PV. The prevalence of first pass isolation, remaining gaps between the left atrium (LA) and PV after circumferential ablation and dormant conduction after completion of PVI were also investigated in each of the six areas (Fig. 2). Radiofrequency application time in each side of the PVI and total procedure time were also calculated.
Follow-up
Follow-up was performed at 1, 3 and 6 months after the procedure, and every 3 months thereafter. At each visit, 12-lead ECG, and 24-h Holter monitoring were performed. Recurrence was defined as documentation of atrial tachycardia, or AF lasting > 30 s recorded by 12-lead ECG or 24-h Holter monitoring. AAD were continued for 3 months after PVI and discontinued thereafter. The long-term efficacy was assessed clinically on the basis of the clinical symptoms, surface 12-lead ECG, and 24-h Holter monitoring.
Statistical analysis
A Chi-square test was used for comparisons of categorical variables, which are expressed as numbers and percentages. Continuous variables are presented as mean ± standard deviation, and were compared using Student’s t-test. All analyses were performed using SPSS for Windows, version 25.0 (SPSS Inc., Chicago, IL, USA), and all statistical tests were two-sided. A P-value of < 0.05 was considered statistically significant (All data analyzed during this study are included in the Additional file 1).
Results
Patient characteristics
The patient characteristics are summarized in Table 1. There were no significant differences in sex, age, type of AF, body mass index, BNP, estimated glomerular filtration rate, left atrial dimension, left atrial volume index, or left ventricular ejection fraction between the two groups (Table 2).
Comparison of procedural characteristics between the Conventional and HP-SD settings
In the RPV, first pass isolation was achieved more frequently in the HP-SD group than in the Conventional group (81% vs. 65%, P = 0.027) (Table 3). However, the rate of first pass isolation in the LPV did not differ between the two groups (83% vs. 80%, P = 0.657) (Table 3). The remaining gaps after circumferential ablation were fewer in the right superior PV (4% vs. 21%, P = 0.001) and the left inferior PV (1% vs. 8%, P = 0.032) areas in the HP-SD group compared to the Conventional group (Fig. 3). The dormant conduction tended to be fewer in the HP-SD group than in the Conventional group in the RPV (1% vs. 6%, P = 0.062) (Fig. 4). In addition, radiofrequency application time in each side of the PV was shorter in the HP-SD group than in the Conventional group (RPV,
Distribution of the remaining gaps after circumferential ablation in each PV. The remaining gaps after circumferential ablation were fewer in the right superior PV and the left inferior PV areas in the HP-SD group compared to the Conventional group. HP-SD, high-power short-duration; LPV, left pulmonary vein; RPV, right pulmonary vein
12.0 ± 8.9 min vs. 34.0 ± 31.7 min, P < 0.001; LPV, 10.6 ± 3.6 min vs. 25.7 ± 22.3 min, P < 0.001). The total procedure time was also shorter in the HP-SD group than in the Conventional groups (171.8 ± 40.8 min vs. 193.1 ± 45.3 min, P = 0.002).
Clinical outcome after PVI
During a follow-up period of 753 days, recurrence of atrial arrhythmia was documented in 15 patients (11%). With respect to the comparison of ablation outcome between the two groups, atrial arrythmia recurrence seemed to be more frequent in the Conventional group (16.3% vs. 7.1%, P = 0.083). The Kaplan–Meier time-to-event curves for recurrence showed that the recurrence rate in the Conventional group seemed to be higher than that in the HP-SD group (Log rank; P = 0.289) (Fig. 5). Regarding major ablation-related complications during PVI, pericardial effusion was observed in two patients in the Conventional group but in no patients in the HP-SD group. The other complications were never occurred in both groups.
Discussion
The major findings of the present study were that the HP-SD setting was able to improve the rate of first pass isolation, reduce the remaining gap after circumferential ablation and the radiofrequency application time as well as the total procedure time. Moreover, this setting might reduce the dormant conduction after circumferential ablation and the rate of atrial tachyarrhythmia recurrence.
