Abstract
Background
Constrictive pericarditis (CP) is characterized by scarring and loss of elasticity of the pericardium. This case demonstrates that mixed martial arts (MMA) is a previously unrecognized risk factor for CP, diagnosis of which is supported by cardiac imaging, right and left heart catheterization, and histological findings of dense fibrous tissue without chronic inflammation.
Case presentation
A 47-year-old Caucasian male former mixed martial arts (MMA) fighter from the Western United States presented to liver clinic for elevated liver injury tests (LIT) and a 35-pound weight loss with associated diarrhea, lower extremity edema, dyspnea on exertion, and worsening fatigue over a period of 6 months. Past medical history includes concussion, right bundle branch block, migraine headache, hypertension, chronic pain related to musculoskeletal injuries and fractures secondary to MMA competition. Involvement in MMA was extensive with an 8-year history of professional MMA competition and 13-year history of MMA fighting with recurrent trauma to the chest wall. The patient also reported a 20-year history of performance enhancing drugs including testosterone. Physical exam was notable for elevated jugular venous pressure, hepatomegaly, and trace peripheral edema. An extensive workup was performed including laboratory studies, abdominal computerized tomography, liver biopsy, echocardiogram, and cardiac magnetic resonance imaging. Finally, right and left heart catheterization—the gold standard—confirmed discordance of the right ventricle-left ventricle, consistent with constrictive physiology. Pericardiectomy was performed with histologic evidence of chronic pericarditis. The patient’s hospital course was uncomplicated and he returned to NYHA functional class I.
Conclusions
CP can be a sequela of recurrent pericarditis or hemorrhagic effusions and may have a delayed presentation. In cases of recurrent trauma, CP may be managed with pericardiectomy with apparent good outcome. Further studies are warranted to analyze the occurrence of CP in MMA so as to better define the risk in such adults.
Similar content being viewed by others
Background
This case demonstrates that mixed martial arts (MMA) with recurrent chest wall trauma can be a previously unrecognized risk factor for constrictive pericarditis (CP). CP is characterized by scarring and loss of elasticity of the pericardium. The most common etiologies of CP in Western countries are idiopathic, prior cardiac surgery, and mediastinal radiation [1]. Although uncommon, blunt trauma should be considered as an initiating cause of CP and can have a delayed presentation [2]. MMA is a contact heavy sport causing blunt trauma, based upon standing combat, grappling and ground fighting, and striking opponents by incorporating various martial arts techniques from around the world. The cardiac risks facing MMA athletes is largely unknown, since studies tend to be focused on musculoskeletal injury or head trauma [3]. Therefore, objectives of the case are as follows: to assist readers in recognizing MMA as a risk factor for CP; to diagnose CP that is potentially secondary to recurring trauma leading to hemorrhagic effusions; to recognize that in the case of repeated blunt trauma, delayed presentation of CP may be seen; to identify liver abnormalities secondary to right heart failure as a unique presentation of CP in addition to dyspnea on exertion and peripheral edema; and to select appropriate testing for CP in the setting of elevated jugular venous pressure and liver abnormalities.
Case presentation
A 47-year-old Caucasian male former MMA fighter from Nevada presented to liver clinic for elevated liver injury tests (LIT) and a 35-pound weight loss associated with nausea, vomiting, and diarrhea. He endorsed symptoms of lower extremity edema, dyspnea on exertion, and fatigue which had worsened over 6 months and was treated with diuretics. Physical exam revealed jugular venous pressure (JVP) of 15 cm with a steep y descent, without Kussmaul’s sign. He had hepatomegaly with a firm liver edge 4 cm below the right costal margin. He had normal S1 and S2 without additional sounds. He had no evidence of pleural effusion or ascites but did have trace peripheral edema.
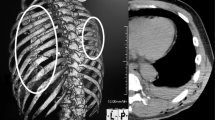
The patient has a past medical history of concussion, migraine headache, hypertension, chronic pain related to musculoskeletal injuries and fractures secondary to MMA competition, and right bundle branch block (Fig. 1). Past surgical history is notable for fracture surgeries, diagnostic right knee scope, right shoulder surgery, and foot surgery. He reported a 20-year history of performance enhancing drugs (PEDs), including testosterone use that ceased 8 months prior to presentation. Notably, the patient had an 8-year history of being a professional MMA fighter and a 13-year history of MMA fighting with recurrent trauma to the chest wall.