Previous findings about HP-SD ablation
Recently, several studies have revealed the efficacy of HP-SD ablation. Okamatsu, et al. showed that HP-SD ablation could shorten the time to complete circumferential PVI and improve the rate of first pass isolation. [6] In addition, an extremely low rate of complications was demonstrated by using HP-SD ablation. [6, 8]
Procedural characteristics of HP-SD ablation
Our data also revealed that HP-SD ablation could shorten radiofrequency application time and total procedure time. AI was calculated with ablation time, radiofrequency power, catheter stability and CF. [12, 13] To achieve the targeted AI, the needed radiofrequency application time would be shorter when using HP-SD setting compared to conventional setting. Moreover, the reduced remaining gap might shorten the procedure time by avoiding the additional radiofrequency application. Thus, it was acceptable that both radiofrequency application time and procedure time were shortened in the HP-SD group.
The results of the present study showed the trend of lower prevalence of remaining gaps after circumferential PVI in the HP-SD group. Mulder et al. revealed that local LA wall thickness was associated with acute PV reconnection after PVI. [14] In addition, our previous report showed that the presence of hypertension induced heterogeneous LA wall hypertrophic change and LA remodeling. [15] Therefore, it might be possible that the LA wall thickness might affect the existence of remaining gaps after circumferential PVI.
Clinical implication
The safety of AI guided ablation has already been validated in some reports. [7, 12, 13] In the present study, the major complication during PVI was pericardial effusion, which occurred in two patients of the Conventional group but in no patients of the HP-SD group; and no other complications occurred in either group. No major complication in the HP-SD group in the present study suggests that the safety of the HP-SD setting. Moreover, the advantage of HP-SD setting has been shown to prevent collateral tissue damage by shortening the resistive heating phase. [8, 16] Especially, the prevention of esophageal complication is very important because esophageal mucosal injury has the potential to advance to left atrial-esophagus fistula, which is a fatal complication associated with PVI. [17, 18] As we had reported the advantage of HP-SD setting in preventing esophageal complications, we should take advantage of this setting in regard to safety. [19, 20]
Study limitations
There are several limitations to the present study. First, it was a single center observational study; therefore, further evaluation in a multicenter, randomized control study is necessary. Second, the follow-up period was relatively short in the HP-SD group. A large-scale study with a longer follow up period is needed to investigate further. Third, the present study showed the favorable trend of the long-term efficacy when using HP-SD setting. However, there was a lack of data about the AF duration of each patient in both groups, and the impact on the long-term efficacy in the present study might be limited.
Conclusions
The results of the present study indicate that the HP-SD setting might contribute to improve the rate of first pass isolation and reduce the radiofrequency application time as well as total procedure time. Moreover, the HP-SD setting might contribute to reduce the recurrence of atrial tachyarrhythmia after PVI using HP-SD setting.
Availability of data and materials
All data analyzed during this study are included in the supplementary information files.
References
Benjamin EJ, Wolf PA, Silbershatz H, et al. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–52.
Lloyd-Jones DM, Wang TJ, Leio EP, et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation. 2004;110:1042–6.
Machino-Ohtsuka T, Seo Y, Ishizu T, et al. Relationships between maintenance of sinus rhythm and clinical outcomes in patients with heart failure with preserved ejection fraction and atrial fibrillation. J Cardiol. 2019;74:235–44.
Okada M, Tanaka N, Oka T, et al. Clinical significance of left ventricular reverse remodeling after catheter ablation of atrial fibrillation in patients with left ventricular systolic dysfunction. J Cardiol. 2021;77:500–8.
Katayama H, Shibata A, Doi A, et al. Successful catheter ablation improves exercise tolerance in persistent atrial fibrillation patients, especially those with reduced ventricular contraction, preserved atrial function, or a high CHADS2 score. J Cardiol. 2020;75:529–36.
Okamatsu H, Koyama J, Sakai Y, et al. High-power application is associated with shorter procedure time and higher rate of first-pass pulmonary vein isolation in ablation index-guided atrial fibrillation ablation. J Cardiovasc Electrophysiol. 2019;30:2751–8.