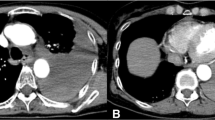
Differential diagnosis included pulmonary hypertension, chronic pulmonary emboli, heart failure, cirrhosis, and constrictive pericarditis. As patient initially presented to liver clinic, preliminary work-up showed hepatic congestion with ALT and AST of 1155 U/L and 219 U/L respectively. Abdominal computerized tomography demonstrated hepatomegaly with steatosis, ascites, a dilated inferior vena cava, and a calcified pericardium (Fig. 2). During liver biopsy, the patient had documented right atrial pressure (RAP) of 12 mmHg, free hepatic vein pressure of 12 mmHg, wedged sinusoidal pressure of 13 mmHg, and hepatic vein portal vein gradient of 1 mmHg, diagnostic of post hepatic portal hypertension secondary to right heart failure. These findings warranted referral to cardiology, where echocardiogram revealed abnormal septal motion suggestive of ventricular interdependence (Additional file 1: Video S1, Additional file 2: Video S2, Additional file 3: Video S3). Cardiac magnetic resonance imaging (MRI) revealed marked thickening of the pericardium, MRI signal void consistent with pericardial calcification, and profound late gadolinium enhancement on both standard and high-resolution inversion recovery images. There was no significant myocardial late gadolinium enhancement, suggesting that any potential inflammatory involvement of the myocardium has resolved without myocardial scar formation (Fig. 3). Right and left heart catheterization revealed normal coronary arteries and normal pulmonary artery pressures (Table 1) with right ventricle-left ventricle (RV-LV) discordance, consistent with constrictive physiology (Fig. 4).
Cardiac magnetic resonance imaging. A Steady state free precession (“cine”) imaging demonstrates circumferential thickening of the pericardium (indicated with white arrows) and significant signal void consistent with calcification B Phase sensitive inversion recovery imaging approximately 10 min after administration of gadobenate dimeglumine demonstrates marked circumferential late gadolinium enhancement (indicated with white arrows) suggesting presence of inflamed and/or scar tissue C Double inversion recovery (“black blood”) imaging demonstrates marked circumferential thickening of the pericardium (indicated with white arrows) D Whole heart high resolution 3D inversion recovery imaging demonstrates pronounced late gadolinium enhancement of the entire pericardium (indicated with white arrows). No significant late enhancement of the myocardium was seen, suggesting lack of myocardial involvement or that any associated inflammatory process involving the myocardium has resolved without myocardial scar formation
Pericardiectomy was performed via sternotomy with cardiopulmonary bypass. During the procedure, calcification and thickening of the pericardium was evident (Fig. 5). The pericardium was removed from phrenic-to-phrenic nerve anteriorly and was removed in the oblique sinus and posterior to the phrenic nerve on the left. Histology confirmed pericardial tissue with fibrosis and inflammation consistent with chronic pericarditis (Fig. 6). These changes can be seen in chronic pericarditis of various etiologies; however, there was no evidence of acute or granulomatous inflammation, and the dense fibrosis without chronic inflammation implicated repeated trauma as a potential etiology, where in the setting of lack of other risk factors, appears to be the likely cause. Fungal and bacterial cultures were negative.
After pericardiectomy, the patient’s hospital course was uncomplicated. At his 7-week post-operative clinic visit, the patient reported he had returned to jogging and no longer had any of his previous symptoms of lower extremity edema, dyspnea on exertion, fatigue, diarrhea secondary to small bowel edema, or hepatic congestion. In the absence of poor predictors, a good prognosis is anticipated, and patient is now NYHA functional class I.
Discussion and conclusions
The most common etiologies of CP in Western countries are idiopathic, prior cardiac surgery, and mediastinal radiation [1]. Infectious or inflammatory causes do not appear to be the etiology in this case. The patient did not describe any syndrome of acute pericarditis and did not live in a region where, for example, tuberculosis is more prevalent. His hepatitis screening was negative. He also did not have clinical or serological evidence of autoimmune disease, despite a thorough work-up, no history of cardiac surgery, radiation, or relevant infections, but does notably have 20 years of repeated chest trauma due to MMA fighting, thereby implicating repeated chest trauma as the most likely etiology for his CP. Admittedly, pericardial thickening and calcification often represents a burned-out stage in pericarditis regardless of etiology, by the time a patient undergoes pericardiectomy, there may be little evidence of active inflammation.
MMA is certainly a cause of recurrent blunt trauma, as it is a contact heavy sport based upon standing combat, grappling and ground fighting, and striking opponents by incorporating various martial arts techniques. Little is known regarding cardiac risks for MMA fighters, with the majority of studies has been focused on musculoskeletal or head injuries [3]. In this case, the patient stated that he was kicked, kneed, and punched in the ribs approximately 50 times in a 2-h practice, occurring 3 to 4 times a week for several years. Although uncommon, blunt trauma should be considered as a potential initiating cause of CP and can have a delayed presentation [2, 4]. This rare phenomenon has been documented sporadically in the literature, most notably in the case of a boxer with recurrent trauma to the chest wall [5]. This case expands our understanding of the physical consequences of MMA fighting and raises additional questions about the clinical sequelae of PEDs in sports [6, 7].
Right and left heart catheterization remain the gold standard in the diagnosis of CP and is primarily characterized by the near equalization of end-diastolic pressures in all chambers and the pulmonary artery [8] (Table 1). In addition to RV-LV discordance on catheterization, right atrial tracing may demonstrate a prominent y descent (Fig. 4B). Transthoracic echocardiogram (TTE) has been shown to be diagnostic of CP in 70% of cases. Both cardiac MRI and TTE are capable of distinguishing CP from other forms of heart failure, including restrictive cardiomyopathy [9,10,11], as in this case presented here.