Wielandts JY, Kyriakopoulou M, Almorad A, et al. Prospective randomized evaluation of high power during CLOSE-Guided pulmonary vein isolation: the POWER-AF Study. Circ Arrhythm Electrophysiol. 2021;14:e009112.
Winkle RA, Mohanty S, Patrawala RA, et al. Low complication rates using high power (45–50 W) for short duration for atrial fibrillation ablations. Heart Rhythm. 2019;16:165–9.
Hijioka N, Kamioka M, Matsumoto Y, et al. Clinical impact of insulin resistance on pulmonary vein isolation outcome in patients with paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol. 2019;30:479–86.
Kimata A, Nogami A, Yamasaki H, et al. Optimal interruption time of dabigatran oral administration to ablation (O-A time) in patients with atrial fibrillation: integrated analysis of 2 randomized controlled clinical trials. J Cardiol. 2021;77:652–9.
Inoue K, Hirano K, Kumagai K, et al. Long-term efficacy and safety of anticoagulation after atrial fibrillation ablation: data from the JACRE registry. J Cardiol. 2021;77:263–70.
Das M, Loveday JJ, Wynn GJ, et al. Ablation index, a novel marker of ablation lesion quality: prediction of pulmonary vein reconnection at repeat electrophysiology study and regional differences in target values. Europace. 2017;19:775–83.
Hussein A, Das M, Chaturvedi V, et al. Prospective use of Ablation Index targets improves clinical outcomes following ablation for atrial fibrillation. J Cardiovasc Electrophysiol. 2017;28:1037–47.
Mulder MJ, Kemme MJB, Hagen AMD, et al. Impact of local left atrial wall thickness on the incidence of acute pulmonary vein reconnection after Ablation Index-guided atrial fibrillation ablation. Int J Cardiol Heart Vasc. 2020;29:100574.
Kamioka M, Hijioka N, Matsumoto Y, et al. Uncontrolled blood pressure affects atrial remodeling and adverse clinical outcome in paroxysmal atrial fibrillation. Pacing Clin Electrophysiol. 2018;41:402–10.
Castrejón-Castrejón S, Martínez Cossiani M, Ortega Molina M, et al. Feasibility and safety of pulmonary vein isolation by high-power short-duration radiofrequency application: short-term results of the POWER-FAST PILOT study. J Interv Card Electrophysiol. 2020;57:57–65.
Pappone C, Oral H, Santinelli V, et al. Atrio-esophageal fistula as a complication of percutaneous transcatheter ablation of atrial fibrillation. Circulation. 2004;109:2724–6.
Dagres N, Kottkamp H, Piorkowski C, et al. Rapid detection and successful treatment of esophageal perforation after radiofrequency ablation of atrial fibrillation: lessons from five cases. J Cardiovasc Electrophysiol. 2006;17:1213–5.
Kaneshiro T, Kamioka M, Hijioka N, et al. Characteristics of esophageal injury in ablation of atrial fibrillation using a high-power short-duration setting. Circ Arrhythm Electrophysiol. 2020;13(10):e008602.
Kaneshiro T, Amami K, Hijioka N, et al. Significance of contact force on esophageal thermal injury during relative high-power short-duration ablation of atrial fibrillation. Circ Arrhythm Electrophysiol. 2021;14(6):e009897.
Acknowledgements
None.
Funding
The authors did not receive support from any organization for the submitted work.
Author information
Authors and Affiliations
Contributions
NH, TK and MK made contributions to the conception, design of the work, the acquisition/analysis/interpretation of data and drafting the manuscript. TN, KA, MN, SY, TI made contributions to data collection and approval of article. YT made contributions to critical revision of article and approval of article. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocol was approved by the ethical committee of Fukushima Medical University. Written informed consent was obtained from all study subjects.
Consent for publication
Not applicable.
Competing interests
The authors declare that there is no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
The results of patient characteristics, procedural details of ablation and follow-up data after ablation procedures.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Hijioka, N., Kaneshiro, T., Nehashi, T. et al. Procedural characteristics of pulmonary vein isolation with high-power short-duration setting compared to conventional setting. BMC Cardiovasc Disord 22, 14 (2022). https://doi.org/10.1186/s12872-022-02459-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-022-02459-2