The 5-year survival after pericardiectomy ranges from 65 to 90% depending on etiology. Predictors of poor outcome are prior radiation, renal insufficiency, poor ventricular function, higher pulmonary artery pressures, NYHA class IV, low serum sodium, ascites, and hyperbilirubinemia, all of which were absent in this patient [12].
CP can be a sequela of recurrent pericarditis or hemorrhagic effusions and may have a delayed presentation. Here, we present a case of CP most likely caused by recurrent trauma to the chest wall secondary to MMA fighting. In cases of recurrent chest wall trauma, CP may be managed with pericardiectomy with apparent good outcome. Further studies are warranted to analyze the occurrence of CP in MMA to better define the risk in such adults. In the case of individuals who have experienced recurrent or significant chest wall trauma, there must be a higher index of suspicion when considering CP on the differential diagnosis.
Availability of data and materials
The datasets during and/or analyzed during the current study are included within the manuscript and the supporting files. Additional data will be made available from the corresponding author on reasonable request.
Abbreviations
- ALT:
-
Aspartate transaminase
- AST:
-
Alanine transaminase
- CP:
-
Constrictive pericarditis
- CT:
-
Computed tomography
- PED:
-
Performance enhancing drug
- ECG:
-
Electrocardiogram
- LV:
-
Left ventricle
- RV:
-
Right ventricle
- LIT:
-
Liver injury tests
- MMA:
-
Mixed martial arts
- MRI:
-
Magnetic resonance imaging
- NYHA:
-
New York Heart Association
- RAP:
-
Right atrial pressure
- TTE:
-
Transthoracic echocardiogram
References
Syed F, Schaff H, Oh JK. Constrictive pericarditis—a curable diastolic heart failure. Nat Rev Cardiol. 2014. https://doi.org/10.1038/nrcardio.2014.100.
Anderson EM, Jaroszewski DE, Arabia FA. Blunt trauma as a suspected cause of delayed constrictive pericarditis: a case report. J Med Case Rep. 2011. https://doi.org/10.1186/1752-1947-5-76.
Bledsoe GH, Hsu EB, Li G, et al. Incidence of injury in professional mixed martial arts competitions. J Sport Sci Med. 2006;5:136.
Goldstein S, Yu PN. Constrictive pericarditis after blunt chest trauma. J Am Heart Assoc. 1965. https://doi.org/10.1016/0002-8703(65)90426-6.
Ooi A, Douds AC, Kumar EB, Nashef SAM. Boxer’s pericardium. Eur J Cardiothorac Surg. 2003. https://doi.org/10.1016/S1010-7940(03)00579-7.
Grimes JM, Ricci LA, Melloni RH. Glutamic acid decarboxylase (GAD65) immunoreactivity in brains of aggressive, adolescent anabolic steroid-treated hamsters. Horm Behav. 2003. https://doi.org/10.1016/s0018-506x(03)00138-7.
Momaya A, Fawal M, Estes R. Performance-enhancing substances in sports: a review of the literature. Sports Med. 2015. https://doi.org/10.1007/s40279-015-0308-9.
Nishimura RA. Constrictive pericarditis in the modern era: a diagnostic dilemma. Heart. 2001. https://doi.org/10.1136/heart.86.6.619.
Ling LH, Schaff HV, dal-Bianco J, et al. Detection of constrictive pericarditis: a single-centre experience of 523 surgically confirmed cases [abstract]. J Am Coll Cardiol. 2009;53:eA176.
Miranda WR, Oh JK. Constrictive pericarditis: a practical clinical approach. Prog Cardiovasc Dis. 2017. https://doi.org/10.1016/j.pcad.2016.12.008.
Oh JK, Hatle LK, Seward JB, et al. Diagnostic role of Doppler echocardiography in constrictive pericarditis. J Am Coll Cardiol. 1994. https://doi.org/10.1016/0735-1097(94)90514-2.
Busch C, Penov K, Amorim PA, et al. Risk factors for mortality after pericardiectomy for chronic constrictive pericarditis in a large single-centre cohort. Eur J Cardiothorac Surg. 2015. https://doi.org/10.1093/ejctx/ezv322.
Acknowledgements
Not applicable.
Funding
Dr. Ryan and his research team are supported by the Gordon Family.
Author information
Authors and Affiliations
Contributions
MF, JR, BW, and RT reviewed the literature and prepared the rough draft of the manuscript. RT, JR, and SM were directly involved in patient care and medical decision making. KT, BW, and SI analyzed and interpreted the echocardiogram and cardiac images. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethics approval and consent to participate was obtained.
Consent for publication
A written informed consent was obtained from the patient for publication of this manuscript and the accompanying images.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Video S1. Transthoracic echocardiogram (TTE) demonstrates abnormal septal motion indicative of ventricular interdependence.
Additional file 2: Video S2. TTE Parasternal Long axis demonstrates septal bounce.
Additional file 3: Video S3. TTE Parasternal Short axis demonstrates septal bounce.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ferrel, M.N., Iriana, S., Raymond Thomason, I. et al. Constrictive pericarditis in the setting of repeated chest trauma in a mixed martial arts fighter. BMC Cardiovasc Disord 21, 561 (2021). https://doi.org/10.1186/s12872-021-02378-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-021-02378-8